Impaired Fracture Healing in Sarco-Osteoporotic Mice Can Be Rescued by Vibration Treatment Through Myostatin Suppression
ABSTRACT
Sarcopenia is highly prevalent in fragility fracture patients and is associated with delayed healing. In this study, we investigated the effect of low-magnitude high-frequency vibration (LMHFV) on osteoporotic fracture with sarcopenia and the potential role of myostatin. Osteoporotic fractures created in sarcopenic SAMP8, non-sarcopenic SAMR1 were randomized to control or LMHFV (SAMP8, SAMR1, SAMP8-V, or SAMR1-V) groups. Healing and myostatin expression were evaluated at 2, 4, and 6 weeks post-fracture. In vitro, conditioned-media were collected from myofibers isolated from aged and young SAMP8 or C2C12 myoblasts with or without LMHFV. Osteoblastic MC3T3-E1 under osteogenic differentiation were treated with plain or conditioned-medium (±myostatin propeptide). LMHFV significantly enhanced callus formation was in non-sarcopenic SAMR1 mice; but the enhancement effect was not significant in SAMP8 mice at week 2. Myostatin expressions in callus and biceps femoris of SAMP8 group were significantly higher all groups with significant negative correlation with callus size (R2 = 0.7256; p = 0.0004). Mechanical properties (week 4) and callus remodeling (week 6) were inferior in SAMP8 versus SAMR1 and were significantly enhanced by LMHFV. Alkaline Phosphatase (ALP) and Runx2 expression of MC3T3-E1 was lower in aged myofiber compared with young, but upregulated by LMHFV or myostatin inhibition; also confirmed with C2C12. LMHFV enhanced early callus formation, microarchitecture, callus remodeling and mechanical properties of fracture healing in both SAMP8 and SAMR1; however, more effective in non-sarcopenic SAMR1 mice. Impaired fracture healing in sarcopenic SAMP8 mice is attributed by elevated myostatin expression in callus and muscle, which correlated negatively with callus formation. © 2019 Orthopaedic Research Society. Published by Wiley Periodicals, Inc. J Orthop Res 38:277-287, 2020
Ageing causes a significant functional decline in both skeletal muscle and bone quality leading to sarcopenia and osteoporosis, respectively. Sarcopenia is a geriatric syndrome characterized by a progressive loss of skeletal muscle mass and function1 with high prevalence in the older population worldwide.2, 3 The prevalence of sarcopenia is also very high in osteoporotic fracture patients: 84.1% of men and 41.9% of women with fragility hip fracture in Chinese elderly2; 81.1% of men and 44.7% women with hip fracture in Japan4; 71% of hip fracture patients in Australia 5 and 58% of the older women following a hip fracture in Italy.6 Sarcopenia alone7 as well as co-existence with osteoporosis (sarco-osteoporosis) in community-dwelling people result in a significantly higher risk of fractures.8 Therefore, managing sarcopenia has become a major target to prevent osteoporotic fractures.9
Bone repair is a complicated process involving various cells, signaling molecules and growth factors, and adjacent muscles has been postulated to play important roles in fracture healing.10 The lack of intact muscle around the fracture site leads to poor vascularization, hindered cytokine release, and recruitment of stem cells.11, 12 Our previous study has demonstrated that osteoporotic fracture healing in sarcopenic mice showed significantly delayed healing compared with those without sarcopenia.13 Mechanical forces exerted by skeletal muscles are essential for stimulating bone metabolism and bone repair.14 Low-magnitude high-frequency vibration (LMHFV)15 was effective in enhancing both normal and ovariectomy (OVX)-induced osteoporotic bone fracture healing in a rat model by promoting callus formation, mineralization, and remodeling.15, 16 On muscle, a randomized controlled trial on 710 community elderly showed that LMHFV was effective in fall prevention with improved muscle strength and balancing ability,17 indicating that LMHFV is useful in counteracting the loss of muscle strength and mobility. Our pre-clinical study also showed that LMHFV could significantly improve the contractibility and cross-sectional area of type IIa muscle fibers, suppress satellite cell pool reduction and myostatin expression, thus retarding the progress of sarcopenia in sarcopenic animal model.18
Myostatin is a potent myokine that inhibits muscle growth19 and a key mediator of sarcopenia.20 It is also a negative factor in osteogenic differentiation of bone marrow-derived mesenchymal stem cells (bMSC).21 The expression of myostatin was significantly downregulated in primary murine satellite cells from CD1 mice and C2C12 myoblasts treated by low-amplitude high frequency (30 Hz) vibration.22 On the basis of these evidences, we hypothesized that osteoporotic fracture healing might be negatively affected by the elevated expression of myostatin in sarcopenia and could be reversed by LMHFV treatment.
MATERIALS AND METHODS
Animals
SAMP8 mice were used as a sarco-osteoporosis animal model which sarcopenia onsets at month 8 with senile osteoporosis as previously reported.13, 18 Non-sarcopenic SAMR1 mice23 were used as control mice with senile osteoporosis only. SAMR1 is a well-known control strain of SAMP8 and age-matched SAMR1 mice was proven without sarcopenia phenotype compared with SAMP8 in our previous13 and other studies.24, 25 Animals were supplied by the Laboratory Animal Service Center (LASEC), the Chinese University of Hong Kong.
All animals were kept under a 12:12 h light: dark cycle with food and water ad libitum. The research protocol was approved by the Animal Experimentation Ethics Committee of the Chinese University of Hong Kong (Ref:15/074/GRF-5-C).
Animal Model and Experimental Design
A total of 100 animals (50 SAMP8 mice and 50 SAMR1 mice) were included in this study. A closed fracture model was created based on our previously established protocol.13 Briefly, 8-month-old mice were put under general anesthesia by intraperitoneal injection of ketamine and xylazine (100 mg/kg and 4 mg/kg body weight, respectively). A 1.5-inch, 25-gauge needle (BD Medical Technology, Franklin Lakes, NJ) was surgically inserted for internal fixation of the right femur through the patellofemoral groove. Closed diaphyseal fracture was then created by a 3-point bending device. Temgesic 0.1 mg/kg was given to the mice for 3 days post-surgery for analgesia.
The fractured SAMP8 or SAMR1 mice were randomized to either control (SAMP8 n = 25 and SAMR1 n = 25) or LMHFV treatment (SAMP8-V n = 25 and SAMR1-V n = 25) groups. Mice in the control groups were put on the LMHFV platform with power off. Those in vibration groups were given LMHFV treatment (35 Hz, 0.3 g; g = gravitational acceleration) 20 min/day and 5 days/week starting from day 1 postoperatively according to our established protocol, which the mice could start full weight-bearing with hindlimb.15, 16, 26
Fractured mice were sacrificed at week 2 post-fracture (n = 5 per group), week 4 (n = 10 per group), and week 6 (n = 10 per group) for further assessments.
Radiographic Analysis
Fracture healing was monitored weekly by a high-resolution digital radiography system (MX-20; Faxitron, Tucson, AZ) with an exposure of 32 kV for 5 s. Callus width and area of the fractured femur was measured at each time point using ImageJ (NIH, Bethesda, MD) as previously reported.27, 28
Microcomputed tomography (MicroCT) Analysis
Femora specimens were scanned by the microCT system (VivaCT 40; Scanco Medical, Brüttisellen, Switzerland) at 70 keV and 114 μA covering 3 mm proximal and distal to the fracture line at a resolution of 10.5 μm as previously reported.28, 29 Region of interest (ROI) was selected from two-dimensional CT images, followed by segmentation of mineralized tissues with Gaussian filter at Sigma = 1.2 and Support = 2. Total tissue volume (TV), total bone volume (BVt), BVt/TV were evaluated.
Histomorphometry
Fractured femora were fixed, decalcified, paraffin embedded followed sagittal sections at 5 μm for hematoxylin and eosin (H&E) staining as previously described.13, 30 For quantitative analysis, images were captured under a light microscope (DM5500B; Leica, Wetzlar, Germany) with the ROI defined 1.5 mm proximal and distal to the fracture line. Callus tissues within the ROI were quantified using ImageJ (NIH, Bethesda, MD) as previously described.13, 15
Immunohistochemistry
Myostatin, tumor necrosis factor-α (TNF-α) and bone morphogenetic protein-2 (BMP-2) were reported to involve in bone-muscle crosstalk.31 Primary antibodies against these factors (Abcam, Cambridge, MA) were applied followed by the detection with a commercially available HRP/DAD kit (EXPOSE; Abcam). For myostatin, TNF-α and BMP-2 detection, a secondary goat-anti-rabbit AlexaFluor-488 antibody was used (Invitrogen, Thermo Fisher Scientific, Carlsbad, CA). Images were captured on a fluorescence microscope and the positive area fraction was quantified using ImageJ (NIH, Bethesda, MD) as previously described.26, 32
Mechanical Testing
Mechanical properties of the femora were examined by a three-point bending test at week 4 and 6 post-fracture according to our established protocol.13, 16 Three-point bending test can provide the distribution of internal loading similar to physiology for mechanical characterization at region of interest. This technique is a highly directional dependent that is the limitation when compared with a torsional test that is not affected by the orientation of asymmetric calluses.
Before mechanical testing, the femora were thawed overnight at room temperature and positioned horizontally with the anterior side upwards, centered on supports 10 mm apart on the material testing machine (H25KS; Hounsfield Test Equipment, Salfords, UK) with a 50 N load cell-driven down at constant velocity at 5 mm/min. The ultimate load, stiffness, and energy to failure values were calculated using built-in software (QMAT Professional; Tinius Olsen, Horsham, PA) normalized against contralateral intact femora controls.
Myofibers Isolation, Culture and Conditioned Medium (CM) Collection
Myofibers from 3-month (Young) and 10-month-old (Aged) SAMP8 mice were isolated and cultured as described.33 Briefly, extensor digitorum longus (EDL) was dissected and transferred to F10 solution with collagenase at 1,000 units/ml (Gibco, Carlsbad, CA) and incubated in a 37°C water bath for digestion until muscles started to loosen and myofibers became visible. The muscle was flushed with warm F10 medium to a new dish using a large-bore glass pipette until fibers were gently released. Myofibers were isolated and transferred to a new dish with F10 medium under a dissecting microscope; repeated for two more times until all dead myofibers and debris were removed. About 50 myofibers were transferred to each dish and cultured at 37°C, 5% CO2. The isolated myofibers were divided into four groups: control groups (Aged-Ctrl and Young-Ctrl) received no treatment and vibration groups (Aged-VT and Young-VT) were given daily LMHFV treatment (35 Hz, 0.3 g) 20 min/day for two times (n = 3 per group) with the culture plate placed directly on the vibration platform. After 48-h culture, serum-free CM was collected and centrifuged at 500 g for 10 min and stored at −80°C until use.
Myoblasts C2C12 Culture and CM Collection
Murine myoblasts C2C12 cells were cultured in high glucose Dulbecco's Modified Eagle's Medium (DMEM) (Gibco) with 10% fetal bovine serum (FBS) (Gibco) and 1% Penicillin-Streptomycin-Neomycin (PSN) Antibiotic Mixture (Gibco), at 37°C in a controlled 5% CO2 incubator with humidified atmosphere. Cells were maintained until approximately 70% confluence throughout the culture period. The cells were divided into four groups: control group (C2C12-Ctrl) received no treatment and vibration group (C2C12-VT) were given LMHFV treatment as described above (n = 3 per group). After 48-h culture, serum-free CM was collected and centrifuged at 500 g for 10 min and stored at −80°C until use.
Cell Culture
Murine osteoblastic MC3T3-E1 cells were cultured in high glucose alpha Modified Eagle's medium (α-MEM) (Gibco) with 10% FBS (Gibco) and 1% PSN (Gibco). Osteogenic differentiation medium contained 10 mM β-glycerolphosphate, 50 μg/ml of ascorbic acid and 15% FBS. At day 8, MC3T3 cells were co-cultured with the collected CM with or without recombinant myostatin propeptide (100 ng/ml; PeproTech, Rocky Hill, CT) (-PRO groups),34 that was a myostatin inhibitor by binding to myostatin,35 followed by alkaline phosphatase staining after 48 hours using PCIP/NBT color development substrate (Promega, Madison, WI) and collected for further assessments.
Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
Total RNA was extracted from the harvested MC3T3-E1 cells using RNAiso Plus (TaKaRa, Shiga, Japan) according to the manufacturer's instructions. A first-strand complementary DNA (cDNA) was synthesized from 1 μg of the total RNA using RT Master Mix kit (TaKaRa) followed by qRT-PCR (Power SYBR Green PCR Master Mix; Thermo Fisher Scientific) according to the manufacturer's instructions. Expression of Runx2, a key regulator of osteoblastic differentiation and another pro-differentiation factor osteocalcin (OCN) were evaluated36 (n = 6 for each parameter).
- Runx2-F′ TTCAACGATCTGAGATTTGTGGG;
- Runx2-R′ GGATGAGGAATGCGCCCTA.
- OCN-F′ CTGACCTCACAGATCCCAAGC
- OCN-R′ TGGTCTGATAGCTCGTCACAAG
- GAPDH-F′ TGGCCTTCCGTGTTCCTAC;
- GAPDH-R′ GAGTTGCTGTTGAAGTCGCA.
The conditions of RT-PCR were as follows: denaturation at 95°C for 10 s, 40 cycles at 95°C for 10 s, and 60°C for 30 s. The dissociation stage was added to the end of the amplification procedure. No non-specific amplification was confirmed by the dissociation curve analysis. Data were analyzed by the ΔΔCt method against the housekeeping gene of glyceraldehyde 3-phosphate dehydrogenase (GAPDH).32
Western Blot
Cells were lysed on ice for 30 min in the lysis buffer containing 50 mM Tris-HCl, pH7.4, 150 mM NaCl, 1% Nonidet P-40, and 0.1% sodium dodecyl sulfate (SDS) supplemented with protease inhibitors (10 mg/ml leupeptin, 10 mg/ml pepstatin A, and 10 mg/ml aprotinin). For western blot analysis, 50 μg of protein sample was resolved on 10% SDS polyacrylamide gel electrophoresis (SDS-PAGE) and electrotransferred onto nitrocellulose membranes (Whatman, Piscataway, NJ). The primary antibodies used were anti-Runx2 (1:1,000) (Abcam) and anti-OCN (1:500) (Abcam). GAPDH (1:1,000) (Abcam) was used as a loading control. Horseradish peroxidase (HRP)-conjugated secondary antibodies were used at 1:1,000 dilution. The antigen-antibody complexes were made visible by using syngene chemiluminescence detection system (GeneGnome). Immunoreactive bands (n = 3) were quantitatively analyzed in triplicate by normalizing the band intensities against GAPDH on scanned films by ImageJ software.37
Statistics
All data were expressed in mean ± standard deviation (SD). Statistical analyses were performed using SPSS 20.0 software (IBM, New York, NY). One-way analysis of variance was used for evaluating the differences among the groups separately at different time points, followed by Bonferroni post hoc tests for pairwise comparison, as time since fracture induction was not considered an independent variable due to the known temporal changes for measured parameters. The correlation was performed by the coefficient of determination. p < 0.05 was regarded as statistically significant.
RESULTS
LMHFV Partly Restored Osteoporotic Fracture Healing in Both Sarcopenic and Non-Sarcopenic Mice
The delayed osteoporotic fracture was found in sarcopenic SAMP8 compared with non-sarcopenic SAMR1 in our previous study.13 The effect of LMHFV on osteoporotic fracture with or without sarcopenia was examined in this study. As shown in the serial radiographs and callus measurement (Fig. 1A–C), at week 2 post-fracture, SAMP8 group showed significantly smaller callus area than SAMR1 (p = 0.023) and SAMR1-V (p = 0.000). LMHFV slightly enhanced callus area in the sarcopenic SAMP8-V group compared with SAMP8 group, yet without significant difference (p = 0.074). In non-sarcopenic SAMR1, LMHFV significantly enhanced the callus area compared with SAMR1 group (p = 0.026). At week 6 post-fracture, SAMP8 group showed significantly larger callus area than SAMR1 (p = 0.023) and SAMR1-V (p = 0.014) groups. Callus area was decreased in SAMP8-V group compared with SAMP8 group but was not statistically significant.
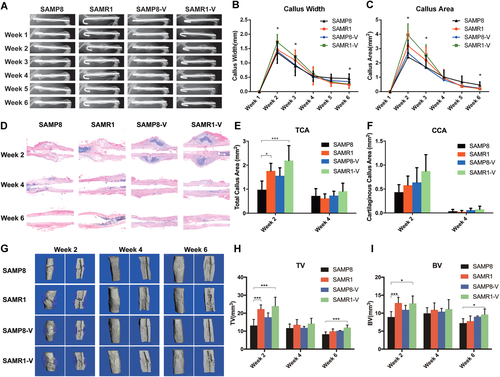
Low-magnitude high-frequency vibration (LMHFV) could partly enhance osteoporotic fracture healing in both sarcopenic and non-sarcopenic mice. (A) Representative radiographic images of osteoporotic fracture healing from week 1 to week 6; n = 10 for each group. (B) Quantification of callus width and (C) callus area. (D) Representative images of callus sections stained with H&E of all groups at week 2, 4, and 6 post-fracture (×10 magnification). (E) Quantification of total callus area (TCA) and (F) CCA at week 2 and 4 post-fracture; n = 5 per group per time point. (G) Representative three dimensional (3D) microCT images of fracture callus at week 2, week 4 and week 6 post-fracture. (H) TV of all groups (I) BV of all groups. n = 5 for each group per time point. *0.01 ≤ p < 0.05, ***p < 0.01 for comparison between groups at the same time point. [Color figure can be viewed at wileyonlinelibrary.com]
Histomorphometric analysis showed similar results (Fig. 1D–F). At week 2 post-fracture, the total callus area (TCA) was significantly smaller in SAMP8 group compared with SAMR1 group (p = 0.046) and SAMR1-V group (p = 0.002). TCA was also increased after LMHFV treatment in SAMP8-V group compared with SAMP8 group, yet not significant. At week 4 post-fracture, TCA and the cartilaginous callus fraction became insignificant among groups. At week 6 post-fracture, callus was remodeled, and the size was decreased. The callus became imperceptible in all groups and hence were not quantified. The cartilaginous callus area (CCA) showed a similar trend without significant difference.
MicroCT data further substantiated the radiographic and histologic findings (Fig. 1G–I). At week 2 post-fracture, TV of SAMP8 group was significantly lower than SAMR1 (p = 0.002) and SAMR1-V (p = 0.001) groups. BV of SAMP8 group was also significantly lower than SAMR1 (p = 0.005) and SAMR1-V (p = 0.01) groups. However, BV/TV of SAMP8 group was significantly higher than SAMR1 (p = 0.035) group and SAMR1-V (p = 0.005) group. At week 4 post-fracture, no significant difference was observed among groups. Surprisingly, at week 6 post-fracture, BV/TV of SAMP8 group was significantly higher than SAMR1 (p = 0.003) and SAMR1-V (p = 0.029) group, while BV/TV of SAMP8-V group was significantly higher than SAMR1 (p = 0.001) and SAMR1-V (p = 0.015) group (Table 1).
| TV | BV | BV/TV | ||
|---|---|---|---|---|
| Week 2 | SAMP8 | 13.13 ± 3.41 | 8.93 ± 1.52 | 0.69 ± 0.07 |
| SAMR1 | 22.30 ± 2.31* | 12.84 ± 1.52* | 0.58 ± 0.05* | |
| SAMP8-V | 17.73 ± 2.43 | 10.92 ± 1.25 | 0.62 ± 0.07 | |
| SAMR1-V | 23.94 ± 4.95* | 12.73 ± 2.05* | 0.54 ± 0.05* | |
| Week 4 | SAMP8 | 11.71 ± 2.29 | 9.94 ± 1.61 | 0.85 ± 0.04 |
| SAMR1 | 13.57 ± 2.92 | 10.93 ± 1.92 | 0.81 ± 0.06 | |
| SAMP8-V | 11.81 ± 0.80 | 10.40 ± 0.91 | 0.88 ± 0.02 | |
| SAMR1-V | 14.18 ± 2.96 | 11.14 ± 2.64 | 0.78 ± 0.04 | |
| Week 6 | SAMP8 | 8.30 ± 1.30 | 7.23 ± 1.24 | 0.87 ± 0.03 |
| SAMR1 | 9.93 ± 1.34 | 7.80 ± 1.43 | 0.78 ± 0.05* | |
| SAMP8-V | 10.23 ± 0.14 | 9.00 ± 0.23 | 0.88 ± 0.03** | |
| SAMR1-V | 11.98 ± 1.39* | 9.62 ± 1.52* | 0.80 ± 0.04* | |
- Values are expressed in mean ± standard deviation. One-way analysis of variance (ANOVA) among groups.
- * p < 0.05 compared with SAMP8 group.
- ** p < 0.05 compared with SAMR1 group.
LMHFV Promoted Re-Establishment of Mechanical Properties in Both Sarcopenic and Non-Sarcopenic Mice
As the gold standard of fracture healing, mechanical properties were measured by three-point-bending test at week 4 and week 6 post-fracture. At week 4 post-fracture, the relative ultimate load of the femora in SAMR1 group was significantly higher than SAMP8 group (p = 0.021). After LMHFV treatment, the relative ultimate load of the femora in SAMP8-V and SAMR1-V groups were significantly higher than those in SAMP8 group (p = 0.001, p = 0.001, respectively) (Fig. 2A). The relative stiffness of the femora in SAMR1 was significantly higher than SAMP8 group (p = 0.001). SAMP8-V and SAMR1-V groups were significantly higher compared with SAMP8 group (p = 0.014, p = 0.004, respectively) (Fig. 2B). Relative energy to failure in SAMP8-V and SAMR1-V groups were significantly higher than SAMP8 group (p = 0.002, p = 0.001, respectively) and SAMR1 group (p = 0.039, p = 0.010, respectively) (Fig. 2C). No significant differences in relative ultimate load, stiffness, and energy to failure were observed among groups at week 6 post-fracture (Fig. 2A–C).
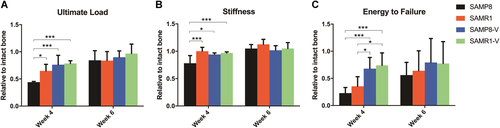
Low-magnitude high-frequency vibration (LMHFV) accelerated re-establishment of mechanical properties in sarcopenic and non-sarcopenic mice. (A) Ultimate load, (B) stiffness, and (C) energy to failure of the callus relative to the contralateral intact femur at weeks 4 and 6. n = 5 per group per time point. *0.01 ≤ p < 0.05, ***p < 0.01 for comparison between groups at the same time point. [Color figure can be viewed at wileyonlinelibrary.com]
Myostatin Expression Could be Suppressed by LMHFV and Correlated Negatively With Callus Formation
Myostatin is a key cross-talk myokine between muscle and bone detected in both the fracture callus and adjacent bicep femoris (BF). Myostatin expression in the callus was significantly higher (p = 0.0001) in SAMP8 group compared with SAMR1 group at week 2 post-fracture. LMHFV significantly reduced its expression (p = 0.0002) in SAMP8-V compared with SAMP8 group (Fig. 3A and B). Meanwhile, the expression of myostatin in the BF adjacent to the fracture site was also significantly higher (p = 0.0170) in SAMP8 group versus SAMR1 group at week 2; similarly, LMHFV significantly reduced myostatin expression (p = 0.0220) in the BF muscle in SAMP8-V compared with SAMP8 (Fig. 3C and D). A significant positive linear correlation between myostatin expression in the BF muscle and in the callus was found at week 2 post-fracture (R2 = 0.7837, p = 0.0001, Fig. 3E). A significant negative linear correlation (R2 = 0.7256, p = 0.0004) between TCA and myostatin expression in the callus was observed at week 2 post-fracture (Fig. 3F). However, at week 4 and 6 post-fracture, no significant difference was observed among groups and the myostatin expression was low in all groups (Supplementary Fig. S1). The expression of BMP2 and TNF-α were found not different (Supplementary Fig. S2).
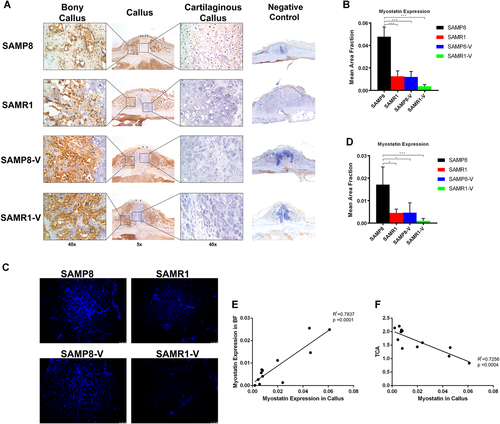
Myostatin expression was suppressed by low-magnitude high-frequency vibration (LMHFV) and correlated negatively with callus formation capacity. (A) Immunohistochemistry (IHC) staining of myostatin and (B) quantification of the expression of myostatin in fracture callus (n = 5 per group) at week 2 post-fracture. (C) Immunofluorescence (IF) staining of myostatin (blue fluorescence) and (D) quantification of the expression of myostatin in BF muscle next to the fracture site (n = 5 per group) at week 2 post-fracture. (E) Correlation between myostatin expression in the BF muscle and in the callus at week 2 post-fracture. (F) Correlation between total callus area (TCA) and myostatin expression in the callus. *0.01 ≤ p < 0.05, ***p < 0.01 for comparison between groups at the same time point. [Color figure can be viewed at wileyonlinelibrary.com]
Negative Effects of Aged Myofibers on Osteogenic Differentiation of Osteoblasts Was Partly Reversed by LMHFV Treatment or Myostatin Propeptide
Muscle and bone are closely linked in the motor unit and therefore hard to exclude influence from one another in vivo. A CM culture approach was utilized to further investigate the direct effect of sarcopenic muscle on the bone with or without LMHFV intervention. Alkaline Phosphatase (ALP) staining showed that osteogenic differentiation of MC3T3-E1 cultured in CM of aged-SAMP8 myofibers (Aged-Ctrl) was poorer, as compared with CM of young myofibers (Young-Ctrl). In contrast, when cultured in the CM with LMHFV treatment, osteogenesis was rescued (Fig. 4A and B) as shown between Aged-Ctrl and Aged-VT groups. Surprisingly, ALP staining showed that osteogenesis was suppressed in Young-VT group compared with Young-Ctrl group. Runx2 messenger RNA (mRNA) and protein expression were also significantly lower (p = 0.0091, 0.0090, respectively) in Aged-Ctrl group compared with Young-Ctrl group. LMHFV significantly upregulated Runx2 mRNA and protein expression in Aged-VT group compared with Aged-Ctrl group (p = 0.0004, 0.0001 respectively) (Fig. 4C–E). Similar to the ALP results, LMHFV treatment downregulated the Runx2 mRNA expression in Young-VT group compared with Young-Ctrl group. As shown in the animal part, LMHFV suppressed the myostatin expression in muscle tissues (Fig. 3).22 The effect of myostatin propeptide, a myostatin inhibitor,34 on the osteogenic difference of MC3T3-E1 was further evaluated. Results showed that myostatin propeptide could enhance osteogenesis of MC3T3-E1 as shown by the ALP signals in Aged-PRO group compared with Aged-Ctrl group (Fig. 4A and B). Myostatin propeptide addition also upregulated Runx2 mRNA and protein expression (p = 0.0020, 0.0022, respectively) (Fig. 4C–E). For OCN mRNA expression, no significant difference among groups was observed (Fig. 4F).
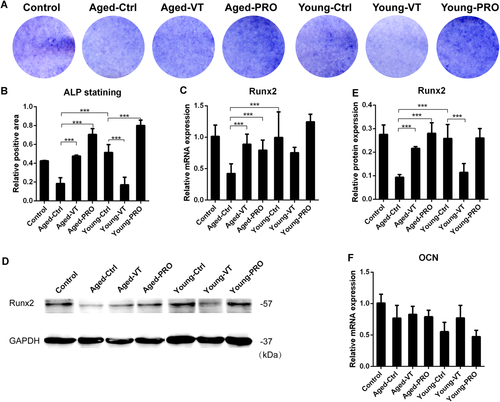
Negative effects of aged myofibers conditioned medium on osteogenic differentiation of osteoblasts was partly reversed by low-magnitude high-frequency vibration (LMHFV) or myostatin propeptide. (A) ALP staining of all groups. (B) Relative positive area of ALP staining. (C) Relative messenger RNA (mRNA) expression of Runx2 of all groups (n = 6 per group). (D) Western blot of Runx2 of all groups. (E) Relative expression of Runx2 protein of Western blot (n = 3 per group). (F) Relative mRNA expression of osteocalcin (OCN) of all groups (n = 6 per group). *0.01 ≤ p < 0.05, ***p < 0.01 for comparison between groups at the same time point. [Color figure can be viewed at wileyonlinelibrary.com]
Osteogenic Differentiation of Osteoblasts Was Enhanced by the CM of C2C12 Myoblasts With LMHFV Treatment or Myostatin Propeptide
To reconfirm the observed interaction between myofibers and osteoblastic MC3T3-E1, an adherent muscle cell line C2C12 was used. ALP staining showed that the osteogenic differentiation of MC3T3-E1 cultured with CM from the C2C12-VT group was slightly enhanced compared with C2C12-Ctrl group. Runx2 mRNA and protein expression levels were also significantly upregulated (p = 0.0463, 0.0002, respectively) in C2C12-VT group compared with C2C12-Ctrl group. Myostatin propeptide enhanced osteogenic differentiation in C2C12-PRO group (Fig. 5A and B) and upregulated Runx2 mRNA and protein expression (p = 0.0303, 0.0029, respectively) in C2C12-PRO compared with C2C12-Ctrl group (Fig. 5C–E). OCN mRNA expression was significantly upregulated (p = 0.0034) after myostatin propeptide addition when comparing between C2C12-Ctrl and C2C12-PRO groups (Fig. 5F).
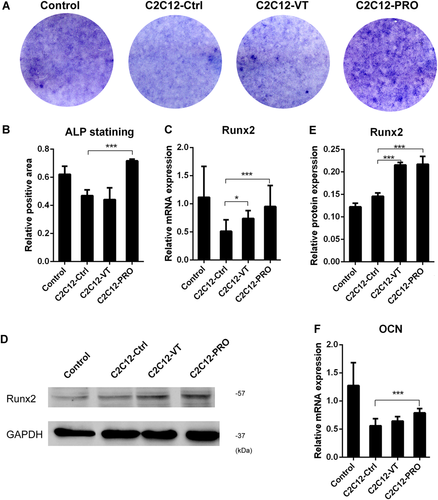
Osteogenic differentiation of osteoblasts was enhanced by the conditioned medium (CM) of C2C12 myoblasts treated with low-magnitude high-frequency vibration (LMHFV) or myostatin propeptide. (A) ALP staining of all groups. (B) Relative positive area of ALP staining. (C) Relative messenger RNA (mRNA) expression of Runx2 of all groups (n = 6 per group). (D) Western blot of Runx2 of all groups (E) Relative expression of Runx2 protein of western blot (n = 3 per group). (F) Relative mRNA expression of osteocalcin (OCN) of all groups (n = 6 per group). *0.01 ≤ p < 0.05, ***p < 0.01 for comparison between groups at the same time point. [Color figure can be viewed at wileyonlinelibrary.com]
DISCUSSION
This study aims to investigate the effect of LMHFV on osteoporotic fracture healing in the presence of sarcopenia and examine the potential role of myostatin. Our in vivo results confirmed that LMHFV could enhance both sarco-osteoporotic and osteoporotic fracture healing, while sarcopenia affected the efficacy of LMHFV on fracture healing. Also, myostatin was shown to play a potential key role between skeletal muscle and the formation of fracture callus in vivo, which was further substantiated by our in vitro results between myofibers and MC3T3-E1 osteoblast cell line.
LMHFV was shown to enhance the early stage of osteoporotic fracture healing in both sarcopenic SAMP8 and non-sarcopenic SAMR1 mice and the efficacy of LMHFV on callus formation might be affected by the presence of sarcopenia in SAMP8 mice, indicating the important role of skeletal muscle in the healing process. It was reported that sarcopenia onset causes deterioration of muscle and may decrease its ability to respond to mechanical stimulations.38 Also, as skeletal muscle is crucial for mechanotransduction and interaction with bones, loss of muscle stiffness would cause aberrant mechanotransduction.39 Hence, the affected efficacy of LMHFV in sarcopenic SAMP8 mice may be due to compromised muscle quality in the early stages of osteoporotic fracture healing. However, our data confirmed that LMHFV could enhance the mechanical properties of the healed bone, the gold standard of fracture healing, in SAMP8-V compared with SAMP8. At the late stage of osteoporotic fracture healing, the smaller callus in SAMP8-V compared with other groups indicated that the remodeling phase was accelerated by LMHFV. However, mechanical properties showed no difference among all groups at week 6 post-fracture. It is not surprising that the fractures in all groups would heal and show no difference of mechanical properties during remodeling phase, which was in accordance with a previous murine fracture study that demonstrated no difference in the later healing period with or without estrogen receptor deletion.40 With validation of the in vitro experiments, comparison between Aged-Ctrl and Aged-VT groups (Fig. 4) showed that LMHFV treatment on myofibers could enhance the osteogenic marker Runx2 and ALP expression in osteoblasts, which was also confirmed with the C2C12 cell line (Fig. 5, C2C12-Ctrl vs. C2C12-VT). These data suggested that LMHFV could affect osteogenesis through stimulating muscle fibers, leading to enhanced osteoporotic fracture healing as observed in the animal experiments. In addition, LMHFV significantly decreased the expression of myostatin in both cartilaginous callus and adjacent BF muscle (Fig. 3A–D). The enhancement of callus formation was not only caused by LMHFV directly on the fractured bone tissue, but also by the cascade reaction from the decreased expression of myostatin in the muscle induced by LMHFV during the early stage of osteoporotic fracture healing.
Skeletal muscle is known to be a large organ that can release myokines to promote fracture healing.12, 41 Myostatin is a well-known potent inhibitor of muscle growth19 and a key mediator of human sarcopenia.20 Although assessments of myostatin in human serum or plasma for sarcopenia is still controversial,42 it is still a highly potential biomarker43 or therapeutic target of sarcopenia. Targeting myostatin and its receptor showed a promising direction of effective treatments for cachexia and muscle wasting diseases.44 It was reported that myostatin was highly expressed in the round mature layer of chondrocytes and to a lesser extent, in the flat proliferating chondrocytes during the early stage of fracture healing.45 In this study, myostatin expression was found at much higher level in sarco-osteoporotic SAMP8 mice compared with SAMR1 group at early stage of fracture healing and was negatively associated with callus formation during osteoporotic fracture healing (Fig. 3). LMHFV could suppress the myostatin expression in both callus and skeletal muscle in vivo with enhanced fracture healing. This was also supported by in vitro results that LMHFV partly reversed the negative effects of sarcopenic myofibers on the osteoblastic differentiation. These data matched with our previous findings that LMHFV treatment could decrease myostatin level in the serum of SAMP8 mice.18 The role of myostatin was also substantiated by a study that gene expression (Sox-5, BMP-2), total osseous tissue area, callus size and callus strength were increased in myostatin-deficient fracture mice, as compared with wide-type mice.46 Meanwhile, Hamrick's group reported that blocking the myostatin pathway by myostatin propeptide could improve the fracture repair and enhance muscle regeneration in a fibula osteotomy mice model with deep penetrant musculoskeletal injury.21 In our study, a significant negative linear correlation between callus size and myostatin expression in the callus at week 2 post-fracture revealed that myostatin should play an inhibitory role in callus formation. Although the direct effect of the myostatin from skeletal muscle adjacent to the fracture site on callus formation is difficult to delineate in vivo, the significant positive linear correlation between myostatin expression in the bicep femoris and callus at week 2 post-fracture demonstrated its important role between skeletal muscle and bone. The higher expression of myostatin in sarcopenic SAMP8 mice might have decreased the maturation of chondrocytes and endochondral ossification of the callus at the early stage. Supported by other studies, myostatin was shown to have inhibitory effects on the osteogenic differentiation of MSCs,47 osteoblastic differentiation, and mineralization of osteoblasts48 involved in the fracture healing process. Further evidences are required to consolidate the role of myostatin in muscle-bone interaction. Blocking the myostatin pathway by myostatin propeptide in the CM from aged myofibers (Fig. 4, Aged-Ctrl vs. Aged-PRO) or myoblasts (Fig. 5, C2C12-Ctrl vs. C2C12-PRO) significantly enhanced osteogenic differentiation of the osteoblasts. In contrast, the difference of Runx2 expression became insignificant in the CM of young myofibers (Fig. 4, Young-Ctrl vs. Young-PRO). These results also confirmed that blocking myostatin in the presence of sarcopenia might be a potential therapeutic strategy to enhance the osteoporotic fracture healing.
Sarcopenia and osteoporosis are well known to be highly associated and they share many common risk factors and biological pathways,49 which estrogen depletion may play a key role in pathophysiology. This should be contributed by constant cross-talking of muscle and bone within a muscle-bone unit. Meanwhile, during osteoporotic fracture, there is a lack of evidence on how sarcopenia affects bone healing. Interestingly, our previous studies16, 50 demonstrated that osteoporotic fractured bone was very sensitive to mechanical signals in the form of vibration, as compared with age-matched non-osteoporotic bone, which is contradictory to general belief. Estrogen receptor (ER) is therefore proposed to play a critical role, as ER is also a signaling molecule in mechanotransduction. A few of ours and other recent studies successfully had validated the concept that ER-α is crucially involved in vibration-enhanced osteoporotic fracture healing26, 32, 51, 52 and the effect was influenced by estrogen level.53 However, these studies rarely involved the evaluation of skeletal muscles and thus, the future research direction should focus on the interplay among sarcopenia, osteoporotic fracture healing, estrogen levels and vibration treatment in order to formulate effective treatment for the musculoskeletal system as a whole.
Skeletal muscle and bone are closely connected and is impossible to separate them to investigate the influence of LMHFV treatment separately on muscle or bone in animal experiments. Our previous study has shown that osteoporotic fracture healing was delayed in sarcopenic SAMP8 compared with non-sarcopenic SAMR1.13 The in vitro results of this study further supported that the CM of aged muscle fibers downregulated Runx2 and ALP expression of the osteoblasts compared with young muscle fibers (Fig. 4, Aged-Ctrl vs. Young-Ctrl). These indicated that the osteogenic potential of osteoblasts was impaired in the presence of sarcopenia, which might explain the delayed osteoporotic fracture healing in vivo. On the other side, although in vitro study provided the mechanistic clue that LMHFV might downregulate the expression of myostatin in skeletal muscle to facilitate osteoporotic fracture healing, lack of myostatin knock-down approach was the limitation of this study, which should be conducted in the future to further validate their relationship.
In conclusion, delayed osteoporotic fracture healing was associated with the presence of sarcopenia in SAMP8 mice that might be attributed by the elevated expression of myostatin in the callus and skeletal muscle at the fracture site. LMHFV was effective in enhancing osteoporotic fracture healing through suppressing the myostatin expression at the fracture site and the adjacent muscles, while sarcopenia negatively affected the efficacy of LMHFV to certain extent. Inhibition of myostatin in myofibers to facilitate the osteogenesis in osteoblastic cells was a possible mechanism through which LMHFV enhanced sarco-osteoporotic fracture healing. Myostatin is a potential therapeutic target to treat osteoporotic fracture in the presence of sarcopenia.
AUTHORS’ CONTRIBUTION
W.H.C., S.K.C. conceived and designed the analysis. N.Z., Y.N.C., J.W. collected the data and performed analysis. N.Z. and R.M.Y.W. wrote the paper. All authors critically reviewed the manuscript.
ACKNOWLEDGMENTS
This work was supported by the OTC Research Grant (Ref. No. 2014-WHKS), General Research Fund (Ref: 14113018), Direct Grant for Research (2018.057) and Theme-based Research Scheme (Ref: T13-402/17-N).




