Assessing the safety of an epiphyseal plate biopsy in a translational lamb model
Abstract
The literature demonstrates that obtaining a biopsy of the physis may be beneficial for diagnostic purposes. A small biopsy of the epiphyseal plate may allow for earlier detection of certain conditions and be used to monitor the healing of diseased and/or damaged physes. However, due to the fear of a growth arrest in a growing child, biopsies are not currently performed. In this study, we investigated the effects of a biopsy of the epiphyseal plate in 3-month-old lambs. A total of 4.2 mm biopsy samples were captured in the proximal tibiae and distal femora physes. The lambs were monitored 12- and 24-week post-biopsy. Computed tomography (CT) and micro-CT scans were obtained to determine if any angular deformities occurred, while scanning electron microscope (SEM) and histological analysis were utilized to assess the bone response due to the biopsy. The contralateral limbs served as unaltered controls for direct comparison within each lamb. The data demonstrated no signs of angular deformities following a 4.2 mm biopsy of the physis. Bone growth/elongation was confirmed by CT, SEM, and fluorochrome analyses and indicated that the lambs were in fact immature and still growing at the time of the biopsy. Clinical Significance: This investigation demonstrated that a small biopsy of the epiphyseal plate can be obtained safely without the cause of growth arrest and angular deformities. The ability to precisely diagnose, treat, and/or monitor at-risk children at an earlier timepoint by way of a biopsy sample could be an important advancement in regard to researching diseased and/or damaged physes.
1 INTRODUCTION
The epiphyseal growth plate is the main site that allows for the growth/elongation of long bones.1 Likewise, it's also considered to be the most fragile area of growing bones, due to its poor ability to regenerate itself after injury.2 This inferior ability to heal often results in structural disorganization and bone bridging if significant damage occurs. Consequently, injuries to the physis in children have the potential for growth arrest, which may result in angular deformity.3-5 Thus, any diagnosis in regard to physis health is performed non-invasively by radiography, computed tomographic (CT), and/or magnetic resonance imaging (MRI) due to the concerns with disruption to the physis.6 These diagnostic techniques can be valuable; however, they lack the higher resolution and cellular detail that may be needed to obtain critical information on how to diagnose and treat diseased and/or damaged physes. A possible solution may be to capture a small biopsy of the physis for histological and/or RNA sequencing analyses. Bone biopsies are considered to be a safe and cost-effective technique to diagnose bone disorders, abnormality, infection, or distinguish bone tumor from other conditions.7, 8 These bone biopsy samples can be captured in an outpatient setting under local anesthesia with minimal complications. However, despite the potential benefits of obtaining a bone biopsy of the physis, it is not commonly practiced in the clinical setting. This is possibly due to the limited literature and unknown level of risks that come with capturing a portion of the physis in a young growing child.
While the literature is limited in regard to the overall safety of a biopsy, it demonstrates that valuable histological information can be obtained by a biopsy sample in compromised physes. For example, a biopsy was captured of a physis bone bridge in a 6-year-old female that developed a varus deformity due to an ankle injury.9 The biopsy was captured at the time of the patient's angular corrective surgery. Histological analysis of the biopsy allowed for greater insight into the cause of the varus deformity; data suggested that fibrous tissue still permitted longitudinal growth, yet complete growth arrest occurred only after the fibrous scar was fully ossified. Deformity was suspected radiographically at ~6 months and was obvious by 12 months post-injury. Therefore, the question arises, would a biopsy at 6 months allow for earlier intervention and treatment before the obvious angular deformity?
Biopsies of the proximal femoral epiphyseal plate were captured in a separate study from eight patients (aged 11–13 years) with slipped capital femoral epiphysis (SCFE).10 The biopsies were captured during epiphysiodesis surgery. Histological analysis revealed that a SCFE plate is 2× thicker than a normal healthy physis. The analysis also revealed the resting zone was thinner and the chondrocytes within the proliferating and hypertrophic zones were arranged in large clusters separated from each other by loose septae containing longitudinal clefts. The chondrocytes were also smaller and more elongated than normal. Lastly, the authors stated that the proximal femoral growth plate undergoes several histological changes that precede the slipping of the epiphysis.
These clinical experiences support the value of a biopsy at an early timepoint for histological analysis before delayed radiographic confirmation. Additional studies in humans and animals have also demonstrated valuable findings in biopsies obtained of the physis at the time of corrective surgery.11, 12 All authors reported no complications in the patients/animals following biopsy. However, the biopsies were all captured at the time of corrective surgery, which doesn't confirm the actual safety of a biopsy of the physis without corrective surgery.
One human study did not involve corrective surgery at the time of biopsy; tibial physes of children with a variety of growth disorders were biopsied.13 The authors describe in great detail the diverse structures of the physis for pituitary dwarfism, congenital myxedema, Turner's and Marfan's syndromes, achondroplasia, polyepiphyseal, spondylo-epiphysealdysplasia as well as dysplasia epiphyseal punctata. A noteworthy example of the findings was from a 7-year-old patient with achondroplasia. Biopsies were obtained before and after treatment with anabolic steroids for 3 months. During the 3-month time period the child grew 3 cm. The biopsy before treatment showed that the physis was disorganized with extensive fibrosis. The chondrocytes within the physis appeared to organize in columns and islets after treatment. The ability to see the organizing structure of the physis following treatments supports the potential value of obtaining a biopsy of suspected diseased and/or damaged physes. Animal studies have shown similar results when biopsying dogs with endochondral ossification disorders.14 As with the corrective surgery, the authors reported no adverse outcomes due to the biopsies. However, little to no analysis was performed on the actual limbs that were biopsied.
Over 50 years of data demonstrates that a biopsy of the physis may be beneficial; allowing for early detection of certain conditions and could be used to monitor the healing of diseased and/or damaged physes via histological and/or RNA sequencing analyses.15-17 The literature suggests a biopsy to be safe; however, the true safety of obtaining a biopsy of the epiphyseal plate remains scientifically unproven. In this study, we investigated the effects of a biopsy of a healthy epiphyseal plate in 3-month-old lambs. The purpose of the study was to determine the safety of obtaining a biopsy in a pediatric/juvenile model and confirm that the damage to the physis from a biopsy does not result in a growth arrest leading to angular deformity.
2 MATERIALS AND METHODS
2.1 Supplies, instruments, and reagents
Surgical supplies and medications including drapes, tubing, fluids, analgesics, and anesthetics were provided by the University of Utah Comparative Medicine Center. Surgical tools and histological processing materials were provided by the Bone and Joint Research Laboratory. Jamshidi needles were purchased from BD (Becton, Dickinson and Company). Calcein green and xylenol orange were purchased from Sigma Aldrich. Micro-CT was performed on a Quantum FX (PerkinElmer Inc.). Scanning electron microscopy (SEM) was performed using a JEOL JSM-6610 (Peabody). Sanderson's rapid bone stain (SRBS) was purchased from Dorn & Hart Microedge Inc.
2.2 Animal model
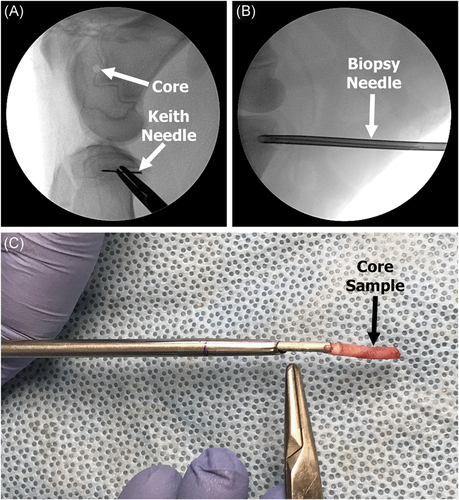
Three-month-old lambs (n = 10) were purchased from K Bar Livestock to simulate the condition and bone growth of a child. Lambs were selected for this investigation due to the similarities in bone growth and remodeling capabilities compared to humans, as well as their ability to produce growth deformities with disruption of the physis.18-21 The lambs were randomly divided into two groups: 12- and 24-week time points (Table 1). All animal work was performed at the University of Utah following local institutional animal care and use committee (IACUC) approval. As previously described, a small biopsy was captured on the medial side of the distal femora and proximal tibiae.22 To increase the accuracy of capturing a uniform biopsy core, a Keith needle (which served as a guide) was first inserted into the physis with the assistance of fluoroscopy. Next, a hollow 4.2 mm (8G) Jamshidi needle was driven over the Keith needle capturing a small transverse biopsy sample of the physis (Figure 1). The 4.2 mm biopsy size was selected following cadaveric work, as it demonstrated it was able to capture the whole epiphyseal plate (resting to ossification zone). A transverse approach through the perichondrial ring was done intentionally in an attempt to model a high-risk scenario for physeal closure by removing the whole epiphyseal plate (resting to ossification zone) rather than the more common oblique route.12, 14 The biopsy resulted in a defect similar to a Type 6 fracture; however, on a much smaller scale.23 The contralateral limbs were not biopsied and served as unaltered control limbs to analyze for signs of angular deformity.
| Timepoint | Biopsied | Non-biopsied |
|---|---|---|
| Time = 0 | – | n = 1 |
| 12 weeks | n = 4 | n = 4 |
| 24 weeks | n = 5 | n = 5 |
| Total | n = 9 | n = 10 |
- Note: The tibia and femur were both biopsied in the same limb.
Calcein green (10 mg/kg) was injected intravenously (IV) in all lambs on two separate occasions24; 12-week lambs were injected 16 and 5 days before euthanasia, while 24-week lambs were injected at ~12 weeks with an 11-day gap between the two injections. The 24-week lambs were additionally injected with xylenol orange (80 mg/kg) 16 and 5 days before euthanasia.22 The use of calcein green and xylenol orange in the same lamb was to differentiate the area of bone modeling at both timepoints. This allowed for confirmation as to whether or not uniform growth/elongation occurred. One lamb was unable to recover following anesthesia; it had severe pneumonia with a cranioventral lung distribution and was euthanized early. This lamb was used as a “Time = 0” specimen to determine the percentage of physis biopsied (Table 1). The remaining n = 9 lambs responded well to the biopsy with minimal signs of distress and/or pain, with the exception of one 24-week lamb that demonstrated a fever. That lamb was given a course of antibiotics and recovered without issues. At the end of the 12- and 24-week timepoints the animals were humanely euthanized and both the biopsied and contralateral limbs were harvested to assess any structural abnormalities that may have developed following a small 4.2 mm biopsy of the physis.
2.3 Computed tomography (overlay analysis)
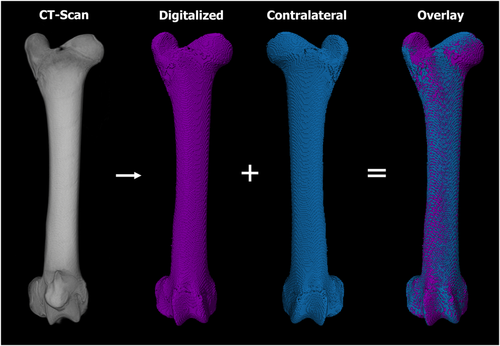
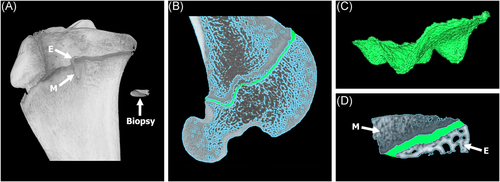
Following euthanasia, the limbs were placed in an upright freezer for a minimum of 48 h to ensure the limbs were at full extension. CT scans were captured of the entire limbs at a 130 kVp tube voltage, 212 μA tube current, and 0.6 mm slice thickness. Following imaging, the digital scans were loaded in Amira v6.4 (Thermo Fisher Scientific) for 3D overlay analysis to determine if any angular deformity occurred as a result of the biopsy. More specifically, soft tissue was extracted using grayscale thresholding leaving only the skeleton. Next, the biopsied and contralateral limbs were isolated and converted to colored 3D masks. The 3D mask of the contralateral limb was mirrored and overlaid with the 3D mask of the biopsied limb for analysis (Figure 2). The advantage of using CT scans compared to the standard radiography analysis, was that the 3D modeling capabilities of the CT scans allowed for the ability to view the limb in all planes/angles. Standard post-mortem radiography, by way of a table top X-ray cabinet, is limited to only two-dimensional anterior-posterior and medial-lateral views and can exhibit artifact due to alignment issues. In addition to the overlay analysis, the lengths of the limbs were also measured to confirm growth/elongation had occurred between the 12- and 24-week timepoints.
2.4 Micro-CT (% physis biopsied)
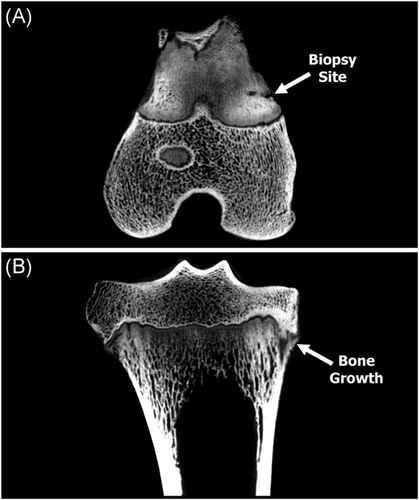
Following CT scanning, limbs were thawed, disarticulated, all soft tissue was dissected, and the bone was resected leaving only the proximal tibiae and distal femora. CT scans provided a broad view of biopsied limbs; however, higher resolution scans were needed to observe potential bone disruption due to the biopsy as well as to determine the approximate percentage of physis removed (% physis). Resected limbs and biopsy samples were scanned using a QuantumGXmicro-CT (PerkinElmer) with a tube voltage of 90 kVp and tube current of 180 μA.25 The field-of-view (FOV) varied due to the small size of the biopsy samples. The proximal tibiae and distal femora were scanned at a 60 mm FOV, while the biopsy cores were scanned at a 10 mm FOV, scans were imported into Seg3D (https://sci.utah.edu).26 The physis (cartilaginous disc) was highlighted and extracted in both the tibia and femur of the time = 0 limb to obtain a baseline volume of a 3-month-old lamb's physis. The physis was then extracted from all 18 biopsy samples. The volume of physis within the core samples was compared to the total volume of physis of the tibia or femur from the time = 0 lambs to determine the approximate percentage of the physis biopsied (Figure 3).
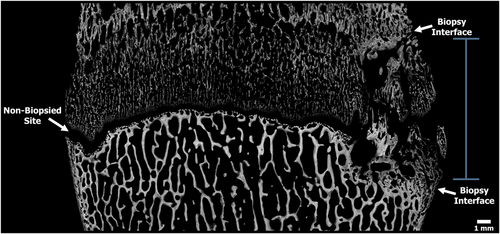
The micro-CT scans were also used to assess any bone bridging or disturbance that occurred as a result of the biopsy. The 60 mm FOV of the tibiae and femora were imported into FIJI (Image J) analysis software, which allowed for qualitative 3D rendered and cross-sectional analyses (Figure 4).22
2.5 Histological processing and imagining
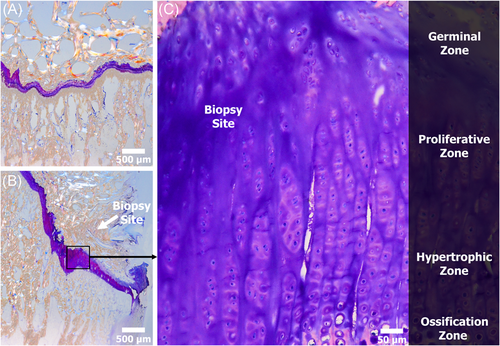
Following micro-CT imaging the proximal tibia and distal femora were fixed in 10% neutral buffered formalin, dehydrated in ethanol, infiltrated, and embedded in poly-methyl-methacrylate (PMMA) to allow for undecalcified histological analyses.27, 28 The polymerized specimens were sectioned coronally parallel to the biopsy site and ground by hand using a variable-speed grinding wheel (Buehler Incorporated) until the biopsy sites were grossly visible. Once the correct plane/biopsy site was located; the specimens were polished to an optical finish to allow for SEM imagining.
The polished specimens were coated with a thin conductive layer of carbon for ~30 s with a high vacuum turbo carbon coater 208C (Ted Pella) for backscattered electron (BSE) imaging.29 The BSE detector allows for high-resolution images of bone morphology, as well as the ability to observe complex structure mineral content of the epiphyseal plate.30 Images were captured at 20× magnification with a resolution of 2560 × 1920 pixels across the entire physis. Post image processing was performed using Microsoft Research Image Composite Editor (MRICE) to mosaic/stitch the BSE images together creating a high-resolution overhead view of the physis (Figure 5).31
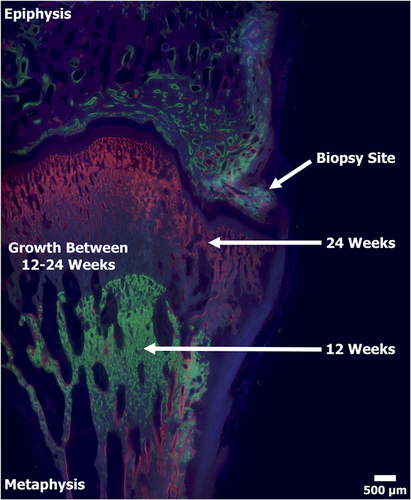
The thin layer of carbon was removed following SEM imaging and the specimens were adhered with ExaktTechnovit 7210 to individual plastic slides using a precision adhesive press (EXAKT Technologies). The specimens were then ground to a final thickness of ~100 µm and viewed under a mercury lamp microscope to display the fluorochrome double-labeled bone (Figure 6).22, 28 Sections were then stained with SRBS and viewed under a light microscope (Nikon E600; Nikon Inc.) equipped with associated image capturing software (Figure 7).22, 32
2.6 Statistical analysis
Outcome measures were compared statistically using SPSS 17.0 software and using independent t test with alpha level 0.05.
3 RESULTS
3.1 CT and micro-CT
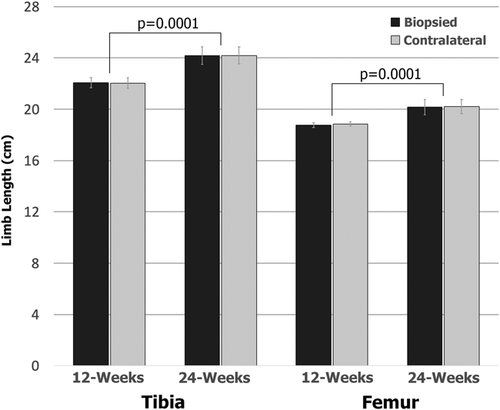
Results from the CT overlay analysis demonstrated no obvious signs of angular deformities due to the biopsy. The 3D reconstructed contralateral limbs successfully aligned with the biopsied limbs at both timepoints with no signs of obvious angular deformities (Figure 2). CT analysis further demonstrated a significant (p = 0.0001) increase in length of the tibia and femur when comparing the 12- and 24-week timepoints (Figure 8). The tibiae revealed a greater increase in length (2.1 cm) compared to the femora (1.4 cm). The increase in length in both the tibia and femur implies that the lambs were in fact immature and still growing at the time the biopsy was obtained. No differences were observed when comparing the limb lengths of the biopsied and contralateral limbs at both timepoints; confirming growth arrest did not occur (Figure 8).
Volumetric analysis from time = 0 micro-CT scans demonstrated the volume of a physis of a 3-month-old lamb was 619 and 699 cubic mm for the femur and tibia, respectively. When compared to the captured biopsy samples, it was determined that a 4.2 mm core resulted in ≤ 1% (femur = 4.4 ± 1.8 mm, tibia = 3.1 ± 1.4 mm) of the total physis biopsied for all n = 18 biopsy samples. However, the size and location of the biopsy samples were not uniform; 44% of the biopsies contained the whole epiphyseal plate (resting to ossification zone), while the remaining 56% of the biopsies contained only partial of the epiphyseal plate (i.e., physis + epiphysis bone or physis + metaphysis bone). The analysis revealed that the larger biopsy samples (bone + physis) did not correlate with capturing the whole epiphyseal plate (Table 2). The data also demonstrated that the biopsy samples from distal femora had a slightly higher volume (19.5 ± 6.6 mm) compared to the proximal tibiae (16.9 ± 7.3 mm) samples (Table 2). However, the difference was not statistically significant (p = 0.449) and was most likely due to variation in the depth of the biopsy needle.
| Biopsy volume (mm) | |||
|---|---|---|---|
| Limb # | Timepoint | Femur | Tibia |
| 1 | 12 weeks | *24.6 | *29.4 |
| 2 | 12 weeks | *12.8 | 21.8 |
| 3 | 12 weeks | 16.0 | *8.3 |
| 4 | 12 weeks | 13.0 | *21.6 |
| 5 | 24 weeks | 11.9 | 18.9 |
| 6 | 24 weeks | 19.4 | 20.0 |
| 7 | 24 weeks | 26.4 | *14.6 |
| 8 | 24 weeks | *30.0 | 6.8 |
| 9 | 24 weeks | *21.4 | 11.2 |
| Mean | 19.5 | 16.9 | |
| Stdev | 6.6 | 7.3 | |
| Median | 19.4 | 18.9 | |
| Min | 11.9 | 6.8 | |
| Max | 30.0 | 29.4 | |
- Note: The * indicates core samples that contained the whole epiphyseal plate.
Micro-CT analyses indicated the presence of a bone response from the biopsy in 100% of the 12-week limbs and in 80% of the 24-week limbs. The bone response appeared unorganized with limited signs of callous bone bridging in the 12-week limbs (Figure 4). By 24 weeks the bone response appeared to begin maturation with signs of bone consolidation. The extent of bone response varied from lamb to lamb, but overall the response remained localized to only the biopsy region. The percentage of physis biopsied did not correlate with the observed bone responses. More specifically, a larger biopsy core sample and/or capturing the whole versus only a partial epiphyseal plate did not determine the extent of the bone response.
3.2 SEM, fluorochrome labels, and histological analysis
Qualitatively, the SEM, fluorochrome labels, and histological analyses all coincided with the CT and micro-CT findings. Specifically, the higher resolution BSE images demonstrated bone consolidation as well as unorganized bone growth within the biopsy defect (Figure 5). This suggests that the biopsy defect did not adversely affect the ability of the host bone to remodel and begin to fill the created void due to the biopsy procedure. Although new bone growth was observed within the defect, no signs of connectivity (bone bridging) between the epiphysis and metaphysis were observed at either time point (12 or 24 weeks). As with the micro-CT scans, the bone response varied per lamb but remained localized at the biopsy site.
Fluorochrome labels confirmed that bone within the biopsied defect and adjacent regions was viable and actively remodeling in both the 12- and 24-week specimens. Labels further demonstrated distinct parallel lines from the calcein green and xylenol orange labels in the 24-week specimens. Parallel lines showed that the bone growth/elongation was uniform and correlated with the lack of angular deformities. The labels also demonstrated a gap (non-labeled bone) between 12-week calcein green labels and 24-week xylenol orange labels, confirming bone growth/elongation occurred between the two timepoints (Figure 6). The 12-week samples only received one label type (calcine green), therefore the growth comparison was unable to be performed on the 12-week group. The epiphysis region demonstrated distinct double labels at the seams of the trabeculae, while the metaphysis was modeling at such a high rate the entire area was labeled (Figure 6).
Histological analysis coincided with SEM analysis revealing predominantly unorganized cartilage within the biopsy site. The smaller bone responses observed in the SEM analysis resulted in a similar structure/response as native unaltered physis. In contrast, the larger bone responses observed in the SEM analysis resulted in a displaced physis within the small biopsied region. The analysis also revealed the physis was still in a healing state at the 24-week timepoint. While healing within the biopsy region was still considered to be immature, the adjacent regions remained unaltered and healthy (Figure 7).
4 DISCUSSION
The physis is considered a delicate structure. Biopsies may allow for improved diagnostics, but must not result in deformities downstream. In this investigation, the CT analysis revealed that all n = 9 biopsied limbs (tibia + femur) overlaid and aligned with their respective contralateral limbs. Determining the tibia and femur growth amounts—2.1 and 1.4 cm, respectively—between the 12- and 24-week time points were critical; this confirmed that the biopsy did not result in a growth arrest of the physis and validated the age selection of the lamb. Had no limb growth/elongation occurred, all data would essentially be meaningless in terms of growth arrest and angular deformity potential. The sheep limb growth rates were higher than what might be found in a human child; tibia and femur grow ~1 cm per year.33 Importantly, the sheep limb lengthening amounts did not include the initial 12-week growth from the time of initial biopsy, meaning a greater amount of growth/elongation occurred overall, but still provided a useful window of data collection.
Nearly all lambs demonstrated some extent of bone response as a result of the biopsy. The extent of the bone response did not appear to be influenced by the size of the biopsy in this study. Why some lambs had a greater bone response compared to others is unclear. Nevertheless, all responses were considered minor and remained localized to only the biopsied region. Histological analyses revealed bone growth/consolidation and disorganized physis at the biopsy site that appeared to be maturing by the 24-week timepoint.
The biopsy technique was determined to be inadequate for patient care. Although a biopsy sample was captured in 100% of the lambs, only 44% of the biopsy samples contained the whole epiphyseal plate. While this is an improvement from Loiacono et al.12 who demonstrated only a 23% success rate with capturing the epiphyseal plate in mules using a biopsy needle, the approach would need improvement to be used in clinical application. The utilization of a Keith needle as a guide appeared efficient in targeting the physis, however, may not be recommended for patient use; the needle pierces the physis and could potentially distort/damage a biopsy sample, thus requiring a second biopsy. The length of the biopsy also varied; this was due to the biopsy needle's inability to capture/snip a uniformed sample. The depth of the needles was kept consistent, however, the sample that was ultimately removed varied. A solution to these issues may be utilizing new advancements in bone biopsy systems. For example, a CT-guided bone biopsy drill or robotically assisted MRI approach may be beneficial for obtaining a complete biopsy sample.34, 35 The development of new surgical devices may also be necessary to ensure that safe and uniform biopsy samples can be captured repeatably. Biopsy samples perpendicular to the physis may also be recommended to ensure full capture of the epiphyseal plate (resting to ossification zone) and to minimize overall damage.
Several limitations accompanied this investigation. First, only one age group was biopsied. In immature lambs, growth disturbances can occur if the physis is damaged/altered.19-21 However, CT and histological analyses in this study showed that the physis of a 3-month-old lamb was well defined with mature trabeculae in the epiphysis. This could model younger and older children, but does not validate a biopsy in an infant or toddler, whose epiphyses would still contain predominately cartilaginous tissue. It is also unclear how a biopsy would affect those in their late adolescence/young adulthood. Second, the study only used a single biopsy size (4.2 mm), which resulted in ~1% of the physis being captured. Whether a larger biopsy size would produce a growth arrest and angular deformity is unclear. Rabbit studies have shown the approximate size of injury required to cause a growth disturbance is 7%–9% of the total physis.36, 37 However, the goal of this study was not to create a growth disturbance, but rather, could a biopsy for diagnostic purpose be obtained without any adverse effects? Third, the lambs were only monitored 24 weeks post-biopsy, resulting in 9-month-old lambs. While the growth that occurred within those 24 weeks simulates ~3–4 years of growth in a child, sheep are not considered skeletally mature until 1–1.5 years of age.38 Lastly, it's important to note that the biopsies were obtained from healthy physes; it is unclear if the results would remain the same if performed on “sick” physes. Additional research is warranted.
5 CONCLUSION
In summary, this lamb study added onto work we published previously with a physeal defect in rabbits to assess the potential of angular deformity.22 The results from these investigations validate observations from previous biopsy studies—that a low volume biopsy of the ephyseal plate may be obtained safely without influencing growth arrest and/or angular deformities. Histological analysis is not commonly performed on physes for diagnostic purposes in the clinical setting or in the research field to evaluate treatment options. This lack of in-depth knowledge of the physis may be leaving a void in clinical decision-making. The ability to precisely diagnose, treat and/or monitor at-risk children at an earlier timepoint by way of a biopsy sample could be an important advancement in regard to researching diseased and/or damaged physes.
ACKNOWLEDGMENTS
Work was performed with resources and the use of facilities at the George E. Wahlen Department of Veterans Affairs. The contents do not represent the views of the U.S. Department of Veterans Affairs, the United States Government or the Department of Defense. The authors thank Brooke Kawaguchi, Ryan Rasmussen, Nicholas Taylor, Mary Dickerson, and the University of Utah Comparative Medicine Center staff for their technical support. Funds to support this study were provided by the Department of Orthopaedics, University of Utah (Sherman S. Coleman Endowment Fund).
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
Peter Stevens established the hypothesis, study design, surgical approach, performed surgeries, and supported manuscript editing. Richard T. Epperson supported study design, performed histological analyses, and drafted the initial manuscript. Dustin L. Williams supported study design, managed animal logistics, IACUC preparation, provided daily oversight and manuscript editing. Brady Rogers and John Maxwell supported histological analyses and manuscript editing.




