Meniscus cell regional phenotypes: Dedifferentiation and reversal by biomaterial embedding
Abstract
Meniscus injuries are common and a major cause of long-term joint degeneration and disability. Current treatment options are limited, so novel regenerative therapies or tissue engineering strategies are urgently needed. The development of new therapies is hindered by a lack of knowledge regarding the cellular biology of the meniscus and a lack of well-established methods for studying meniscus cells in vitro. The goals of this study were to (1) establish baseline expression profiles and dedifferentiation patterns of inner and outer zone primary meniscus cells, and (2) evaluate the utility of poly(ethylene glycol) diacrylate (PEGDA) and gelatin methacrylate (GelMA) polymer hydrogels to reverse dedifferentiation trends for long-term meniscus cell culture. Using reverse transcription-quantitative polymerase chain reaction, we measured expression levels of putative meniscus phenotype marker genes in freshly isolated meniscus tissue, tissue explant culture, and monolayer culture of inner and outer zone meniscus cells from porcine knees to establish baseline dedifferentiation characteristics, and then compared these expression levels to PEGDA/GelMA embedded passaged meniscus cells. COL1A1 showed robust upregulation, while CHAD, CILP, and COMP showed downregulation with monolayer culture. Expression levels of COL2A1, ACAN, and SOX9 were surprisingly similar between inner and outer zone tissue and were found to be less sensitive as markers of dedifferentiation. When embedded in PEGDA/GelMA hydrogels, expression levels of meniscus cell phenotype genes were significantly modulated by varying the ratio of polymer components, allowing these materials to be tuned for phenotype restoration, meniscus cell culture, and tissue engineering applications.
1 INTRODUCTION
Meniscus injuries are highly prevalent, affecting millions of people each year.1 Loss of meniscal function has been linked to early-onset osteoarthritis within 10–20 years.2 However, due to poor healing potential, especially in the inner zone of the meniscus, partial meniscectomy remains the most common surgical treatment for meniscus injuries.3 For these reasons, novel ways to treat meniscus injuries and/or augment surgical repair are greatly needed. To this end, efforts at tissue engineering and biological stimulation of repair have been increasingly investigated in recent years. However, these efforts are hindered by a lack of knowledge regarding the cellular biology of the meniscus and a lack of well-established methods for studying meniscus cells in vitro.
Only a few studies can be found in the literature describing expression level phenotypic changes of meniscus cells during monolayer expansion.4-9 Of the studies that do exist, even fewer report on differences between inner and outer zone meniscus cells7-9 and comparisons with fresh tissue,10 and there is a lack of data comparing tissue explant culture to isolated cells. Inner and outer regions of the meniscus are significantly different in terms of vascularity and extracellular matrix (ECM) composition.11 In addition, the cells in these regions have different phenotypic characteristics based on morphology both in situ12 and when cultured in monolayer.13 On the basis of these morphological characteristics and differences in their regional ECM composition, outer zone cells are often described as “fibroblast-like,” while inner zone cells are described as “chondrocyte-like.”11 The genes most commonly used as phenotype markers for studies of meniscus cell dedifferentiation are commonly associated with the articular chondrocyte phenotype, including type I collagen (COL1A1), type II collagen (COL2A1), aggrecan (ACAN), and SOX9. However, COL2A1 and ACAN may not be ideal for the evaluation of meniscus cells, as they are expressed at significantly lower levels in meniscus tissue.8 While some studies have shown a strong downregulation of COL2A1 with meniscus monolayer culture,4-6 in a recent study utilizing microarray to compare inner zone meniscus tissue to monolayer cultured cells, of the chondrocyte phenotype genes only COL1A1 and SOX9 were found among the most significantly differentially regulated genes.10 Furthermore, COL2A1 and ACAN were notably absent from the top 20 downregulated transcripts. Interestingly, the authors identified and validated several less-studied genes as potential phenotype markers of inner zone cells, which may be more relevant to meniscus cells, including chondroadherin (CHAD), cartilage intermediate layer protein (CILP), and cartilage oligomeric matrix protein (COMP). To address these gaps in knowledge, we have characterized phenotypic changes during monolayer culture in primary meniscus cell gene expression of both traditionally used phenotype marker genes, as well as less-studied targets identified in meniscus cells, and compared this data to fresh tissue and tissue culture expression levels. In addition, we investigated the potential of hydrogel materials for meniscus cell culture and phenotype restoration by directly comparing expression profiles of embedded meniscus cells and meniscus tissue in culture.
Attempts to reverse dedifferentiation patterns have not yet yielded a widely accepted method for maintaining meniscus cell phenotype, and, thus, each lab utilizes their own biomaterials or techniques,4-6 and due to the lack of well-defined expression profiles of meniscus cells, it is difficult to evaluate the efficacy of various cell culture techniques. A number of methods have been investigated, such as the culture of cells on a variety of modified or biomimetic surfaces,4, 5 three-dimensional (3D) culture supported by biomaterials,5, 14 microaggregate culture,6 and varying the oxygen tension of culture conditions.5, 6, 15 It is increasingly appreciated that the physical 3D environment can control cellular phenotype, and biomaterials can be engineered for this purpose.16 In particular, substrate stiffness, the availability of cellular adhesion motifs, and matrix degradability are parameters that have been shown to modulate cellular phenotype.16 We investigated the utility of poly(ethylene glycol) diacrylate (PEGDA) and gelatin methacrylate (GelMA) hydrogels as substrates for 3D meniscus cell culture. PEGDA is a synthetic photocrosslinkable polymer of poly(ethylene glycol) subunits. The stiffness of PEGDA is tunable and the polymer is generally described as “biologically inert” because it contains no cellular adhesion motifs.17, 18 Nevertheless, PEGDA has been successfully used to embed many different cell types for various applications, usually with high cell viability.19-21 GelMA is a photocrosslinkable polymer derived from denatured collagen. Therefore, it retains native ECM cellular adhesion motifs and is cell degradable, but lacks the tunable mechanical properties of PEGDA.18, 22 We hypothesized that a hybrid of these materials could provide a physical 3D environment capable of reversing the dedifferentiation trends of primary meniscus cells in monolayer culture.
The goals of this study were (1) to establish baseline expression profiles and dedifferentiation patterns of inner and outer zone primary meniscus cells, and (2) to evaluate the utility of PEGDA/GelMA polymer hydrogels to reverse dedifferentiation trends for long-term meniscus cell culture.
2 MATERIALS AND METHODS
2.1 Tissue harvest and culture
Skeletally mature 2–3-year-old female porcine knee joints were obtained from a local abattoir. Tissue explants were harvested from the inner and outer regions of medial menisci (N = 12), using 5-mm-diameter biopsy punches, and trimmed to approximately 2 mm thick. Samples were either immediately frozen in liquid nitrogen (“fresh tissue,” n = 6), or maintained in complete culture media composed of Dulbecco's modified Eagle's medium, high glucose (DMEM-HG; Catalog # 11995073; Gibco), 10% fetal bovine serum (FBS; Product # 35-010-CV; Corning), 1X nonessential amino acids (Catalog # 11140050; Gibco), 1X antibiotic/antimycotic (Catalog # 15240062; Gibco), 10 mM HEPES (Cat # 15630080; Gibco), 40 μg/ml l-proline (SKU # H54409; Sigma-Aldrich), and 50 μg/ml ascorbic acid (SKU # A8960; Sigma-Aldrich) for 3 days, then frozen in liquid nitrogen (“tissue explant,” n = 6).
2.2 RNA extraction
Explants were pulverized in TRIzol (Catalog # 15596026; Life Technologies) under liquid nitrogen using a freezer mill (Model 6875; SPEX SamplePrep). Then RNA was extracted by TRIzol/chloroform separation following the manufacturer's protocol and column purified (Catalog # 48300; Norgen Biotek Corp.).
2.3 Cell isolation and monolayer culture
Meniscus cells were isolated from the outer or inner zones (approximately outer one-third and inner two-thirds, respectively) of the medial meniscus (N = 6) by sequential digestion of minced tissue with 0.5% (wt/vol) pronase (SKU 53702; EMD Millipore Corp.) digestion for 1 h and 0.2% (wt/vol) collagenase type I (Catalog # LS004197; Worthington Biochemical Corp.) digestion for 16 h in DMEM-HG with 1X antibiotic/antimycotic and 10% FBS.23 Isolated cells were seeded at 5 million cells per 10-cm tissue culture plate and maintained in complete culture media. Media was replaced every 2–3 days and cells were passaged at 90% confluence (5–6 days for Passage 1 and then 2 days for subsequent passages). RNA was extracted by column purification (Catalog # 48300; Norgen Biotek Corp.) at the time of the first, second, and third passage (p1, p2, and p3).
2.4 Reverse transcription-quantitative polymerase chain reaction
RNA samples were reverse-transcribed using SuperScript VILO complementary DNA Synthesis Kit (Catalog # 11754050; Life Technologies). Then, quantitative polymerase chain reaction (qPCR) was performed using PowerUP SYBR Green Master Mix (Catalog # A25776; Thermo Fisher Scientific Baltics UAB) and the StepOne Plus Real-time PCR System (Model 4376374; Applied Biosystems). Relative fold change was determined by the 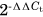 method24 using 18S as a reference gene. Results are presented as fold change relative to the mean of the indicated group.
method24 using 18S as a reference gene. Results are presented as fold change relative to the mean of the indicated group.
2.5 Hydrogel preparation
PEGDA and GelMA precursors were synthesized as previously described.21, 25 Polymer precursors were prepared as 10% (wt/vol) solution and mixed at 8:2, 6:4, 5:5, and 4:6 ratios to yield final concentrations of 10% PEGDA (10P), 8% PEGDA/2% GelMA (8P2G), 6% PEGDA/4% GelMA (6P4G), 5% PEGDA/5% GelMA (5P5G), and 4% PEGDA/6% GelMA (4P6G), respectively. Lithium phenyl(2,4,6-trimethylbenzoyl) phosphinate (LAP; SKU 900889; Sigma-Aldrich) photoinitiator was prepared as a 75 mg/ml aqueous solution and added to the precursor solutions before photopolymerization to yield a final concentration of 0.75 mg/ml. The precursor solutions containing LAP were then added to a custom cylindrical mold (4 mm diameter, 2 mm height) and photopolymerized by exposure to UV light at 365 nm for 1 min.
2.6 Equilibrium modulus
The equilibrium modulus of hydrogel constructs (n = 8/group) was determined by stress relaxation testing at 5%, 10%, 15%, and 20% strain in unconfined compression. Construct height and width were measured using a precision camera system (iMetrum), and then constructs were submerged in a phosphate-buffered saline (PBS) bath for testing. An Electroforce 3220 load frame (TA Instruments) and 250 g load cell (Model 31; Honeywell) were used. A preload of 2 g was applied for 15 min, and then samples were compressed at a rate of 0.01 mm/s to each strain level and allowed to equilibrate for 30 min. The equilibrium modulus was calculated as the slope of the stress/strain curve at the four-strain levels tested.
2.7 Cell encapsulation
Monolayer expanded porcine meniscus cells pooled from three subjects were trypsinized at p2, counted, centrifuged, and resuspended in PEGDA/GelMA solution at a density of 10 million cells/ml. Constructs were maintained in complete culture media, with media replaced every 2–3 days. A subset of samples (n = 3/group) were collected after 48 h in culture for viability measurements and then frozen in PBS for subsequent digestion and quantification of DNA content. The remaining samples were collected after 2 weeks in culture, and immediately processed for RNA extraction (n = 3/group) or frozen in PBS for DNA content analysis (n = 3/group) or histological staining (see Figure S2). RNA was extracted by manual pestle homogenization of hydrogel constructs and column purification (Norgen). Reverse transcription (RT)-qPCR was performed as described above.
2.8 Viability
Viability was assessed with the Live/Dead Viability/Cytotoxicity Kit (Catalog # L3224; Molecular Probes), according to the manufacturer's instructions. Constructs were stained with 4 μM calcein AM and 4 μM ethidium homodimer-1 in PBS and imaged with a fluorescent microscope (Model IX83; Olympus). Live and dead cells were quantified using CellSens Dimension software (Olympus).
2.9 DNA content
Hydrogel constructs collected and frozen at 48 h postembedding or after 2 weeks in culture were manually homogenized and digested in papain (SKU P4762; Sigma-Aldrich) solution (125 μg/ml in phosphate-buffered EDTA solution) at 65°C for 16 h.23, 26-29 DNA concentration was measured by Quant-iT Picogreen dye (Catalog # P11496; Molecular Probes). Total DNA was calculated and divided by construct wet weight to obtain a DNA/wet weight for each sample (n = 3/group). The DNA/wet weight values at 2 weeks were divided by the average DNA/wet weight of the 48-h samples to obtain the fold change in DNA content.
2.10 Statistical analyses
Expression data were log transformed to correct for skewness inherent to relative expression data and meet statistical model assumptions. For dedifferentiation RT-qPCR results, one-way analysis of variance (ANOVA) followed by Bonferroni corrected post hoc analyses were performed to select relevant comparisons: all inner zone samples to each other, all outer zone samples to each other, and fresh inner to fresh outer tissue. Hydrogel gene expression, DNA content, and mechanical testing data were analyzed by one-way ANOVA followed by Tukey corrected post hoc analyses. Alpha was set a priori as less than 0.05.
3 RESULTS
3.1 Meniscus cell dedifferentiation
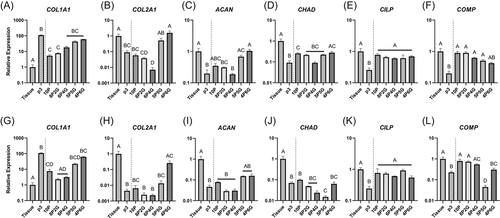
COL1A1 showed a robust increase in expression with monolayer passage in both inner and outer zone cells, increasing approximately 18-fold at Passage 1 and 80 to 100-fold at subsequent passages, relative to tissue explant culture (Figure 1A). Outer zone fresh tissue expression of COL1A1 was significantly higher (p = 0.02) than inner zone, although this six-fold difference was modest compared to the changes observed with monolayer culture. Lower mean expression of COL2A1 was observed in later passage monolayer cells, but these differences were surprisingly not significant for inner zone cells (Figure 1B). COL2A1 expression of outer zone monolayer cells at all passages was significantly downregulated compared to fresh tissue or tissue explant culture (p < 0.01). There was no difference detected between inner and outer zone fresh tissue expression of COL2A1. ACAN expression was downregulated in monolayer culture for both inner and outer zone cells compared to fresh tissue (Figure 1C, p < 0.01). Expression in outer zone cells at all passages and inner zone cells at Passage 3 was significantly downregulated compared to the corresponding tissue explant culture (p < 0.05). While outer zone fresh tissue ACAN expression appeared lower than inner zone expression, no significant difference was detected (p = 0.07). Expression of SOX9 (Figure S1) was significantly downregulated in tissue explant culture and monolayer compared to fresh tissue (p < 0.001), but there were no apparent expression differences between tissue explant culture and Passage 3 monolayer cells or between inner and outer zone fresh tissue.
CHAD (Figure 1D), CILP (Figure 1E), and COMP (Figure 1F) all showed significant decreases in expression in both inner and outer zone monolayer cultured cells compared to fresh tissue (p < 0.001) and no significant differences were detected between inner and outer zone fresh tissue. In almost all cases, these genes were significantly differentially expressed between fresh tissue, tissue explant, and monolayer culture (p < 0.05), except for CILP (inner zone tissue explant vs. Passage 1) and outer zone CHAD (fresh tissue vs. tissue explant).
3.2 Hydrogels for meniscus cell embedding
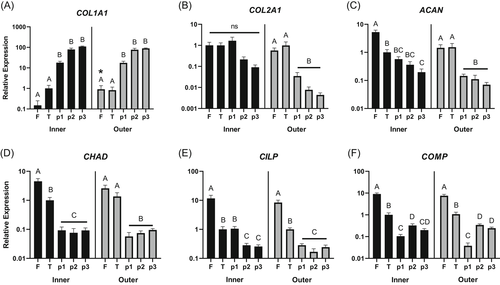
Hydrogels for meniscus cell culture were tested for (1) mechanical properties (equilibrium modulus), (2) viability of embedded meniscus cells, and (3) expression level of the phenotype marker genes described above. The equilibrium modulus ranged from 27 kPa for the 5P5G hydrogels to 40 kPa for 10P hydrogels, and only 10P and 5P5G hydrogels had significantly different equilibrium moduli (Figure 2, p < 0.05).
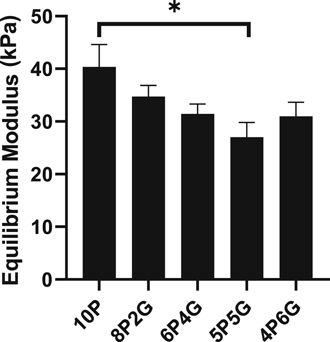
Viability of the cells embedded within the hydrogels varied by gel composition (Figure 3A and Figure S3). The percent cell viability indicated that higher PEGDA content negatively affected cell viability, with 10P hydrogels demonstrating decreased cell viability at 48 h postembedding compared to all other materials for inner zone cells (p < 0.05), and as compared to 6P4G and 4P6G for outer zone cells (p < 0.05). As shown by the fold change in DNA content (Figure 3B), the cellular content of majority-PEGDA hydrogels declined over 2 weeks in culture (fold change < 1). Conversely, 5P5G hydrogels maintained about the same overall level of cellularity during culture (fold change ∼1) and 4P6G hydrogels promoted cellular proliferation (fold change > 1). These differences were significant when comparing majority-PEGDA hydrogels (10P, 8P2G, and 6P4G) to 5P5G or 4P6G for inner zone cells (p < .05), or when comparing majority-PEGDA hydrogels to 4P6G for outer zone cells (p < 0.05).
Embedding meniscus cells in hydrogels of varying PEGDA/GelMA composition had striking effects on meniscus cell gene expression (Figure 4). COL1A1 expression was increased in hydrogels with higher GelMA content. Only 10P and 8P2G for inner zone cells (Figure 4A, p < 0.05), and 10P, 8P2G, and 6P4G for outer zone cells (Figure 4G, p < 0.05) significantly suppressed COL1A1 expression compared to p3 monolayer cells. All hydrogel materials, except 8P2G and 6P4G with outer zone cells, demonstrated significantly higher COL1A1 expression than tissue explant culture (p < 0.05). Higher-GelMA content hydrogels (5P5G and 4P6G for inner zone cells [Figure 4B] and 4P6G for outer zone cells [Figure 4H]) induced expression levels of COL2A1 similar to tissue explant culture and significantly higher than p3 monolayer culture (p < 0.001). Similarly, ACAN expression was not significantly different from tissue explant culture for either inner (Figure 4C) or outer zone cells (Figure 4I) embedded in 5P5G or 4P6G hydrogels. For inner zone cells, 10P and 4P6G embedding induced CHAD expression significantly higher than monolayer (p < 0.05), but 10P was still significantly lower than tissue explant culture (Figure 4D, p < 0.05). For outer zone cells, none of the hydrogels increased CHAD expression compared to monolayer culture (Figure 4J). On the other hand, CILP expression was supported by all hydrogel materials for both inner (Figure 4E) and outer zone cells (Figure 4K); for every group, CILP expression was significantly higher than monolayer culture (p < 0.05) and not significantly different than tissue explant culture. COMP expression trended in the opposite direction from the other ECM component genes, showing explant level expression in high-PEGDA hydrogels and decreasing expression as GelMA composition was increased (Figures 4F and 4L); however, for the inner zone cells (Figure 4F), none of the hydrogel constructs were significantly different from tissue explant culture expression levels.
4 DISCUSSION
Although it is generally accepted that meniscus cells dedifferentiate in monolayer culture, our results expand the understanding of meniscus cell dedifferentiation by providing comparisons between inner and outer zone cells and between fresh tissue and explant tissue culture, for a number of genes generally assumed to be dysregulated in meniscus cell dedifferentiation. In addition, we report decreased expression during monolayer culture of CHAD, CILP, and COMP, which were recently identified as potential markers of meniscus cell dedifferentiation.10 Overall, COL1A1, CHAD, CILP, and COMP show robust and consistent changes in expression with monolayer culture, which make them useful markers of dedifferentiation in meniscus cells. Mean expression levels of COL2A1 and ACAN did decrease with passage number but were less sensitive as markers of dedifferentiation for inner zone cells: downregulation of COL2A1 was not found to be significant, and ACAN expression was not significantly different from tissue explant culture until Passage 3. In this study, we were able to directly compare the gene expression levels of meniscus tissue explant culture and monolayer culture to the expression profile of meniscus cells in different concentrations of hydrogel biomaterials. Our findings show that hydrogel embedding modulates the dedifferentiation process, and depending on the embedding material, is capable of restoring phenotype marker gene expression to levels similar to tissue explant culture. Of the materials tested, higher GelMA containing hydrogels were the most promising for meniscus cell culture, restoring expression levels of most phenotype markers to tissue explant culture levels and promoting the expression of the major ECM components.
One widely accepted definition of articular chondrocyte dedifferentiation is an increase in COL1A1 expression concomitant with decreases in COL2A1, ACAN, and SOX9.30, 31 However, our results showed that of these genes, only COL1A1 showed robust changes in expression level for monolayer cultured meniscus cells of both inner and outer zone. While COL2A1 and ACAN expression did tend to decrease with passage, changes in relative expression were smaller than those reported in articular chondrocytes.30, 31 Furthermore, in this study, outer zone cells showed more robust changes in the chondrogenic gene COL2A1 during monolayer culture. SOX9, considered an indicator of chondrocyte dedifferentiation,30, 32, 33 only showed differences between fresh tissue and all cultured conditions, including tissue explant culture. Therefore, we did not interrogate SOX9 further, as we focused primarily on genes that showed changes in expression between tissue explant culture and monolayer culture, as we considered the tissue explant to be the best comparator for other culture methods. Genes identified as markers of meniscus cell dedifferentiation by microarray of inner zone meniscus cells and tissue (CHAD, CILP, and COMP)10 displayed similar expression patterns between inner and outer zone tissue and decreased markedly in monolayer cultured cells in this study. Our findings match previous reports of COMP expression4, 7; however, to our knowledge, CHAD and CILP have not previously been utilized as phenotype markers for both inner and outer zone meniscus cells.
Meniscus cells are often described as “chondrocyte-like” in the inner zone, and “fibroblast-like” in the outer zone.11 However, of all the genes measured in this study, only COL1A1 expression was significantly different in fresh tissue between inner and outer zone cells. ACAN was nearly significant (p = 0.07), while all other genes had similar expression levels between fresh tissue from the inner and outer zones. Both in our results and in the literature,8, 9 significant changes in COL1A1 expression are observed between inner and outer zone tissue, while differences in expression of chondrogenic phenotype genes, such as COL2A1, ACAN, and SOX9 are relatively small or absent. Previous studies have found significant differences between the inner and outer zone expression of COL2A1 and ACAN.8, 9 However, the difference in COL2A1 expression reported by Upton et al.9 was 5.7 ± 3.4 fold higher in inner versus outer tissue, whereas the reported change in COL1A1 expression was greater than 10,000 fold. This study also reported a similar fold-change in ACAN expression to our results (4.6 ± 1.6-fold and 3.6 ± 0.6-fold, respectively). Son and Levenston8 reported dramatic increases in COL2A1 expression in the inner third of the meniscus and found ACAN expression to be significantly higher in inner regions but still far lower than in cartilage. Subdividing the tissue into thirds rather than inner two-thirds and outer third, as performed in the current study, may have increased the sensitivity to measure differences in COL2A1. However, Son and Levenston8 utilized tissue from 2-week-old calves, which may also explain the observed differences, as patterns of COL2A1 and other ECM gene expression are likely to vary with age and health status of the tissue.34-37 Therefore, although COL2A1 and ACAN expression appear to vary spatially across the meniscus, the use of these genes as phenotype markers may be complicated by their expression being modulated by a number of patient variables, including age and health. Taken together, these results demonstrate that characterizing meniscus cells as chondrocyte-like or fibroblast-like is insufficient. Indeed, a recent study utilizing single-cell RNA-sequencing identified seven unique cell types in the meniscus. Markers of several of the identified cell types, including the major population defined as “fibrochondrocytes,” did not appear to be differently expressed between inner and outer zones,38 which highlights the limitations of the currently accepted descriptions and zones of meniscus tissue.
One challenge in studying the meniscus is the difficulty in defining the precise location of inner versus outer zone tissue. Since there is no clear boundary to distinguish the zones, the selection of tissue for cell isolation or explant culture is subjective. In this study, we defined the outer zone tissue as the outer one-third and the inner zone tissue as the inner two-thirds of the meniscus. This method was consistent between samples and was utilized in previous studies of meniscus regional cellular characterization.5, 6, 10 It is possible that further subdivision of meniscus regions may have yielded further significant differences in gene expression, as observed by Son and Levenston.8 Ultimately, the true spatial variability and distribution of cell types throughout the meniscus remain largely unknown and cannot be established by the bulk-RNA preparation methods used in this paper. Therefore, further characterization of spatial variation in the meniscus cell transcriptome is necessary to understand the distribution and phenotypic characteristics of meniscus-resident cells.
In this study, we used porcine meniscus cells. However, comparisons to the literature suggest that the gene expression patterns revealed in this study generally show concordance across species, with porcine,9 bovine,4, 8 and human5 menisci showing similar gene expression trends in monolayer culture. Furthermore, the identification of CHAD, CILP, and COMP as potential markers of meniscus cell dedifferentiation by microarray was performed using human tissue, and our results confirmed the loss of expression of these genes in porcine tissue, supporting the utility of porcine tissue for studies of meniscus cell phenotyping.
As with dedifferentiation, redifferentiation of articular chondrocytes has been studied more than redifferentiation of meniscus cells. Methods widely accepted for redifferentiation of chondrocytes include 3D culture in agarose or alginate hydrogels and/or treatment with various growth factors, such as transforming growth factor (TGF)-β.33, 39-42 In our experience, treating meniscus cells with TGF-β or agarose embedding results in large increases in COL2A1 expression (unpublished data), which is by no means a return to baseline, or “redifferentiation” of meniscus cells. Although some have proposed using a ratio of COL2A1/COL1A1 as an indicator of dedifferentiation,6 as shown by our results, changes related to dedifferentiation would be driven almost entirely by changes in COL1A1. Furthermore, redifferentiation strategies that increase COL2A1 expression could restore the ratio but would fail to restore the baseline phenotype.
The lack of established culture methods to maintain the differentiated meniscus cell phenotype may be largely due to the fact that most currently available methods are attuned to driving a chondrogenic phenotype, as well as the lack of clearly defined markers of the meniscus cell phenotype to use as benchmarks. It is known that cell shape, substrate stiffness, and availability of cellular adhesion sites have profound effects on the phenotype of cells in culture, and indeed these properties have been shown to be effective in modulating the phenotype of chondrocytes.21, 43 Therefore, we tested the hypothesis that varying mixtures of PEGDA, which is a synthetic polymer often described as “biologically inert” and GelMA, which is a collagen-derived polymer retaining native cellular adhesion motifs, would alter the phenotype of embedded meniscus cells, and that the right ratio would provide an environment that returns phenotypic markers to tissue explant culture levels. In this study, gene expression was measured after 2 weeks in hydrogel embedded culture. This was chosen to give adequate time for the reversal of monolayer-induced dedifferentiation, which was observed over a period of 10 days during monolayer culture (time from cell isolation to Passage 3). In addition, prior work has shown that 2 weeks is sufficient time for hydrogel-embedded chondrocytes to produce a pericellular matrix,44, 45 which is important for cells to sense and respond to their physical environment, and, therefore, may be important for phenotype maintenance mediated by material embedding.
The varying PEGDA/GelMA ratio hydrogels had markedly different effects on the meniscus phenotype markers measured in this study. Higher GelMA content favored the expression of major ECM genes COL1A1, COL2A1, and ACAN. For inner zone cells, COL2A1 and ACAN returned to expression levels similar to tissue explant culture in 5P5G and 4P6G materials. Unfortunately, higher GelMA containing gels appeared to be less effective in returning COL1A1 expression to baseline levels. COMP, which is also a major ECM component in both cartilage and meniscus, showed an opposite trend, with expression decreasing with increasing GelMA concentration. Outer zone cells displayed similar overall trends in expression to inner zone cells, but the materials tested seemed less effective at returning their expression levels to tissue explant culture levels, suggesting that other materials or approaches may be necessary for the culture of outer zone cells.
Mechanical testing of the hydrogel materials revealed only modest differences in stiffness, suggesting that the effect of cellular adhesion sites or some other property of the GelMA/cell interaction is driving phenotypic changes rather than differences in stiffness. Overall, the stiffness of these hydrogels is similar to materials used to encapsulate chondrocytes for mechanobiology and tissue engineering research,46 so they should be suitable for experiments of mechanical stimulation similar to those performed on chondrocyte-embedded agarose constructs. Viability and DNA content measurements showed that higher GelMA content hydrogels supported greater cell survival and 4P6G hydrogels promoted cellular proliferation. Overall, these materials were partially successful in halting or reversing the dedifferentiation trends in the phenotype markers measured in this study. 5P5G and 4P6G materials returned most of the phenotype markers to near-tissue explant culture levels for inner zone meniscus cells; however, these materials did not significantly reduce COL1A1 expression relative to p3 monolayer culture. For tissue engineering applications, 4P6G hydrogels seem to promote maximum ECM component expression and cellular proliferation. However, COL1A1 expression seems to increase and COMP expression decreases with increasing GelMA concentration. Therefore, higher GelMA ratios may further diverge from the meniscus cell phenotype. By directly comparing expression levels to tissue explant culture, we have shown that it is possible to reverse dedifferentiation trends by embedding meniscus cells in biomaterial hydrogels. However, much like previous attempts,4, 5, 15 returning COL1A1 expression to baseline remains a challenge. Additional work is still needed to further define meniscus cell phenotype, factors driving dedifferentiation, and methods to maintain a native meniscus cell phenotype in vitro for studies of meniscus cell biology and tissue engineering.
Overall, we identified COL1A1, CHAD, CILP, and COMP as useful markers of meniscus cell dedifferentiation. Our findings provide new details on the meniscus cell dedifferentiation process, including comparisons between inner and outer zone tissue, tissue explant culture, and isolated cells. However, there remains a dearth of knowledge regarding the phenotypes of meniscus cells, including clear definitions of inner and outer zone cell identities, and whether multiple subpopulations exist beyond these major delineations. Attempts to reverse or halt meniscus cell dedifferentiation by embedding meniscus cells in PEGDA/GelMA hydrogels are promising, exhibiting a partial reversal of the dedifferentiation phenotype and returning several marker genes to near tissue explant culture levels. Our findings fill important gaps in knowledge that lay the foundation for future studies of meniscus cell biology and meniscus tissue engineering.
ACKNOWLEDGMENTS
This study was supported in part by NIH Grants AR073221, AR074800, and AR065527, an Orthopaedic Research and Education Foundation Grant with funding provided by the Musculoskeletal Transplant Foundation, and the Urbaniak Sports Science Institute. The authors would like to thank Nivedita Sangaj for technical assistance in hydrogel preparation.
CONFLICT OF INTERESTS
The authors declare that there are no conflicts of interests.
AUTHOR CONTRIBUTIONS
Study design and conceptualization: Amy L. McNulty, Benjamin Andress, and Shyni Varghese. Data collection and processing: Benjamin Andress and Jason H. Kim. Preparation of manuscript and figures: Benjamin Andress. Clinical expertize: Annunziato Amendola. Data analysis and interpretation: Benjamin Andress, Hattie C. Cutcliffe, Louis E. DeFrate, Adam P. Goode, and Amy L. McNulty. All authors contributed to manuscript editing and critical revision and have read and approved the submitted version.




