MEK inhibition preferentially suppresses anchorage-independent growth in osteosarcoma cells and decreases tumors in vivo
Takatsune Shimizu and Kiyomi Kimura contributed equally to this study.
Abstract
Osteosarcoma is the most common high-grade malignancy of bone, and novel therapeutic options are urgently required. Previously, we developed mouse osteosarcoma AXT cells that can proliferate both under adherent and nonadherent conditions. Based on metabolite levels, nonadherent conditions were more similar to the in vivo environment than adherent conditions. A drug screen identified MEK inhibitors, including trametinib, that preferentially decreased the viability of nonadherent AXT cells. Trametinib inhibited the cell cycle and induced apoptosis in AXT cells, and both effects were stronger under nonadherent conditions. Trametinib also potently decreased viability in U2OS cells, but its effects were less prominent in MG63 or Saos2 cells. By contrast, MG63 and Saos2 cells were more sensitive to PI3K inhibition than AXT or U2OS cells. Notably, the combination of MAPK/ERK kinase (MEK) and PI3K inhibition synergistically decreased viability in U2OS and AXT cells, but this effect was less pronounced in MG63 or Saos2 cells. Therefore, signal dependence for cell survival and crosstalk between MEK–ERK and PI3K–AKT pathways in osteosarcoma are cell context-dependent. The activation status of other kinases including CREB varied in a cell context-dependent manner, which might determine the response to MEK inhibition. A single dose of trametinib was sufficient to decrease the size of the primary tumor and circulating tumor cells in vivo. Moreover, combined administration of trametinib and rapamycin or conventional anticancer drugs further increased antitumor activity. Thus, given optimal biomarkers for predicting its effects, trametinib holds therapeutic potential for the treatment of osteosarcoma.
1 INTRODUCTION
Osteosarcoma is the most common primary high-degree malignancy of bone in childhood and adolescence. Although recent advances in treatment combining surgery and chemotherapy have improved prognosis, ~30% of patients still do not survive over the long term, mainly due to uncontrollable metastasis.1-4 Accordingly, novel options for overcoming therapeutic resistance are urgently required.
Previously, we developed a mouse model of osteosarcoma by overexpressing c-MYC in bone marrow stromal cells derived from Ink4a/Arf-null mice.5 When inoculated into C57BL/6 syngeneic mice, these highly tumorigenic cells, designated AXT cells, rapidly form lethal tumors with metastatic lesions that mimic human osteoblastic osteosarcoma.6-10
Anoikis is a form of programmed cell death induced in normal cells by detachment from the original extracellular matrix (ECM).11, 12 Cancer cells can escape anoikis, enabling them to grow without ECM and develop metastatic lesions. Such anchorage-independent growth under nonadherent conditions is a hallmark of highly malignant tumors. Consistent with their high metastatic capacity in vivo, AXT cells also can proliferate in nonadherent cultures.
Tumor cells adapt their metabolism to their environment to survive and proliferate,13-16 using environmental nutrients opportunistically or activating cell-intrinsic adaptive metabolic pathways to sustain viability.15, 17, 18 Accordingly, metabolite levels could serve as an indicator of the environment in which tumor cells are located.
Using capillary electrophoresis–mass spectrometry (CE-MS), we found that the levels of metabolites in AXT cells from in vivo samples were more similar to those in cells grown under nonadherent than adherent conditions in vitro. This observation suggested that drugs that inhibit nonadherent cell growth might exert an antitumor effect on osteosarcoma in vivo. A drug screen identified MAPK/ERK kinase (MEK) inhibitors that preferentially suppress cell growth under nonadherent conditions. Among these compounds, trametinib exerted a potent antitumor effect even at low doses. Trametinib confers a long-term benefit in patients suffering from unresectable or metastatic melanoma with BRAF V600E or V600K mutation.19-21 However, although a few experiments have implied that this drug might be efficacious against osteosarcoma,22, 23 the antitumor effect of trametinib in that context remains to be confirmed. Hence, in this study, we examined the effects of MEK inhibition by trametinib on osteosarcoma cells in vitro and in vivo.
2 METHODS
2.1 Cell culture
Mouse osteosarcoma AXT cells were established through the overexpression of c-MYC in bone marrow stromal cells (BMSCs) derived from Ink4a/Arf null mice.5 AXT cells or human osteosarcoma cells obtained from ATCC were cultured in iscove's modified dulbecco's medium (IMDM) (Nacalai Tesque) or McCoy's 5A medium (Thermo Fischer Scientific), respectively, supplemented with 10% fetal bovine serum (FBS) under 5% CO2 at 37°C.6, 10 Mouse wild-type bone marrow stromal cells (WT BMSCs) were established as previously described.5 Briefly, bone marrow cells from C57BL/6J mice were obtained by flushing femurs and tibias with PBS and hematopoietic cells were depleted using autoMACS (Miltenyi Biotec, Bergisch Gladbach) with biotin-conjugated antibodies to CD45 and lineage-specific antigens. Adherent cells were cultured in IMDM supplemented with 20% FBS and used as BMSCs within 10 passages.
2.2 Reagents
Trametinib (#T-8123 LC laboratories) and rapamycin (#R-5000, LC laboratories) were reconstituted in dimethyl sulfoxide (DMSO) at a stock concentration of 20 mM. Suppliers of other reagents are listed in Table S1.
2.3 Cell proliferation assay
Cells were trypsinized, collected, and washed with a serum-free medium. Then AXT cells or human cells (1 × 103 in 50 µl of IMDM or McCoy's 5A medium, respectively, supplemented with 10% FBS) were transferred to each well of a 96-well cell culture plate. Ultra–low-attachment surface plates (Corning #7007) were used for nonadherent culture. Cells were incubated for 1 h before the addition of 50 µl of the corresponding medium supplemented with test reagents at twice the desired final concentrations. After incubation, cell viability was measured with the use of a Cell Titer Glo Assay Kit (Promega). Assays were performed at least in triplicate and data are expressed as means ± SD relative (fold) to the corresponding control value for cells incubated in the absence of test agents.
2.4 Animal care
All animal care and procedures were performed in accordance with the guidelines of Hoshi University (approval number: 29-118). Mice were housed in ventilated cages (floor area 501 cm2; five mice per cage) with ALPHA-dri bedding (Shepherd Specialty Papers) under specific pathogen-free conditions. Mice were fed a standard chow diet and water ad libitum with daily inspection to ensure that they were not under distress throughout the experiments. Rooms were temperature controlled at 22°C and kept on a 12-h light/dark cycle.
2.5 Tumor xenograft model
The detailed schedules were shown in Figure 6. To establish tumor xenografts, AXT cells suspended in 100 μl of IMDM were injected subcutaneously and bilaterally into the flanks of 7-week-old female syngeneic C57BL/6J mice (SLC) under anesthesia with inhalation of 2% isoflurane (Wako). One day after cell inoculation, all mice were gathered and randomly placed in the cages assigned to each treatment. Trametinib and rapamycin were orally, adriamycin was intravenously, and cytarabine was intraperitoneally administered once a day.
The endpoints were set as follows: (1) The mean tumor diameter does not exceed 20 mm. (2) Combined tumor burden is less than 15% body weight (~20 g of 7-week-old mice). (3) No ulceration, infection, or necrosis of tumor. (4) Body weight loss was less than 20% from baseline weight. The major and minor axes of the bilateral tumors were measured, and the estimated tumor weight was calculated from the following formula with reference to the guidelines of Washington State University (https://iacuc.wsu.edu/documents/2017/12/tumor-burden-guidelines.pdf/).
Estimated tumor weight (mg) = tumor volume (mm3) = d 2 × D/2, where d and D were the major and minor diameter in mm, respectively. The maximum tumor volume of mice for which the endpoints were applied was 102 × 25/2 + 102 × 10/2 = 1750 mm (8.8%) in Figure 6A-E or 132 × 24/2 + 82 × 10/2 = 2348 mm (11.7%) in Figure 6F-I. The actual measured values of these mice (=the maximum value) were 2.37 g (11.9%) and 2.75 g (13.8%), respectively. Two mice were accidentally dead after subcutaneous injection of AXT cells and oral administration of trametinib. Before analyses, the mice were euthanized with an intraperitoneal injection of a lethal dose (100 mg/kg) of pentobarbital sodium (Tokyo Kasei Kogyo).
2.6 Immunoblot analysis
Cells were disseminated and cultured overnight and then treated with the indicated concentrations of reagents. The cell lysate was prepared with 2 × Laemmli sample buffer (Bio-Rad) supplemented with β-mercaptoethanol. Immunoblot analyses were conducted according to standard semi-dry transfer procedures using 5%–20% gradient precast polyacrylamide gels (ePAGEL, ATTO) with primary antibodies listed in Table S2.
2.7 Phospho-kinase array
Kinase activation was screened using the Phospho-kinase Array Kit (R&D Systems). U2OS and MG63 cells were cultured under nonadherent conditions for 1 day and then treated with 500 nM trametinib for 3 h. Five hundred micrograms of total cell lysate was used per sample. Signal intensities were quantitated using the ImageJ software,24 and data are expressed as means normalized against the intensities of reference spots (Table 1).
2.8 Detection of apoptotic cells by flow cytometry
Cells were collected, washed with ice-cold PBS, and stained with propidium iodide and allophycocyanin-conjugated annexin V (eBioscience). Stained cells were assayed by flow cytometry on a FACSVerse (BD Biosciences). Data were analyzed with the FlowJo software (Tree Star).
2.9 Cell-cycle analysis
Cells were trypsinized, washed with PBS, fixed with 70% ethanol for ≥48 h at −20°C, washed twice with ice-cold PBS, and stained with PBS containing 10 μg/ml propidium iodide and 20 μg/ml RNase. The DNA content of at least 10,000 singlet cells was analyzed by flow cytometry.
2.10 Reverse transcription (RT) and real-time PCR analysis
Total RNA extraction, RT, and real-time PCR analyses were performed using the NucleoSpin RNA kit and PrimeScript reverse transcriptase (Takara).6, 8, 10 The sequences of PCR primers are provided in Table S3. For preparation of in vivo samples, the left lung was suspended with lysis buffer and disrupted using BioMasher (Nippi). For evaluation of circulating tumor cells, total RNA was extracted from 200 μl blood with the NucleoSpin RNA Blood kit (Takara). Tumor cells were quantitated based on the level of Gfp mRNA relative to Actb mRNA.
2.11 Metabolome analysis
AXT cells were cultured for 24 h under adherent or nonadherent conditions. Adherent cells (1.8 × 106) on the dish or nonadherent cell pellets (2.1 × 106) were washed twice with 10 ml of 5% mannitol and supplemented with 1 ml methanol containing 25 μM each of three internal standards (IS; l-Methionine, 2-(N-morpholino)-ethanesulfonic acid (MES), and d-camphor-10-sulfonic acid [CSA]). Adherent cells were collected with cell scrapers. After vortexing, 400 μl supernatant was mixed with 400 μl CHCl3 and 200 μl milli-Q water. After centrifugation, 400 μl of the aqueous layer was filtered through an HMT 5 kDa ultrafiltration tube (Human Metabolome Technologies), and the filtrate was dehydrated and used for CE-MS.25 For in vivo samples, AXT cells (5 × 105) suspended in 100 μl IMDM were injected subcutaneously into the flanks of 7-week-old female syngeneic C57BL/6J mice on Day 1. Primary tumors (25.0–52.1 mg) and lung metastases (3.0–28.2 mg) were resected after euthanasia on Days 23 or 31; normal tissues were removed under a stereomicroscope. Samples were immediately frozen with liquid nitrogen and stored at −80°C. Then the charged metabolites were extracted and quantified by CE-MS as previously described.25
2.12 Statistical analysis
All assays were performed at least in triplicate. Unless indicated otherwise, quantitative data are expressed as means ± SD relative to the control value. Data were analyzed with Student's t test, and a p < 0.05 was considered statistically significant. (*, p < .05; **, p < .005; NS, not significant).
3 RESULTS
3.1 MEK inhibitors potently decrease osteosarcoma cell viability under nonadherent conditions
Mouse osteosarcoma AXT cells can grow under both adherent and nonadherent culture conditions, although growth rates are higher in the former case (Figure 1A,B). Under nonadherent conditions, several tyrosine kinases that could activate the MAPK pathway and support cell growth were still activated in AXT cells (Figure S1A). To assess the similarities between the two in vitro culture conditions and the in vivo environment, we focused on metabolite levels. Specifically, we calculated the correlations between in vitro and in vivo tumor cells in the levels of 82 metabolites detectable by CE-MS (Figures 1C and S1B, Table S4).
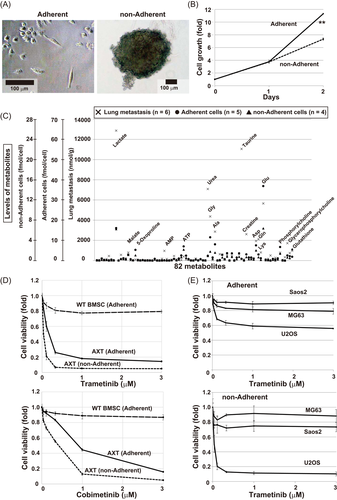
The correlation between in vivo primary lesion and lung metastases was quite high (correlation coefficient, 0.917), although lactate, glycine, and taurine were more abundant in metastases, suggesting that glycolysis and protein synthesis were upregulated in metastatic cells (Figure S1B). In addition, in vitro adherent and nonadherent culture conditions were also very similar (correlation coefficient, 0.914). The correlation between metastases and adherent or nonadherent cells was 0.543 or 0.673, respectively, suggesting that nonadherent conditions were more similar to the in vivo environment than adherent conditions, although metabolism differed substantially between in vitro and in vivo samples.
These findings prompted us to investigate whether drugs that effectively decrease the viability of nonadherent cells are candidates for the treatment of metastatic lesions. To identify drugs capable of efficiently suppressing nonadherent cell growth, we screened 364 drugs, including various signaling blockers (kindly provided by JSPS KAKENHI project #JP16H06276); all compounds were used at 0.5 µM. We found that two MEK inhibitors, U0126 and PD98059, could preferentially attenuate nonadherent cell growth with minimal effect on control WT BMSCs (data not shown).
Notably, all seven MEK inhibitors examined inhibited viability more strongly in nonadherent cells, and these effects were dose-dependent (Figures 1D and S1C). Activation of ERK was completely inhibited by all agents at 1 μM (Figure S1D). Among the seven inhibitors, trametinib had the most potent effects even at low concentration, as well as weaker growth inhibition in WT BMSCs. Irrespective of the difference in sensitivity, treatment of trametinib completely blocked Erk activation both in AXT and WT BMSCs and did not affect B-Raf activation (Figure S1E). As in AXT cells, trametinib also exerted an antiproliferative effect in human U2OS osteosarcoma cells. However, the effect in Saos2 and MG63 cells was less pronounced (Figure 1E), indicating that the effect of trametinib in osteosarcoma is cell context-dependent.
Therefore, we further investigated the antitumor effect of trametinib to evaluate its therapeutic potential in osteosarcoma.
3.2 Trametinib inhibits cell-cycle progression and induces apoptosis in osteosarcoma cells
To elucidate the mechanisms underlying the antiproliferative effect of trametinib in osteosarcoma cells, we examined cell-cycle status. A 10 h exposure of AXT cells to trametinib increased the fraction of cells in the G1 phase while decreasing the fraction in the S phase under adherent culture conditions (Figure 2A,B). Under nonadherent conditions, the S- and G2/M-phase fractions were lower than in adherent culture. Treatment with trametinib further decreased the S- and G2/M-phase fraction and increased the sub–G1-fraction, indicating the emergence of apoptotic cells.
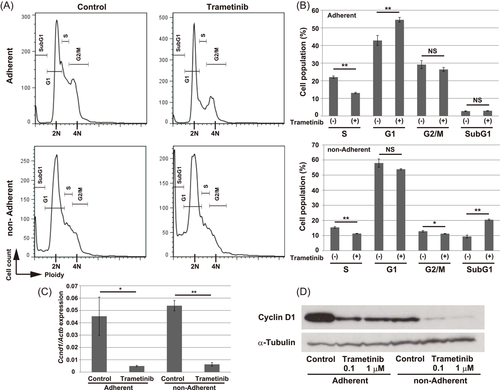
The expression level of Cyclin D1 was significantly decreased at both the mRNA and protein levels by a 6 h treatment of trametinib (Figure 2C,D). Consistent with the cell-cycle profiling, the Cyclin D1 protein level was lower in nonadherent cells than in adherent cells.
Short-term treatment with trametinib was sufficient to induce apoptosis in nonadherent cells, whereas the emergence of apoptotic cells in adherent culture was less prominent (Figure 3A,C). However, a 22 h treatment with trametinib also significantly induced apoptosis in adherent cells (Figure 3B,D). Together, these findings indicate that treatment with trametinib suppressed cell-cycle progression and induced apoptosis in AXT cells; this effect was stronger in nonadherent culture.
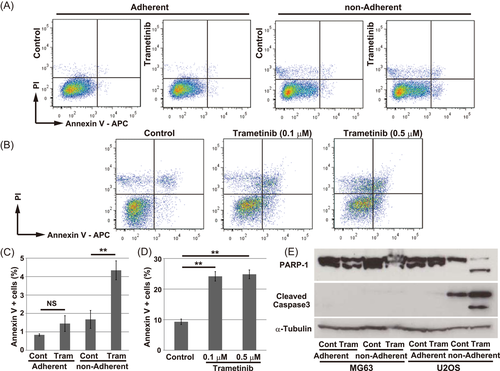
The inhibitory effect of trametinib differed between U2OS and MG63 cells (Figure 1E). However, trametinib significantly attenuated the expression level of CCND1 in both cell types (Figure S2A). An 18 h treatment with trametinib induced the cleaved forms of PARP-1 and Caspase3 under nonadherent conditions in U2OS (Figure 3E), which suggested the emergence of apoptotic cells. As nonadherent U2OS cells were fragile, and many cells were physically damaged during staining procedures, it was difficult to evaluate cell death precisely by flow-cytometry due to the high background (Figure S3A-C). Following treatment with trametinib, however, the PI-positive fraction (indicating damaged cells) was significantly elevated (Figure S3C), and the population of intact cells (as determined by forward-scatter and side-scatter) was markedly reduced (Figure S3G, H). Damaged and apoptotic cells were also more abundant following trametinib treatment of MG63 cells but the effect was less prominent (Figure S3D-F, I, J). These findings were also demonstrated with another MEK inhibitor, cobimetinib (Figures S2B, C and S3K, L). In addition, simultaneous knockdown of MEK1/2 by small interfering RNA was sufficient to enhance apoptosis in U2OS but not in MG63 cells under nonadherent conditions (Figure S3M).
Collectively, MEK inhibition by treatment with trametinib induced apoptosis in osteosarcoma cells, especially under nonadherent culture conditions, but such an effect was dependent on cellular context.
3.3 Alterations of MAPK and PI3K–AKT activation by trametinib treatment in osteosarcoma cells
To clarify the molecular events induced by trametinib treatment, we evaluated kinase activation by immunoblotting. In AXT cells, phosphorylation of Erk1/2 was totally abolished by a 5 h treatment with trametinib (Figure 4A). In adherent cells, trametinib did not affect the activation of mTor or Akt, but attenuated activation of p70S6K and S6 (Figure 4A,B). The phosphorylation levels of mTor, Akt, p70S6K, and S6 were lower in nonadherent cells and were decreased by trametinib in a dose-dependent manner.
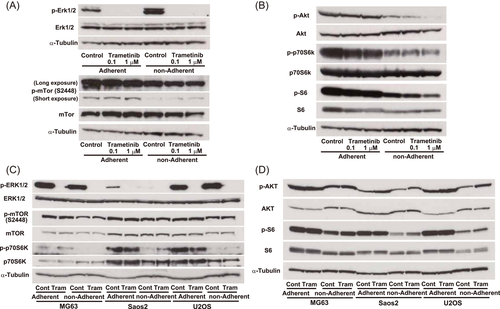
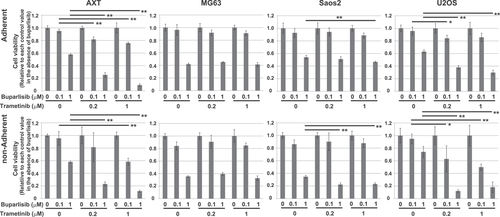
In human osteosarcoma cells, phosphorylation of p70S6K and S6 was reduced in all three cell types under nonadherent conditions, which were similar to those obtained in AXT cells (Figure 4C,D). However, trametinib failed to attenuate AKT activation, and the ERK phosphorylation level in Saos2 cells was lower than in the other two cell types. Activation of B-RAF (Figures S1E and S4A) or C-RAF (data not shown) was not affected by trametinib treatment. As the activation status of AKT or its downstream when the ERK pathway was inactivated differed among culture conditions in a cell context-dependent manner, we next examined the influence of PI3K inhibition on cell growth. Treatment with 1 μM buparlisib, a PI3K inhibitor, suppressed adherent cell growth in all cell types (Figure 5). In AXT and U2OS, sensitivity to buparlisib was identical under adherent and nonadherent conditions. By contrast, in MG63 and Saos2, growth inhibition was greater in nonadherent cells than adherent cells, suggesting a stronger dependence on the PI3K pathway under nonadherent conditions. Combined treatment with buparlisib and trametinib synergistically suppressed cell growth in AXT and U2OS, consistent with a previous report,23 but such strong synergy was not observed in Saos2 and MG63.
| Relative value to the reference spots | |||||
|---|---|---|---|---|---|
| Location | Kinases | MG63 control | MG63 trametinib | U2OS control | U2OS trametinib |
| 1 | ERK1/2 (T202/Y204, T185/Y187) | 0.349 | 0.014 | 0.225 | 0.023 |
| 2 | MSK1/2 (S376/S360) | 0.027 | 0.016 | 0.0501 | 0.053 |
| 3 | AKT1/2/3 (S473) | 0.175 | 0.145 | 0.045 | 0.055 |
| 4 | AKT1/2/3 (T308) | 0.017 | 0.023 | 0.014 | 0.026 |
| 5 | CREB (S133) | 0.195 | 0.244 | 0.244 | 0.486 |
| 6 | HSP27 (S78/S82) | 0.0076 | 0.016 | 0.033 | 0.038 |
| 7 | SRC (Y419) | 0.032 | 0.041 | 0.102 | 0.082 |
| 8 | LYN (Y397) | 0.0081 | 0.0082 | 0.024 | 0.019 |
| 9 | LCK (Y394) | 0.0062 | 0.0061 | 0.022 | 0.016 |
| 10 | STAT2 (Y689) | 0.019 | 0.028 | 0.051 | 0.052 |
| 11 | STAT5a (Y694) | 0.0095 | 0.0079 | 0.025 | 0.024 |
| 12 | FGR (Y412) | 0.0021 | 0.0098 | 0.018 | 0.019 |
| 13 | HCK (Y411) | 0.032 | 0.0404 | 0.084 | 0.041 |
| 14 | PRAS40 (T246) | 0.573 | 0.762 | 0.222 | 0.193 |
| 15 | c-JUN (S63) | 0.041 | 0.035 | 0.028 | 0.159 |
| 16 | WNK1 (T60) | 0.299 | 0.334 | 0.046 | 0.125 |
| 17 | PYK2 (Y402) | 0.014 | 0.022 | 0.0086 | 0.021 |
Together, these findings indicate that the dependency on MAPK or PI3K pathway for survival differs among osteosarcoma cell types that might affect the sensitivity to trametinib.
3.4 Alteration of kinase activation by trametinib treatment of human osteosarcoma cells
To further clarify the mechanisms involved in sensitivity to trametinib, we compared the phosphorylation levels of kinases per unit of protein in U2OS and MG63 cells under nonadherent conditions. Activation of ERK1/2, used as the positive control, was decreased ≥10-fold by trametinib (Figure S4B and Table 1). We identified 17 kinases whose phosphorylation levels differed by ≥twofold between cell types or between the presence or absence of trametinib. The phosphorylation levels of AKT (S473), its downstream target PRAS40, and WNK1 were basically higher in MG63 than U2OS. Conversely, MSK1/2, HSP27, SRC, LYN, LCK, STAT2, STAT5a, and FGR were more highly activated in U2OS than in MG63. Phosphorylation of CREB, c-Jun, and WNK1 was elevated after trametinib treatment in U2OS cells, whereas HSP27 and FGR were activated in MG63. Collectively, these findings show that the activation of multiple kinases is involved in sensitivity to trametinib, in a manner dependent upon cell context.
Among the kinases listed, CREB is a transcription factor with pleiotropic effects. Its transcriptional function is activated after phosphorylation on serine 133.26, 27 In addition, CREB and AP-1, including c-Jun together, bind to CBP after phosphorylation and induce transcription.28-30 Therefore, we further investigated the role of CREB activation in trametinib sensitivity. The phosphorylation levels of CREB and c-Jun were increased by trametinib treatment of U2OS cells (Figure S5A). Creb was also activated in AXT cells, whereas its phosphorylation level was not affected by trametinib (Figure S5B). We next established CREB-knockdown cells in U2OS and AXT cells. Trametinib sensitivity in U2OS-sh1 cells, which express extremely low levels of CREB, was significantly reduced under nonadherent conditions (Figure S5C-E). AXT-sh2 cells exhibited successful knockdown of Creb and were also less sensitive to trametinib under nonadherent conditions (Figure S5F-H). In contrast, knockdown of CREB did not attenuate sensitivity to trametinib under adherent conditions (data not shown). Thus, the activation of CREB can partially increase sensitivity to trametinib under nonadherent conditions.
3.5 Antitumor effect of trametinib in vivo
Finally, we investigated whether trametinib might exert antitumor activity against AXT cells in vivo. Administration of trametinib diminished phosphorylation of ERK in tumors, while MEK and AKT were still activated (Figure S6A), suggesting the effectiveness of trametinib in vivo. However, the mice administered with trametinib did not manifest a significantly longer survival (Figure S6B). Thus, to identify drugs with a synergistic antitumor effect in combination with trametinib, we screened 364 drugs (kindly provided by JSPS KAKENHI project #JP16H06276) in AXT cells. We found rapamycin and trametinib increased each other's antiproliferative effects (Figure S6C), and similar results were obtained with other mTOR inhibitors, such as everolimus and temsirolimus (Figure S6D,E). We injected AXT cells into syngeneic C57BL/6 mice and then treated the animals with trametinib, rapamycin, or both drugs (Figure 6A). As the AXT cells were labeled with GFP, the tumor cells could be isolated from surrounding normal mouse cells in vivo.5, 6, 31 Treatment with trametinib alone induced a significant decrease in primary tumor weight and circulating tumor cells (CTCs) (Figure 6B-D). Rapamycin alone did not exhibit an antitumor effect in vivo and single treatment with trametinib or rapamycin failed to diminish lung metastatic lesions (Figure 6E). In combination, however, trametinib plus rapamycin decreased metastatic lesions, although the effect was not statistically significant.
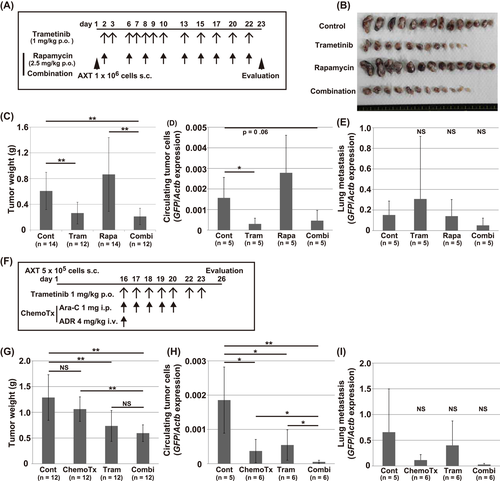
As trametinib preferentially decreased the viability of nonadherent cells, we next examined whether combined treatment with trametinib and existing anticancer drugs that inhibit adherent cell proliferation could efficiently affect tumor growth in vivo. In a previous screen,8, 10 adriamycin and cytarabine (Ara-C) were identified as drugs that potently suppress the viability of adherent cells rather than nonadherent cells (Figure S6F-H). Therefore, we examined the antitumor effects of trametinib in combination with these drugs (Figure 6F).
As before, administration of trametinib alone again significantly decreased primary tumor weight and CTCs but did not affect lung metastasis (Figure 6G-I). Chemotherapy alone was also sufficient to decrease CTCs. Notably, combination treatment further decreased CTCs and lung metastasis to a very low level. Together, these findings show that trametinib alone exerted an antitumor effect in primary lesions and CTCs. In addition, in combination with other agents, it could also decrease metastatic lesions.
4 DISCUSSION
In this study, we first compared the levels of metabolites in in vitro and in vivo samples to identify drugs with anti-osteosarcoma activity. As the pattern of metabolites in tumors in vivo was more similar to nonadherent than adherent cell cultures (Figure 1C), we chose nonadherent cells as the platform for drug screening.
We found that MEK inhibitors, including trametinib, potently decreased the viability of nonadherent osteosarcoma cells, but not adherent cells, in a cell context-dependent manner (Figure 1D,E). Although the mechanisms involved in the preferential effect of trametinib on nonadherent cells have not yet been fully elucidated, the activation levels of several kinases differed according to cell context (Figures 4, and S4, Table 1). Activation of AKT and its downstream target PRAS40 was stronger in MG63 than in U2OS, whereas SRC was more strongly activated in U2OS. Activation of PI3K–AKT is one of the most important signaling pathways involved in supporting anchorage-independent growth in cancer cells.11, 32, 33 Activation of SRC family kinases was reported to allow tumor cells to evade cell death under anchorage-independent conditions through MAPK and PI3K/AKT activation.34, 35 Notably in this regard, a previous report demonstrated differential dependence on survival signals under nonadherent conditions using human intestinal cell systems.35 The authors of that study demonstrated that differentiated cells depend on the SRC, PI3K–AKT, and MEK–ERK pathways for survival, like U2OS cells; by contrast, as in MG63 cells, undifferentiated cells mainly depend on SRC and the PI3K-AKT pathway, but not the MEK–ERK pathway, for survival.
Combined treatment with trametinib and a PI3K inhibitor, buparlisib, also revealed differential dependence on survival signals in osteosarcoma cells (Figure 5). The effects of buparlisib alone on Saos2 and MG63 were stronger under nonadherent conditions, but no such escalation was observed in U2OS or AXT cells (Figure 5). These findings suggested that Saos2 and MG63 are more dependent on PI3K signals for survival, especially in the nonadherent state. Furthermore, the combination of trametinib and buparlisib synergistically suppressed viability in U2OS and AXT, but this effect was much less prominent in Saos2 and MG63. These findings imply close crosstalk between the MEK–ERK and PI3K–AKT pathway in U2OS and AXT cells, but the absence of such a correlation in Saos2 and MG63.
Osteosarcoma consists of heterogeneous populations and the driver oncogenes of this cancer remain to be elucidated.1 Consistent with its nature, the status of p53 or Rb was different in a cell-context-dependent manner. AXT cells harbor a mutant form (R267C at DNA binding domain) of p53 that was highly overexpressed, while p53 was not detected in human osteosarcoma cells without stimuli (Figure S7A). The expression level of CDKN1A, a transcriptional target of p53, was upregulated by the treatment of doxorubicin in U2OS cells (Figure S7B). In contrast, such elevation was not detected in AXT, MG63, or Saos2 cells, consistent with the mutation or deletion of p53 in these cells.36 Rb was detected in AXT, MG63, and U2OS cells by immunoblotting, but not in Saos2 cells, which was also consistent with the mutation of Rb in Saos2 cells (Figure S7A).37 c-MYC that drives tumorigenic activity in AXT cells was expressed in all cell lines used (Figure S7C). Immunohistochemical analysis of a tissue array containing human osteosarcoma specimens revealed that 15 of 53 cases (28.3%) were strongly positive for c-MYC in nuclei and 21 cases (39.6%) were positive for p-ERK (Figure S7D). Ten out of 15 c-MYC positive cases were also p-ERK positive (66.7%), suggesting the frequent activation of ERK in relation to c-MYC expression. However, collectively, these results suggested that p53, Rb or c-MYC status was not correlated with the sensitivity to trametinib in osteosarcoma cells. Therefore, it would be currently difficult to apply trametinib as the first-line therapy for osteosarcoma. However, if optimal biomarkers could be identified that help predict antitumor effect and determine treatment indications, trametinib represents a potential therapeutic option for this disease.
Notably, our in vivo analyses showed that trametinib alone could decrease primary tumor size and CTCs (Figure 6). In addition, the combination of trametinib and rapamycin or conventional chemotherapy attenuated lung metastatic lesions, although this effect was not statistically significant. Collectively, these findings suggested that trametinib could be used as a potential adjuvant in the treatment of refractory osteosarcoma in combination with conventional therapy.
ACKNOWLEDGMENTS
The authors acknowledge Yuji Onizawa (Eisai Co., Ltd. Tsukuba Res. Laboratories) for the advice and discussion on the selection of screening compounds. The authors thank Ikuyo Ishimatsu and Nagisa Kamoshita for technical assistance. The compounds for screening were kindly provided by the Japan Society for the Promotion of Science KAKENHI project JP16H06276 (AdAMS). This study was supported by a KAKENHI grant from the Japan Society for the Promotion of Science (JSPS) (to T.S. #18K07247).
AUTHOR CONTRIBUTIONS
Takatsune Shimizu and Kiyomi Kimura contributed to the conception and design, collection of data, analysis, and interpretation of data, and manuscript writing. Eiji Sugihara, Sayaka Yamaguchi-Iwai, Hiroyuki Nobusue, Oltea Sampetrean, Yuji Otsuki, and Yumi Fukuchi contributed to the collection of data, interpretation of data, and analysis of data. Kaori Saitoh and Keiko Kato contributed to metabolome analysis. Tomoyoshi Soga, Akihiro Muto, and Hideyuki Saya contributed to the conception and design, interpretation of data, and administrative support. Takatsune Shimizu contributed to the final approval of the manuscript.




