Role of Inflammation and the Immune System in the Progression of Osteoarthritis
ABSTRACT
Understanding the molecular drivers and feedback loops of osteoarthritis (OA) may provide future therapeutic strategies to modulate the disease progression. The current paradigm of OA is evolving from a purely mechanical disease caused by cartilage wear toward a complex biological response connecting biomechanics, inflammation, and the immune system. The view of OA as a chronic wound highlights the role inflammation plays and also the body's attempts to repair an ongoing injury. Inflammatory signals, including cytokines such as interleukin-1 and tissue necrosis factor α, surface-expressed pattern recognition receptors such as toll-like receptors 2 and 4, complement factors such as C5, as well as pathogen-associated molecular patterns and damage-associated molecular patterns drive the enzymatic cascade that degrades cartilage matrix in OA. Considering the joint as an entire organ, interactions between the cells that reside in the synovium including macrophages and other immune cells, appear to drive enzymatic activity in cartilage, which, in turn, feeds signals back to the synovium that continues stimulating degradation in a feed-forward loop. This review will explore the potential roles of immune cells such as macrophages and T cells in the synovium in both stimulating and modulating the inflammatory response in OA. © 2019 Orthopedic Research Society. Published by Wiley Periodicals, Inc. J Orthop Res 38:253-257, 2020
According to the Centers for Disease Control and Prevention, more than 30 million patients in the United States have been diagnosed with osteoarthritis (OA). In the progression of this condition, patients can endure severe pain and experience loss of mobility. As a result, work productivity is diminished along with rising costs to the health care system. Currently available treatments prior to joint replacement include intra-articular injections such as corticosteroids or hyaluronic acids that ameliorate symptoms but have not yet been shown to modulate disease progression. The progression of OA as a multifactorial condition is driven by a set of components such as failing joint biomechanics,1 biochemical cascades, and cellular immunity responding to an inflammatory environment. Inflammatory cytokines play an important role in OA progression by stimulating matrix metalloproteinase (MMPs) production, which, in turn, increase matrix degradation.2 Current research now seeks to understand the drivers and the regulation of this inflammation. The body's natural wound healing response manifests itself in the osteoarthritic joint and leads to an increasing interest in how the immune response may influence disease progression.3
The acute phase of the wound healing cascade is marked by a short-lived influx of neutrophils followed by macrophage infiltration and neovascularization. The final step in this wound healing process includes remodeling of the damaged tissue. While the synovium can become vascularized, and increased vascularization is often a component of synovitis,4 cartilage is an avascular tissue. The question then arises, does the body attempt to deploy the wound healing cascade during OA and could the disease be driven by a partial or stalled repair process?
The immune system is categorized into innate and adaptive immunity, and both are linked in the body's defense against various threats such as microbes, injury, or malignancies. Classically, the body's first responders are derived from the innate immunity (or non-antigen-specific responses) and are marked by macrophages and neutrophils, followed by the adaptive immunity (antigen-specific or acquired response) that is comprised of T lymphocytes (T cells), B lymphocytes (B cells), and antibodies.5 The innate immune system is designed to defend and protect the organism when triggered by signals such as damage-associated molecular patterns (DAMPs),6 inflammatory cytokines, or complement factors. For example, while predominately studied in terms of defense against pathogens, innate signaling often depending on Toll-like receptor activation7 has been found in OA cartilage lesions and OA synovial fluid.8 Could identification of injury by the innate and/or adaptive immune system also be inadvertently tied to OA disease progression just as in normal wound injury processes?
Understanding the cells and signals in both wound healing/inflammation and the immune processes may provide an understanding on OA disease progression. Further, these processes could reveal potential therapeutic targets in order to slow the rate of disease progression or enhance the inherent repair mechanisms.
WOUND HEALING—THE ROLE OF THE MACROPHAGE
Inflammation is typically the response of vascularized tissue to injury. Changes in vascular flow and permeability allow an influx of neutrophils to emigrate out of the vasculature and follow a chemical gradient through the process of chemotaxis toward the site of injury. Neutrophils are only present a short time (24–48 h) and are responsible for phagocytosis of microorganisms and foreign bodies.5 Then monocytes migrate from the blood vessels and differentiate into macrophages. These macrophages affect the inflammatory microenvironment with the release of proteases, radical oxygen species, and inflammatory cytokines, among other factors.9 Macrophages also recruit fibroblasts and endothelial vascular cells via release of chemokines.5 New blood vessels and fibroblasts develop granulation tissue that remodels into the local tissue. With persistent stimulus, either from a foreign body, microorganism, or even tissue fragments, the macrophages will continue to elicit inflammatory signaling.5 The OA progression may, in fact, be driven by chronic inflammation in an attempt to repair the damaged tissue.
Macrophages and neutrophils exhibit complex phenotypes in chronic inflammation reflecting the diverging functions in inflammation and healing. Neutrophils have a predominate role in rheumatoid arthritis pathogenesis.10 Macrophage activity, however, has been identified in OA progression. Historically, during the inflammatory stage of wound healing, macrophages have been characterized as M1 macrophages,11 or classically activated macrophages, and are signaled by inflammatory mediators such as tissue necrosis factor-α (TNFα), interferon-γ (IFNγ), or pathogen-associated molecular patterns (PAMPs). Once activated, these macrophages themselves release pro-inflammatory cytokines (interleukin-1 [IL-1], IL-6, IL-12, TNFα) and other tissue-injury signaling. The purpose of the pro-inflammatory signaling is to queue the cleanup and removal of microbes and debris before remodeling can occur. Interestingly, macrophages are also responsible for the downregulation of inflammation and the initiation of tissue repair. In historic literature, the M2 macrophage phenotype was associated with the repair once the cleanup is complete. The M2 cells (or alternative activation pathway) activated by IL-4 and IL-13 release growth and angiogenic factors such as TGF-β, vascular endothelial growth factor (VEGF), and epidermal growth factor (EGF), and also regulate T-cell function, which promotes remodeling of the damaged tissue.12 In recent years, the view on macrophages has been evolving to view phenotypes as not a rigid state but as a plastic diversity between phenotypes, and it is becoming clear that a simplistic definition of M1 versus M2 may not accurately describe the complexities of the cells and their functions.11, 13, 14 Polarization of macrophages is dynamic and often never an “all or none” effect, making the process of understanding the role of polarization challenging as experiments often only provide a single snapshot in time.13
There is a suggestion that complex macrophage phenotypes are present, particularly in the synovium, and may influence OA progression. Synovial inflammation has been correlated to knee OA symptoms, particularly pain.15 Cellular infiltration in acute synovitis is marked predominantly by neutrophils while in chronic synovitis cellular infiltration is marked with macrophages and lymphocytes.4 Cells activated in the synovium continue to produce IL-1 and TNF-α, which, in turn, stimulate chondrocyte production of matrix-degrading enzymes including MMP and aggrecanases.16 There is evidence that inhibiting the activity of IL-1 and TNFα may stop the production of degrading enzymes and consequently slow cartilage destruction. For example, in a mouse model of post-traumatic arthritis (PTA), local administration of recombinant IL-1 receptor antagonist (IL-1ra), an antagonist to IL-1, reduced cartilage degradation and synovial inflammation.17 Similarly, in a rat meniscal tear model, cartilage degradation was reduced in animals treated with an intra-articular injection of an autologous anti-inflammatory product containing IL-1ra, sTNF-RI, and sTNF-RII compared with saline.18 Additionally, depletion of synovial macrophages in mice prior to creation of OA prevented the formation of MMP-induced neoepitopes; thus, linking macrophages to cartilage destruction.19 Likewise, the release of matrix breakdown products into the synovial fluid triggers feedback to cells in the synovium, stimulating further catabolic enzyme production2 This feed-forward loop continues to produce catabolic stimulus to the macrophages, and therefore they do not transition to a remodeling phenotype because they continue to receive the “cleanup” signal. By signaling protease digestion around the chondrocytes, the chondrocytes are released from the matrix and attempt to populate the injured site.20 However, it appears that in particular in the middle and deep zones of cartilage, this repair is too slow and the cell density is too low to overcome the ongoing degradation process.20 Therefore, the synovial macrophages never get an adequate signal to fully stop the inflammatory cleanup process and continue the cycle of producing matrix-degrading proteases. In addition to inhibiting endogenous repair, chondrocyte senescence increases with age and contributes to disease progression by preventing repair. Cellular senescence may be mediated by pro-inflammatory cytokines and proteases. Interestingly, the elimination of senescing chondrocytes in a mouse post-traumatic OA (PTOA) model improved symptoms and repair.21 It is clear that OA disease progression involves cross-talk between the cells, tissues, and synovial fluid present in the joint and that potentially by extracellular vesicles released by local and circulating cells aide in this communication.22
While there is evidence that anti-IL-1 and anti-TNF are successful treatments for rheumatoid arthritis, these therapies failed to meet their clinical trial endpoints in patients with OA. This anti-cytokine therapy may not have been successful due to the redundancy in activity between IL-1 and TNF-α.23 Cytokine approaches that include antagonists to both IL-1 and TNF-α have shown early promise in OA and are currently under further clinical investigation.24-26
Many disease processes result in incomplete or impaired polarization of macrophages to the healing phenotype.27 Polarization is directed by the local microenvironment and cytokine signaling. In an animal model of intervertebral disk lesions, immunofluorescence labeling of M1 and M2 macrophages indicated an increase in the proportion of M1 cells as well as an increase in TNF-α expression, suggesting that M1 polarization plays a critical role in early stages of remodeling.28 In the case of knee OA, a study analyzing M1 macrophages and M2 macrophages in synovial fluid in normal versus OA knees found a higher ratio of M1/M2 in OA versus normal knees and the ratio was significantly correlated to the Kellgren-Lawrence grade.29 Direct confirmation that activated macrophages are present in knee OA was collected by imaging of patients with knee OA with SPECT-CT and Etarfolatide label, which exclusively binds to activated macrophages.30 In this study, 76% of the knees measured positive for activated macrophages. The quantity of activated macrophages significantly correlated to knee pain (p < 0.0001) and radiographic knee OA severity (JSN p = 0.007; osteophytes p = 0.01). Joint fluid aspirations from two patients yielded enough cells for immunohistochemistry revealing macrophages that expressed markers for both M1 (iNOS) and M2 (IL-10) and TBF-β1),30 suggesting that the inflammatory and repair signaling are both being attempted. These results suggest that macrophage polarization may indeed play a role in the control and even progression of OA disease.
INNATE IMMUNITY
Tied closely to the inflammatory pathways and wound healing system is the ability of the body to identify foreign bodies and injured tissue. Many cells types overlap between the systems (Fig. 1), including macrophages, neutrophils, and natural killer cells of the innate system and lymphocytes in the adaptive immune system.5 Activation of the innate immune system occurs when certain molecular structures, called surface-expressed pattern recognition receptors (PRRs), bind to pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs).31 There are many classes of PRRs including Toll-like receptors (TLR), RIG-1-like receptors (RLR), and NOD-like receptors (NLR).31 These receptors can activate the transcription factor nuclear factor kappa B (NF-κB) and mitogen-activated protein kinase (MAPK), which, in turn, induces genes that encode for enzymes and cytokines that result in cartilage catabolism through IL-1β and TNF-α upregulation.31 Specifically, Toll-like receptors TLR 2 and 4 were found upregulated in OA lesions compared to OA cartilage not at the site of the lesion and in normal cartilage.32 Additionally, there is evidence that hyaluronan and fibronectin may act as endogenous damage signals to activate TLR 2 and 4 signaling in OA.7 Once activated, the cells and proteases of the innate immune system will carry out their duties indiscriminately without regard to whether the attack is directed toward a contaminant or toward self.5
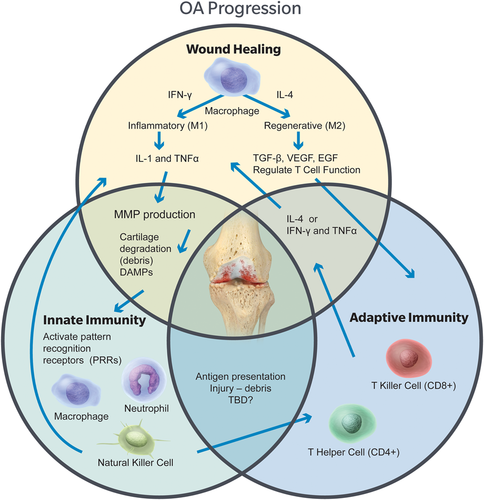
The interactions between the wound healing cascade, innate immunity, and adaptive immunity are linked and may lead to osteoarthritis progression. Each of these processes overlaps and is also dependent on the local environment including trauma, mechanical stress, and tissue damage. [Color figure can be viewed at wileyonlinelibrary.com]
Another important protein component for the innate immune system is the complement system (C1–C9 plus other cofactors).5 Activation of the complement cascade can enhance the immune system causing direct cell death by lysing cell membranes (C5b-9), enhancing neutrophil and macrophage phagocytosis (C3b fragment), increasing vascular permeability and vasodilation (C3a and C5a), increasing leukotriene synthesis (C5a), and by promoting chemotaxis of neutrophils and monocytes (C5a).5 Several complement components have been found in significantly increased abundance in OA synovial fluid compared to healthy synovial fluid.33 Chondrocytes from mice that are genetically deficient for C5 expressed less inflammatory molecules than wild-type-derived cells and furthermore formation of the membrane attack complex (MAC), a transmembrane channel formed from complement molecules onto target cells, is hindered in C5, C6, and CD59a-deficient mice.34 Formation of MAC was also identified in osteoarthritic cartilage and co-localized with MMP-13, further illustrating the important role complement plays in osteoarthritic progression.34 Complement is typically found as plasma proteins and could be in the synovial fluid through filtration from the blood; however, synovial tissues cells can also produce complement.33
The role of the innate immune system is to non-specifically identify invaders, whether as a microbes or tissue fragments, and then activate in order to stimulate clearance of the unwanted material. In the case of OA, continued degradation will expose PRRs in cartilage OA, which may continue to activate the innate immune system.
ADAPTIVE IMMUNE SYSTEM
Following identification of microbes or tissue damage from the non-specific innate immune system, the specific adaptive immune system takes over. The adaptive system includes antibodies, which are proteins that specifically bind antigens; B lymphocytes (B cells), which produces antibodies; and T lymphocytes (T cells), which coordinate the elimination of pathogens. T cells can be broadly categorized into helper T cells (Th cells) and cytotoxic T cells (Tc cells), also known as T killer cells. The Th cells (CD4+), divided generally into Th1, Th2, and Th17 cells, secrete cytokines to stimulate proliferation and differentiation of cells involved in the immunologic response. Tc cells (CD8+) are effector cells that eliminate the targeted cells.5 While the adaptive immune system appears to be designed for the specific identification and removal of intracellular and extracellular microbes, the regulation of the adaptive immune system through the T helper cells has potential to also influence OA progression.
Interestingly, the Th2 cells can influence macrophage polarization by the cytokines secreted. Production of IL-4 directs macrophages to the regenerative M2 phenotype while IFN-γ directs toward the inflammatory M1 phenotype, depending on the dose and timing of the cytokines.13 While predominantly studied in terms of microbe elimination, T helper cells, through cytokine release, guide macrophage to pro-regenerative phenotypes in response to tissue-derived biomaterials in an IL-4-dependent manner,35 providing evidence that the T helper cell has a larger biologic role in controlling inflammation and repair.
The role of the T cells specifically in OA disease progression is an emerging area of investigation when evaluating the role of immune and inflammation cells. There is evidence that T cells and B cells are present in higher numbers in OA organs than in healthy controls.15 A mouse model of PTOA illustrated synovial inflammation infiltrated with macrophages and CD4+ and CD8+ T cell infiltration.36 In OA patients, T helper cells are significantly higher in the synovial fluid and synovial tissue compared to age-matched healthy controls. Specifically, OA synovial fluid had increased the levels of Th1, Th9, and Th17 cells while the synovial tissue had increased Th1, Th17, and cytotoxic T cells. Catabolic cytokines produced from these cells types include IL-2, IFN-γ, and TNF-α.36 While the T cells are clearly present and produce catabolic cytokines that stimulate protease destruction of cartilage matrix, future work may further clarify the role these cells play in OA progression.
CONCLUSION
Understanding OA has evolved beyond its consideration as the result of simple mechanical wear and tear on the joint. First, data suggested how the inflammatory cause cartilage degradation through stimulation of proteases. Now, evaluation is of the cells that control the inflammatory processes and the immune response and how they are responsible for the production and regulation of pro-inflammatory cytokines. Fully understanding the mechanisms of these cellular processes may lead to therapeutic targets to modulate OA progression.
AUTHORS’ CONTRIBUTION
J.E.W.-M.: researched review topic, drafted manuscript, approved the final submission. S.D.S.: critically revised manuscript, approved the final submission.
ACKNOWLEDGMENTS
This review is written a summary of a workshop entitled “The Role of Macrophages and Immune Cells in the Progression of Osteoarthritis” during the Orthopedic Research Society (ORS) Meeting held in Austin, TX on Feb 2–5, 2019. This workshop was supported by the Women's Leadership Forum of the ORS. This review was inspired by the following speakers of the workshop:
Jennifer Elisseeff, PhD, Johns Hopkins University, “The Role of the Adaptive Immune System in Regeneration”.
Steven Olson, MD, Duke University, “Cells and Cytokines Driving Arthritis Progression Following Injury”.
Christopher Little, BVMS, PhD, The University of Sydney Northern Clinical School, “Local versus systemic cellular response in injury and post-traumatic OA”.
Jennifer Woodell-May is a paid employee of Zimmer Biomet.




