Peripheral Blood Stem Cell Therapy Does Not Improve Outcomes of Femoral Head Osteonecrosis With Cap-Shaped Separated Cartilage Defect
Jun Ying, Pinger Wang, and Quanwei Ding contributed equally to this work. Grant sponsor: Natural Science Foundation of China; Grant numbers: 81774332, 81774346, 81873324, and 81873325; Grant sponsor: Natural Science Foundation of Zhejiang Province; Grant numbers: LQ16H270007 and LY18H270004; Grant sponsor: Traditional Chinese Medical Administration of Zhejiang Province; Grant numbers: 2016ZA048, 2018ZA034, 2019ZQ018, and 2018ZZ011; Grant sponsor: Health Commission of Zhejiang Province; Grant number: 2019RC225; Grant sponsor: Opening Project of Zhejiang Provincial Preponderant and Characteristic Subject of Key University (Chinese Traditional Medicine; Grant sponsor: Zhejiang Chinese Medical University; Grant numbers: ZYX2018001 and ZYX2018004; Grant sponsor: Zhejiang Provincial Department of Education Research Project; Grant number: Y201839524; Grant sponsor: Cultivation Program for Innovative Talent Graduate Students; Grant number: 311100G00920.
ABSTRACT
A combination treatment with porous tantalum rod implantation and intra-arterial infusion of peripheral blood stem cells (PBSCs) provides a promise for treating early and intermediate stages of osteonecrosis of the femoral head (ONFH). However, its clinical indications and application restrictions remain unclear. This study aims to determine the clinical, histological, and radiological outcomes of a combination treatment using mechanical support and a targeted intra-arterial infusion of PBSCs for painful ONFH with a cap-shaped separation (CSS) cartilage defect. Compared with the standard pain management (control group), this combination treatment did not improve the Harris Hip Score (HHS) at 36 months. Micro-CT and histologic analyses showed severe focal destruction in all CSS-ONFH femoral heads in both the combination and control groups. Femoral heads showed a higher percentage of bone lesions in the combination treatment group than in the control group. There was no significant difference in osteoclast number in the subchondral bone areas between the two groups. A high level of expression of inflammatory cytokines, including tumor necrosis factor-α and interleukin-1β, was detected in blood vessels around the subchondral bone in both groups. The RANKL/OPG (receptor activator of the nuclear factor-kB ligand/osteoprotegerin) ratio was also similar between the control and combination treatment groups. Our results indicate that this combination treatment is not an effective method for the treatment of patients with painful CSS-ONFH. Moreover, this combination treatment did not inhibit inflammatory osteoclastogenesis in patients with more advanced disease. © 2019 Orthopaedic Research Society. Published by Wiley Periodicals, Inc. J Orthop Res 38:269-276, 2020
Osteonecrosis of the femoral head (ONFH) is a pathological process involving apoptosis of osteocytes leading to necrosis in the subchondral bone and bone marrow of the femoral head. The progression of ONFH frequently involves the collapse of the subchondral bone during remodeling with subsequent deformity of the femoral head, often leading to end-stage osteoarthritis and, eventually, to hip arthroplasty.1, 2 Risk factors for ONFH include trauma, long-term steroid treatment, and alcoholism.3-5 Recent studies demonstrated that ischemia, caused by interruption of the blood supply to the femoral head, is a primary cause of ONFH.6 In addition to osteocyte apoptosis, bone resorption, and adipocyte accumulation are also components of the progression of this disease.1, 7, 8
According to the Association Research Circulation Osseous (ARCO) society, joint preservation, including conservative and surgical treatment, is recommended in stages I and II (early stage; pre-subchondral fracture) ONFH.9 With the onset of more advanced stage III or IV (subchondral bone fracture and femoral head deformity), hip replacement surgery is considered in addition to conservative management.10 We previously determined that a combination treatment of porous tantalum rod implantation and intra-arterial infusion of peripheral blood stem cells (PBSCs) could delay and potentially reverse the progress of early and intermediate stages of ONFH.11 However, we have found that some patients with stage II disease showed evidence of cap-shaped separation (CSS) of cartilage on magnetic resonance image (MRI). These patients presented severe pain symptoms in the hips and failed to respond to the combination treatment. Eventually, some patients chose to have a joint replacement to relieve pain and restore joint functions.
Productions of inflammatory cytokines play an important role in the pathogenesis of ONFH. Interleukin-1 (IL-1) and tumor necrosis factor-α (TNF-α) stimulate osteoclasts and increase local bone remodeling. Plasma concentrations of interleukin-1 (IL-1) and TNF-α were significantly increased in the early stage of the glucocorticoid/endotoxin-induced ONFH model in rats.12 Receptor activator of nuclear factor-κB ligand (RANKL), a member of the TNF superfamily, binds to the receptor RANK and activates osteoclast maturation, leading to bone destruction. Osteoprotegerin (OPG), a decoy receptor for RANKL, which binds to RANKL and prevents its interaction with RANK, thus inhibits bone loss. A high RANKL/OPG ratio indicates bone resorption and is related to bone diseases. The RANKL/OPG ratio is increased in the serum of rats with steroid-induced ONFH.13 Xiong et al.14 found no change in the RANKL/OPG ratio between necrotic and normal tissues in advanced ONFH. However, regarding the unsatisfactory outcomes of combination treatment for CSS-ONFH, the histopathological and structural features of CSS-ONFH and its potential mechanism remain unclear (Table 1).
| Controls | Combination Treatment | p value | |
|---|---|---|---|
| Number (male:female) | 20 (8:12) | 17 (7:10) | N/A |
| Age | 37.3 ± 8.2 | 38.7 ± 8.5 | 0.612 |
| Number of hips | 20 | 17 | |
| Primary HHS | 60.9 ± 8.2 | 62.8 ± 7.6 | 0.469 |
| 9-month HHS | 66.5 ± 6.4 | 75.9 ± 5.9 | <0.001 |
| 18-month HHS | 67.5 ± 5.1 | 76.4 ± 3.3 | <0.001 |
| 36-month HHS | 67.6 ± 4.0 | 69.8 ± 4.3 | 0.342 |
In this study, we evaluated the clinical outcomes of a combination treatment of biomechanical support and transplantation of PBSCs for CSS-ONFH. To determine the mechanism of focal bone destruction in femoral heads with CSS-ONFH, we performed micro-computed tomography (micro-CT) and histologic analyses on samples characterized by CSS-ONFH.
METHODS
Patients
In our prospective study, 37 CSS-ONFH patients (15 males and 22 females, age range 31–64 years) with acute pain and mobility limitation in hips treated at the Department of Orthopedics, The First Affiliated Hospital of Zhejiang Chinese Medical University from January 24, 2012 to December 1, 2013 were recruited into the study. CSS-ONFH was diagnosed by radiographs and magnetic resonance images (MRIs) with two points: (i) microfracture and/or cystic degeneration existed between cartilage and subchondral bone with partial collapse; and (ii) high signal band shadow located separated sites between cartilage and subchondral bone in TW2 MRI images. Patients with a history of tumors, serious infections, hematological disease, metabolic bone diseases, or mental health disorders were excluded. The study was approved by the Ethics Committee of The First Affiliated Hospital of Zhejiang Chinese Medical University according to World Medical Association Declaration of Helsinki and registered in Chinese Clinical Trial Registry(ChiCTR) (registration number: ChiCTR1800016043). Written informed consent was obtained from each patient.
Treatment Groups and Interventions
All hips were divided into the control group (pharmacotherapy) and a combination treatment group according to patients' treatment preferences. Twenty patients (20 hips) preferred conservative treatment with pharmacotherapy. Patients in the control group were treated with acesodyne administration (celecoxib, 1# qd po). Seventeen patients who selected the combination treatment were treated with porous tantalum rod implantation to support the subchondral bone. Seven days later, PBSCs were mobilized in these patients by subcutaneous injections of granulocyte-colony stimulating factor (G-CSF) at a dosage of 10 μg/kg for 4 days and collected using the COBE spectra apheresis system (COBE BCT Inc., Lakewood, CO) with a collection protocol of low extracorporeal volume and high purity of mononuclear cells. Then, 30 ml of the CD34 + cell suspension with a minimum concentration of 8 × l06/l were infused through the medial circumflex femoral artery. The procedure was performed as previously described.11
Clinical Assessment
All patients underwent clinical and radiographic evaluations before treatments. The Harris Hip Score (HHS)15 was measured for all patients and data were compared between the control and combination treatment groups. Total hip arthroplasty (THA) surgery was defined as the endpoint for the evaluation of the efficacy of treatments. Data were collected before pharmacotherapy or porous tantalum rod implantation and at 9-, 18-, and 36-month time points after treatments started. All patients enrolled in the study completed the 36-month follow-up period or had THA performed.
Gross Anatomical Observations and µCT Scanning
The samples were coronally sectioned and gross anatomical structures of the cartilage and trabecular bone were photographed. To obtain high-quality images of the bone structure, the samples were scanned with a μCT scanner (SkyScan 1176; BRUKER μCT N.V, Antwerpen, Belgium) at a 35 lm isotropic resolution. Continuous slices (100) of subchondral bone (35 μm/slice, diameter = 10 mm), starting from the first circular section with a diameter of 10 mm below the subchondral region under the central region of the CSS, were selected as the region of interest (ROI). The parameters of bone volume/total volume (BV/TV), trabecular thickness (Tb.Th), trabecular number (Tb.N.), and bone mineral density (BMD) of the ROI were calculated; indicative of bone regeneration of the necrotic area.
Histochemistry, Immunohistochemistry (IHC), and Immunofluorescence analyses
After μCT scanning, the subchondral bone under the areas of CSS were cut and fixed in 4% paraformaldehyde. The samples were then dehydrated, embedded in paraffin, and sectioned at a thickness of 3 μm. Tissue sections were first deparaffinized and rehydrated, then separately stained with hematoxylin and alcoholic-eosin for 1 min. Digital images were randomly selected at a ×50 magnification. Three independent reviewers counted the numbers of osteocytes and empty lacunae and calculated the ratio of empty lacunae. IHC analysis was also performed to detect the expression of proteins in the tissues. After antigen retrieval using 10 mM Tris EDTA Buffer at 65°C for 1 h, endogenous peroxidase and 5% normal horse serum were incubated on slides to block non-specific binding sites. Then, primary antibodies specific to IL-1β (1:200; Abcam, UK), TNF-α (1:200; Abcam), RANKL (1:200; Abcam), OPG (1:200; Abcam), and CD31 (1:100; Arigo, Taiwan, China) were incubated on slides overnight at 4°C. After overnight incubation, sections were incubated with biotinylated second antibody and enzyme conjugate (Histostain-Plus Kit; Invitrogen, Camarillo, CA) for 10 min, respectively. Sections were then stained with DAB chromogen (Histostain-Plus Kit; Invitrogen) and nuclei were counterstained with Tacha's CAT Hematoxylin (Biocare Medical, Pacheco, CA). In sections that were incubated with rabbit anti-CD31 antibody, goat anti-rabbit immunoglobulin G (1:1,000, ab150077; Abcam) and antifade mountant with 4′,6-diamidino-2-phenylindole (DAPI) (P36931; Invitrogen) were placed on the slides for 30 and 5 min, respectively. Quantification of IHC staining was performed using ImageJ 1.46r Software (National Institutes of Health, Bethesda, MD). The ratio of RANKL/OPG could also be calculated from known OPG and RANKL expressions. Additional sections were stained for tartrate-resistant acid phosphatase (TRAP) to identify osteoclasts. The TRAP staining solution was pre-warmed to 37°C. Then, deparaffinized and rehydrated slides were placed in the warmed TRAP staining solution at 37°C for 30 min. Finally, the slides were counterstained with 0.08% fast-green solution. TRAP-positive multinucleated cells were defined as osteoclasts.16 Three independent reviewers counted the number of TRAP-positive multinucleated osteoclasts by using digital images at ×20 magnification.
Statistical Analyses
Preoperative and postoperative HHS were compared using a paired t test; the Wilcoxon test was used for other numerical variables. Gender and etiology of ONFH were compared between the two groups using the Fisher exact test. Differences were considered significant when p < 0.05. Statistical analyses were performed using the JMP statistical analysis software program (version 11; SAS Institute, Cary, NC).
RESULTS
Clinical and Radiological Results
Thirty-seven patients (22 women and 15 men, 37 hips) with CSS-ONFH participated in this study. These hips were assigned to the control group (pharmacotherapy) and the combination treatment group according to patients' treatment preferences. The mean HHS for the control group and the combination treatment group before treatment was 59.46 ± 8.80 and 62.96 ± 7.23, respectively; this was not significantly different (Fig. 1A; p = 0.202). During the follow-up analysis, the HHSs of the combination treatment group were significantly higher than those of the control group at 9 months and 18 months (p < 0.001). However, HHSs of the combination treatment group declined from the 18-month time point to the 36-month time point. Ultimately, there was no significant difference in HHSs between the two groups at 36 months (p = 0.342). Compared with pharmacotherapy, the combination treatment temporarily relieved pain and improved joint function in patients with CSS-ONFH. However, the long-term effects of pharmacotherapy and combination treatment were similar. With THA as the endpoint, the 36-month survival rate was 35% (95% confidence interval [CI] 15.65–55.19) and 35.3% (95% CI 14.48–57.04) in the control group and the combination treatment group, respectively. In this study, at 9-, 18-, and 36-month follow-up, four, five, and four hips, respectively, in the control group progressed to the subchondral fracture stage and had to eventually undergo THA. In the combination treatment group, 11 hips were found to be clinically failed requiring THA, including two, five, and four hips at 9, 18, and 36 months, respectively. The control group showed a survival probability similar to that of the combination treatment group (Fig. 1B; p = 0.935).
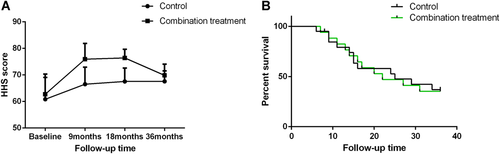
Follow-up results in each treatment group. (A) Harris Hip Score (HHS) in each treatment group in the follow-up period. (B) Kaplan–Meier survival curves of the femoral heads in each treatment group with conversion to total hip arthroplasty (THA) as the endpoint. [Color figure can be viewed at wileyonlinelibrary.com]
Gross Anatomical Changes and μCT Imaging Analysis
In femoral heads obtained from both control and combination treatment groups, articular cartilage separation and damage were obviously observed on radiographic images and general observations (Figs. 2 and 3A). μCT images revealed that most focal subchondral bone destruction and collapse were located at the bearing lateral two-third areas in the femoral heads. The mean ratio of lesion volume to total volume in the control group was higher than that in the combination treatment group (p = 0.032) (Fig. 3B). Quantitative analysis of ROIs revealed that histomorphometric parameters of BV/TV in the control group were significantly higher than those in the combination treatment group (p = 0.0297). However, measurements of Tb.Th, Tb.Sp, and Tb.N showed no significant differences between the control group and the combination treatment group (p > 0.05) (Fig. 3C).
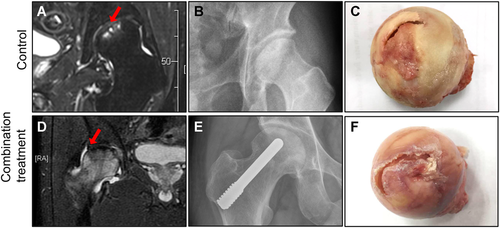
Representative images of a 59-year-old control group patient (A, B, C) and a 51-year-old combination treatment group patient (D, E, F) diagnosed with osteonecrosis of the femoral head (ONFH) characterized by a cap-shaped separation (CSS) cartilage defect by magnetic resonance imaging (MRI). (B and E). X-ray images were taken after 16 months (A) and 17 months (D). Intact femoral heads (C and F) were removed during total hip arthroplasty (THA) surgery. Red arrows: CSS sites on femoral heads. [Color figure can be viewed at wileyonlinelibrary.com]
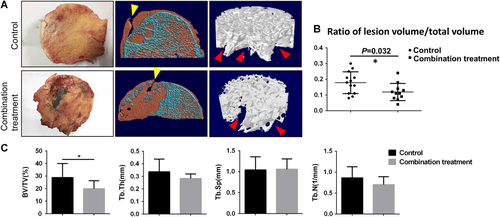
(A) Gross anatomy: coronal view μCT images and magnified regions of interest (ROIs) in representative images of femoral heads from each group. Yellow arrow: the cap-shaped separated sites of cartilage defect (CSS); Red arrow: areas of bone destruction. (B) The percentage of lesion volume to total volume of femoral heads from the control group and the combination treatment group. (C) Micro-computed tomography (μCT) analysis of ROIs in each treatment group. Data are presented as mean ± standard deviation (SD). *p < 0.05, n = 24. [Color figure can be viewed at wileyonlinelibrary.com]
Local Inflammation and Empty Lacunae in the Bone Trabeculae
Adjacent to areas of structural damage to the bone trabeculae, inflammatory cell infiltration was observed in both groups (Fig. 4A). Some osteocytes were pyknotic and empty lacunaes were observed (Fig. 4B). The ratio of empty lacunae in the bone trabeculae was slightly higher in the control group than in the combination treatment group but the difference was not significant (Fig. 4C).
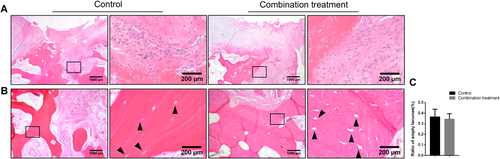
Histologic analysis of the subchondral bone in femoral heads from the control group and the combination treatment group. Hematoxylin and eosin (H&E) staining in subchondral bone areas was performed (A and B). Inflammatory cell infiltration around the bone trabeculae and empty lacunae (black arrows) exists on bone trabeculae. (C) The ratio of empty lacunae of bone trabeculae in the control group and the combination treatment group. Data are presented as mean ± standard deviation (SD). *p < 0.05, n = 24. [Color figure can be viewed at wileyonlinelibrary.com]
Vascular Characteristics Around the Bone Trabeculae
The expression of endothelial marker CD31 was analyzed by immunofluorescence to measure the blood vessels and blood supply in the necrotic area of both groups. Compared with the control group, the combination treatment group exhibited similar staining of CD31 (Fig. 5A). The inflammatory cytokines, TNF-α and IL-1β, were both highly expressed in the walls of blood vessels in subchondral bone sections obtained from both the control group and the combination treatment group (Fig. 5B). A semi-quantitative analysis of the staining intensity showed no difference in TNF-α and IL-1β expression between the two groups (Fig. 5C). These results indicate that abundant blood vessels, with high expression of inflammatory cytokines, are present in the subchondral bone region in both groups.
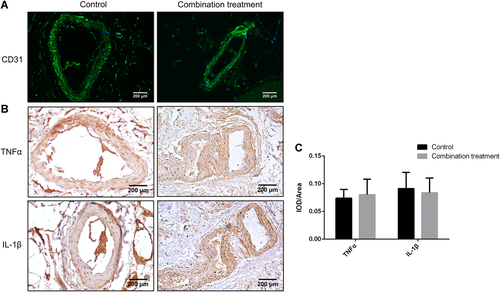
Tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-1β) were highly expressed on blood vessel walls around the subchondral bone areas in both the control group and the combination treatment group. (A) Immunofluorescence analysis of CD31 expression was performed for blood vessels around the trabeculae. (B) Immunochistochemistry (IHC) staining of TNF-α and IL-1β for blood vessels around the trabeculae. (C) Quantitative analysis of the IHC data showed no significant differences in the expression of TNF-α and IL-1β proteins analyzed. Data are presented as mean ± standard deviation (SD). *p < 0.05, n = 24. [Color figure can be viewed at wileyonlinelibrary.com]
Osteoclast Activity and Expression of Bone Resorption-Related Factors Around the Subchondral Bone
To determine differences in osteoclast formation, TRAP staining was performed and found numerous mature osteoclasts in the subchondral bone adjacent to the CSS region (Fig. 6A). However, no significant difference in osteoclast number was found between the control group and the combination treatment group (Fig. 6B) (p > 0.05), suggesting that neither treatment alters osteoclast formation during the development of CSS-ONFH.
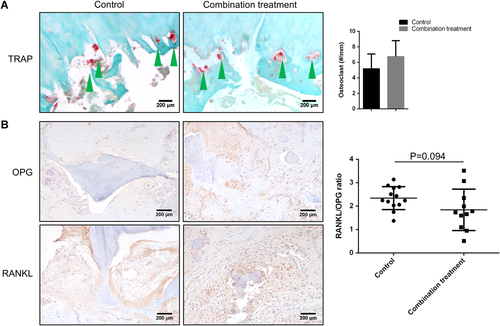
(A) Tartrate-resistant acid phosphatase (TRAP) staining in subchondral bone areas was performed. Mature osteoclasts (green arrows) eroding the subchondral bone in femoral heads from the control group and the combination treatment group were observed. There was no significant difference in osteoclast density in the subchondral bone between the control group and the combination treatment group (B). (C) Immunohistochemistry (IHC) of osteoprotegerin (OPG) and receptor activator of the nuclear factor-κB ligand (RANKL) (brown) adjacent to the subchondral bone. (D) The ratio of RANKL/OPG was similar in the control group and the combination treatment group. Data are presented as mean ± standard deviation (SD). *p < 0.05, n = 24. [Color figure can be viewed at wileyonlinelibrary.com]
To determine the underlying mechanisms of the development of focal bone resorption under the region of CSS-ONFH, IHC analysis of bone destruction-related chemokines and factors was carried out. IHC of RANKL and OPG showed similar expressions of RANKL and OPG in the cells in subchondral bone in the control and combination treatment groups (Fig. 6C). Similarly, there was no significant difference in the RANKL/OPG ratio between the control and combination treatment groups (Fig. 6D) (p > 0.05). These data indicated that combination treatment did not alter the expression of cytokines/growth factors during the process of bone destruction in patients with CSS-ONFH.
DISCUSSION
In a previous report, a combination treatment of porous tantalum rod implantation and intra-arterial infusion of PBSCs showed improved results in patients with early and intermediate stages of ONFH.11 However, our study on one specific ONFH disease type, CSS-ONFH, proved that a combination treatment did not effectively improve the survival probability compared with the results of acesodyne administration. Although the combination treatment did temporarily relieve pain and improve joint functions for CSS-ONFH compared with acesodyne administration, the long-term therapeutic effects were similar. To determine the outcomes of a combination treatment for CSS-ONFH, μCT, and histologic analyses were performed. The μCT analyses showed more severe bone destruction in patients receiving combination treatment compared with patients receiving pharmacotherapy. In addition, fibrous tissues around the damaged subchondral bone had similar expressions of OPG, RANKL, TNF-α, IL-1β, and CD31 in the control group and the combination treatment group.
Glucocorticoid (GC) use and alcoholism are associated with avascular necrosis (AVN) of the femoral head, 50% of which leads to collapse and joint destruction within 3 years of diagnosis.17 The common pathway for ONFH involves interruption of blood supply to the bone, resulting in osteocyte apoptosis and loss of structural integrity, ultimately leading to femoral head collapse.18-20 The transplantation of mesenchymal stromal cells (MSCs) and biomechanical support can often enhance the reparative reaction at the pre-collapse stages of ONFH in patients and animal models.21-25 CD34 has been regarded as a marker of stem cells in studies of PBSC transplantation.26-28 Considering the advantages of PBSCs (peripheral blood CD34+ cells) in terms of easy collection and stimulation of neovascularization and osteogenesis in damaged skeletal tissues, PBSC transplantation has proven to be a selective approach for the treatment of ONFH.29-32 In addition to altered osteoblast and osteoclast activities, inflammation is also involved in the pathogenesis of ONFH.26 In this study, an inflammatory cell infiltration is observed in the subchondral bone area near the necrotic lesion site in both control and combination treatment groups. μCT analyses showed severe bone destruction, in the CSS regions in femoral heads for both groups.
RANKL, a member of TNF superfamily, can mediate terminal differentiation of osteoclasts by cooperating with macrophage-CSF (M-CSF).27 TNF-α promotes RANKL-induced osteoclastogenesis,28 and leads to increased bone resorption. In this study, femoral heads in the combination treatment group showed more bone destruction volume compared with that in the control group. To determine changes in bone destruction, TRAP staining was performed and TRAP-positive osteoclasts were detected around the subchondral bone under the cartilage separation region. Bone homeostasis is maintained by bone resorption, carried out by osteoclasts, and bone formation by osteoblasts. In this study, the RANKL/OPG ratio in the control group was similar to that in the combination treatment group, indicating that combination treatment cannot effectively inhibit bone resorption. The absence of osteocytes within local regions of trabecular bone is a reliable indicator of osteonecrosis. In bone trabeculae of both groups, some osteocytes were pyknotic and empty lacunae were observed. However, there was no significant difference in the ratio of empty lacunae between the control group and the combination treatment group. This data suggests that a combination treatment of porous tantalum rod implantation and intra-arterial infusion of PBSCs did not contribute to the inhibition of bone resorption compared with the control group.
Pathological responses to ONFH include revascularization of the tissue and formation of an inflammatory granulation tissue that initiates a healing response. We detected abundant CD31, an endothelial marker that is mainly expressed by endothelial cells.29 The tissues around the subchondral bone trabeculae had similarly a high expression of CD31 in the control group and the combination treatment group. Previous studies have reported that TNF-α can cause osteoclast-induced bone destruction, as well as inhibition of osteoblast differentiation of MSCs through inhibition of Wnt signaling, the critical pathway stimulating bone formation.30-32 High levels of expressions of TNF-α and IL-1β were observed in the walls of blood vessels in both the control group and the combination group; this is consistent with an inflammatory granulation tissue and a high level of osteoclast bone resorption in our findings.
Although a combination treatment to promote angiogenesis and revascularization of subchondral bone is effective in the early stages of ONFH, factors including age, etiology, osteonecrotic location, and symptoms are also important indicators.33, 34 In this study, unsatisfactory outcomes of a combination treatment for CSS-ONFH in cases of more advanced stages of ONFH show the need for further investigation into the process of inflammation and osteoclast formation in CSS-ONFH.
In conclusion, we found that the focal bone destructions in CSS-ONFH were associated with abundant inflammatory osteoclastogenesis in the combination treatment group. We also found that bone destruction in the combination treatment group was more severe than that in the control group. These findings suggest that the combination treatment of porous tantalum rod implantation and intra-arterial infusion of PBSCs is not an ideal choice to treat CSS-ONFH patients with megalgia symptoms and inflammation-induced osteoclastogenesis.
AUTHORS' CONTRIBUTION
P.T. and H.J. designed the experiments; Q.D. collected patients' informations; J.Y. and P.W. carried out the experiments; J.S. analyzed the experimental results; R.J.O'K. analyzed the clinical results; and Regis J.O'K. and D.C. edited the manuscript. All authors have read and approved the final submitted manuscript.
ACKNOWLEDGMENTS
This study has been supported by Natural Science Foundation of China (Grants Nos. 81774332, 81774346, 81873324, and 81873325), Natural Science Foundation of Zhejiang Province (Grant Nos. LQ16H270007 and LY18H270004); Traditional Chinese Medical Administration of Zhejiang Province (Grant Nos. 2016ZA048, 2018ZA034, 2019ZQ018, and 2018ZZ011); Health Commission of Zhejiang Province (Grant Nos. 2019RC225); Opening Project of Zhejiang Provincial Preponderant and Characteristic Subject of Key University (Chinese Traditional Medicine); Zhejiang Chinese Medical University (Grant Nos. ZYX2018001 and ZYX2018004); Zhejiang Provincial Department of Education Research Project (Grant No. Y201839524); and Cultivation Program for Innovative Talent Graduate Students (Grant No. 311100G00920).




