CCAAT/enhancer binding protein β regulates the expression of tumor necrosis factor-α in the nucleus pulposus cells
ABSTRACT
Tumor necrosis factor alpha (TNF-α) is important in the process of intervertebral disc (IVD) degeneration because of its ability to regulate other inflammatory mediators in autocrine and paracrine fashions. The mechanism responsible for the cell type-specific regulation of TNF-α is not well known. CCAAT/enhancer binding protein β (C/EBP β) is one of the transcriptional factors that is implicated in TNF-α expression. However, it is not known whether cross talk occurs between C/EBP β and the TNF-α pathway in IVD cells. The expression and effect of the C/EBP β mRNA and protein in rat IVD cells was assessed using real-time reverse transcription polymerase chain reaction, immunohistochemical, and immunofluorescence analyses. We present data that show that the C/EBP β mRNA and protein were expressed in rat and human IVDs in vivo. We also found that the expression of TNF-α is regulated by the transcription factor C/EBP β in rat NP cells. The TNF-α promoter was suppressed completely in the presence of the ERK inhibitor PD98059 and the p38 mitogen-activated protein kinase (MAPK) inhibitor SB202190, but not in the presence of the JNK inhibitor SP600125. In addition, gain and loss of function analyses showed that the expression of TNF-α was regulated by C/EBP β through the MAPK pathways. These findings showed that C/EBP β acts as a potent pro-inflammatory mediator by inducing the TNF-α gene at the transcription and protein levels via the ERK1/2 and p38 pathways in rat NP cells. Our findings may open a new avenue toward the understanding of the cellular and molecular mechanisms of IVD cells. © 2015 Orthopaedic Research Society. Published by Wiley Periodicals, Inc. J Orthop Res 34:865–875, 2016.
Abbreviations
-
- TNF-α
-
- tumor necrosis factor alpha
-
- C/EBP
-
- CCAAT/enhancer binding
-
- IVD
-
- Intervertebral disc
-
- NP
-
- nucleus pulposs
-
- AF
-
- annulus fibrosus
-
- LAP
-
- liver-enriched activator protein
-
- LIP
-
- liver-enriched inhibitory protein
The intervertebral disc (IVD) is a unique tissue and complex anatomical structure that consists of three distinct tissues, namely the nucleus pulposus (NP), which occupies its inner portion, the annulus fibrosus (AF), which surrounds the NP, and the cartilaginous endplates, which cover this assembly on both sides (top and bottom). Low back pain (LBP) is often attributed to IVD degeneration, which is synonymously termed degenerative disc disease. The factors that are commonly associated with IVD degeneration include upregulated inflammatory cytokines, loss of resident cell populations, altered biomechanics, uneven loading of the spine, and excessive mechanical stretch of the tissues and cells. Based on this background, IVD degeneration is a complex process, and the underlying mechanical and molecular mechanisms remain poorly understood.
Various messengers that act from within the cell to initiate a variety of signaling evens have been identified.1-3 Several research groups have also suggested that Wnt signaling plays an important role in IVD degeneration.4-9 Furthermore, we have reported the relationship between Tumor Necrosis Factor-α (TNF-α) and Wnt signaling in IVD cells.10 To date, elevated levels of proinflammatory cytokines and other inflammatory mediators have been reported to be present in degenerate IVDs, including TNF-α, IL-1β, IL-6, and prostaglandin E2. Furthermore, patients with chronic LBP caused by disc herniation present higher levels of TNF-α.11, 12 However, the manner in which cross talk between these factors and molecular signaling related to IVD degeneration is achieved remains unclear, and little is known about how TNF-α is regulated in a cell-type-specific fashion.
CCAAT/enhancer-binding proteins (C/EBPs) are a family of basic leucine zipper transcription factors that includes six members as follows: C/EBP α, β, δ, ϵ, γ, and ζ. Among them, C/EBP β was first identified as a nuclear protein that binds to the IL-1β response element in the IL-6 promoter region,13 and has subsequently been reported to regulate various genes involved in cell differentiation, proliferation, survival, immune function, tumor invasiveness, and progression.14-16C/EBP β is also called NF-IL6, NF-M, LAP, IL6-DBP, AGP/EBP, and CRP2. C/EBP β has three major isoforms: 38 kDa (liver-enriched activator protein Star [LAP*]), 36 kDa (LAP) and 20 kDa (liver-enriched inhibitory protein [LIP]) isoforms.17 Previously, it was reported that C/EBP β induced by proinflammatory cytokines such as IL-1β and TNF-α binds directly to the MMP13 and MMP3 promoter regions and stimulates their expression in chondrocytes, resulting in the degradation of cartilage in arthritis.18, 19 Furthermore, some groups reported that the expression of C/EBP β may be important in chronic inflammation associated with a variety of disorders,20, 21 and it was suggested that C/EBP β plays an important role in promoting hypertrophic differentiation of chondrocytes. C/EBP β is also involved in the hypertrophic changes of articular chondrocytes in OA.22 Zhang et al. suggested that C/EBP β and NF-κ β are both involved in the IL-1 β-responsive upregulation of chemokine genes in human chondrocytes.23 Although these studies have shown that C/EBP β stimulates proinflammatory cytokines and chemokines in chondrocytes, it is not fully understood whether C/EBP β influences the expression of such cytokines and the molecular signaling that are characteristic of IVD cells. In the IVD research field, only Wang et al. have reported that in NP cells, positively controls the expression of the TNF-α-dependent chemokine CCL3, and that C/EBP β acts as a negative regulator of cytokine-dependent ADAMTS-4 transcription.24
Although both C/EBP β and TNF-α have been reported to stimulate the degeneration of chondrocytes, their interaction and role in IVD cells remain unknown. Here, we investigated the relationship between C/EBP β activity and TNF-α expression. Our results demonstrate that C/EBP β is an important regulator of TNF-α expression.
MATERIALS AND METHODS
Animal experiments were performed according to a protocol approved by the Animal Experimentation Committee of the University of Tokai (Permit No. 131012 and 142055), Tokyo, Japan.
Reagents and Plasmids
K3-luc (TNF-α promoter element; #11110), pcDNA 3.1 (−) rat C/EBP beta (LAP) (#12558), pcDNA 3.1 (−) rat C/EBP beta (LIP) (#12562), pcDNA3-T7- extracellular signal-regulated protein kinases (ERKs) 1 (#14440), and pcDNA3-HA-ERK2 WT (#8974) were purchased from the Addgene repository (Cambridge, MA). FLAG-tagged wild type (WT) and dominant-negative forms (AF) of human p38α, p38β, p38γ, and p38δ were provided by Dr. Jiahuai Han (Xiamen University, China).25 We used the vector pGL4.74 (Promega, Madison, WI) containing the Renilla reniformis luciferase gene as an internal transfection control. The ERK inhibitor (PD98059, #9900) and p38/mitogen-activated protein kinase (MAPK) inhibitor (SB202190, #8158) were obtained from Cell Signaling Technology (Danvers, MA). The c-Jun N-terminal kinase (JNK) inhibitor (SP600125, #S5567) was obtained from Sigma–Aldrich (St. Louis, MO).
Human NP Tissue Specimens
We obtained informed consent from the patients for the use of their IVD tissues. The participants’ written consent was obtained according to the Declaration of Helsinki. Ethical approval was obtained from the Institutional Ethics Review Board of the Tokai University School of Medicine. Seven patients undergoing surgery for lumbar disc herniation were enrolled in this study. Written informed consent was obtained from the next-of-kin, caretakers, or guardians on behalf of the minor/child participants involved in the study. The patients’ ages ranged from 16 to 66 years (mean age, 41.5 years). We harvested the NP tissue from the disc attached to the intact bottom end plate.
Cell Isolation and Culture
Human NP tissues were digested with TrypLE Express (Gibco, Carlsbad, CA) and then collagenase-P (Roche Diagnostics, Mannheim, Germany), washed, and cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS) (Gibco BRL, Grand Island, NY). Rat NP cells and AF cells were isolated from multiple levels of the lumbar discs of 11-week-old Sprague Dawley rats (n = 32). Primary rat NP and AF cells were isolated as described.9 The NP and AF tissues obtained from the same animal were pooled together. The isolated cells were maintained in DMEM supplemented with 10% FBS and antibiotics, at 37°C in a humidified atmosphere of 5% CO2. Confluent NP and AF cells were harvested and subcultured in 10-cm dishes. Low-passage (<3) cells cultured in monolayers were used for all experiments, because cells obtained from the rat IVD tissues exhibited variable morphology until passage 2 or 3.
Immunofluorescence Staining
Rat NP cells were plated in flat-bottom 96-well plates (3 × 103 cells/well) and incubated for 24 h. Cells were cotransfected with LAP plasmids or the backbone vector. Lipofectamine 2000 (Invitrogen, Carlsbad, CA) was used as the transfection reagent. Subsequently, rat NP cells were fixed with 4% paraformaldehyde, permeabilized with 0.5% Triton X-100 (vol/vol) in phosphate-buffered saline (PBS) for 8 ms, blocked with PBS containing 10% FBS, and incubated overnight at 4°C with antibodies against TNF-α (1:100, Cell Signaling Technology; #3707). The cells were washed and incubated with an anti-rabbit Alexa Fluor 488 (green) antibody (Invitrogen) at 1:200 and with 10 μM 4′, 6-diamidino-2-phenylindole (DAPI) for 1 h at room temperature, for nuclear staining. The samples were observed under a fluorescence microscope interfaced with a digital imaging system. Cells treated with normal IgG (Cell Signaling Technology) at equal protein concentrations were used as negative controls.
Immunohistological Studies
To gain insight into the expression of C/EBP β in the IVD, freshly isolated spines from 3- (young) and 11 (mature) -week-old rats were fixed in 4% paraformaldehyde in PBS, decalcified, and then embedded in paraffin wax. Sagittal sections were deparaffinized in xylene, rehydrated through a graded ethanol series, and stained with hematoxylin. Sections were incubated with antibodies to C/EBP β (Santa Cruz Biotechnology) in 2% bovine serum albumin in PBS at 1:100 overnight at 4°C. Nonimmune IgG was used as a negative control (Cell Signaling Technology) and rat kidney was used as positive control. The sections were washed thoroughly and incubated with a biotinylated universal secondary antibody (Vector Laboratories Canada, Burlington, Ontario, Canada) at 1:20 for 10 min at room temperature. Sections were incubated with a streptavidin—peroxidase complex for 5 min and washed with PBS, and color was developed using 3, 3′-diaminobenzidine (Vectastain Universal Quick Kit; Vector Laboratories) and examined under a fluorescence microscope. The number of positively immunolabeled cells and the total number of cells per high-power field in each section were determined, and the percentage of positively labeled cells was calculated.
Real-Time Reverse Transcription-Polymerase Chain Reaction (RT-PCR) Analysis
After treatment, total RNA was extracted from the cells using the TRIzol RNA isolation protocol (Invitrogen). RNA was treated with RNase-free DNAse I. Total RNA (100 ng) was used as the template for the RT-PCR analyses. The cDNA was synthesized via the reverse transcription of mRNA, as described previously.9 Reactions were arranged in triplicate in 96-well plates using 1 μl of cDNA with SYBR Green PCR Master Mix (Applied Biosystems), to which gene-specific forward and reverse PCR primers for TNF-α were added. The primers were synthesized by Takara Bio Inc. (Tokyo, Japan) or FASMAC Corp. (Tokyo, Japan) and are shown in Table 1 (rat- C/EBP α, β, δ, ϵ, γ, and ζ and TNF-α) and Table 2 (human- C/EBP α, β, δ, ϵ, γ, and ζ). PCR reactions were carried out in an Applied Biosystems 7,500 Fast system, according to the manufacturer's instructions. The expression scores were obtained using the ΔΔCt calculation method. The relative quantification of gene expression in treatment groups vs. controls (cells isolated freshly before culture) was performed using the comparative threshold cycle method, 2^[(CtGAPDH— CtGene)treatment—(CtGAPDH—CtGene)control], where glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as the housekeeping control gene.
| Target | NCBI Number | Forward Primer, 5′-3′ | Reverse Primer, 5′-3′ |
|---|---|---|---|
| C/EBPα | NM_012524.2 | ATAAAGCCAAACAGCGCAAC | CGGTCATTGTCACTGGTCAA |
| C/EBPβ | NM_024125.4 | ACCGGGTTTCGGGACTTGA | CCCGCAGGAACATCTTTAAGTGA |
| C/EBPδ | NM_013154.2 | CATCAGCGTGTGGAGCAG | GCTGGGCAGCTCTTTGAA |
| C/EBPϵ | NM_017095.1 | GACCTCTCCGCCTACATCG | TCATGGCAAAGAGGTCAGAA |
| C/EBPγ | NM_012831.1 | GCCCAAATGAGCAAGCTG | AATGACACTGATCCCGTTCAC |
| C/EBPζ | NM_001108701.1 | CGATGAGGCCAAGAATGG | GCCAGCATAAGGTAATCTTGCT |
| TNF-α | NM_012675.3 | TGAACTTCGGGGTGATCG | GGGCTTGTCACTCGAGTTTT |
| Target | NCBI Number | Forward Primer, 5′-3′ | Reverse Primer, 5′-3′ | |
|---|---|---|---|---|
| C/EBPα | NM_004364.3 | GACATCAGCGCCTACATCG | GGCTGTGCTGGAACAGGT | |
| C/EBPβ | NM_005194.2 | CGCTTACCTCGGCTACCA | ACGAGGAGGACGTGGAGAG | |
| C/EBPδ | NM_005195.3 | GGACATAGGAGCGCAAAGAA | GCTTCTCTCGCAGTTTAGTGG | |
| C/EBPϵ | NM_001805.2 | CCTCTGCGCGTTCTCAAG | GCCGGTACTCAAGGCTATCTT | |
| C/EBPγ | NM_001806.2 | GGAGAGGAAATTGTAGCAGCTTT | AGGTCTCAAGCCCTCTAATGG | |
| C/EBPζ | NM_005760.2 | CATGGCTAACAAAGATAATGCAA | GTTGTGTAGCCAGTCATCACG | |
Western Blot Analysis
Treated rat NP cells were immediately placed on ice and washed with cold PBS. To prepare the total cellular proteins, the cells were lysed with lysis buffer containing 10 mM Tris–HCl (pH7.6), 50 mM NaCl, 5 mM EDTA, 1% Nonidet P-40, complete protease inhibitor cocktail (Roche, IN), 1 mM NaF, and 1 mM Na3VO4. Heat-denatured samples were separated on sodium dodecyl sulfate polyacrylamide gels and electrotransferred onto Immobilon-P polyvinylidene difluoride membranes (Millipore, MA). The membranes were then blocked with blocking buffer (5% BSA and 0.1% NaN3 in PBS), and subsequently incubated overnight at 4°C with the anti- TNF-α (1:1,000, Cell Signaling Technology) and anti-C/EBP β (1:200, Santa Cruz Biotechnology) antibodies diluted in Can Get Signal Immunoreaction Enhancer Solution (Toyobo, Tokyo, Japan). Chemiluminescent signals were visualized with an Immobilon Western Chemiluminescent HRP Substrate (Millipore, MA), and scanned using an Ez-Capture MG imaging system (ATTO, Tokyo, Japan). The western blot data were quantified using Image J pixel analysis (NIH Image software). Western blot data are presented as band intensities normalized to that of the loading control (β-actin). To measure the band intensity, the data shown are representative of at least three independent experiments. The previous study demonstrated that TNF-α protein levels as detected in the supernatants of phorbol 12-myristate 13-acetate (PMA) -stimulated human promyelocytic leukemia cells (HL-60 cells).26 Thus we used as positive controls.
Sequence Analyses
To identify transcriptional regulatory sequences and potential transcription factor binding sites on the TNF-α promoter region, the sequences of human, mouse, and rat were obtained from GenBank. We used the program Alibaba 2.1 provided by TESS (Transcription Element Search System) (http://labmom.com/link/alibaba_2_1_tf_binding_prediction) and an online server TFSEARCH [Searching Transcription Factor Binding Sites (ver. 1.3)]).
Transient Transfection
Transfections were performed using Lipofectamine 2000 (Invitrogen) according to the manufacturer's recommendations. For the siRNA transfection experiment, rat NP cells grown on a 12-well plate were cotransfected with a control or LAP expression vector together with either of the following small interfering (si) RNAs (Signal Silence, Cell Signaling); control (#6568) or p42/44 MAPK(ERK1/2) (#6560) (100–200 nM) using Lipofectamine 2000. Six hours after transfection, the medium was replaced with complete growth medium, and the cells were allowed to recover for 18 h. Cells were then cultured for 24 h, and luciferase activity was measured.
Transfections and Dual-Luciferase™ Assay
Rat NP cells were transferred to 24-well plates at 3 × 104 cells/well 1 day before transfection. Cells were cotransfected with 100–500 ng of expression plasmids or the backbone vector together with the reporter plasmids. Lipofectamine 2000 (Invitrogen) was used as the transfection reagent. Reporter activity was measured 48 h after transfection using the Dual-Luciferase™ reporter assay system (Promega) for the sequential measurements of firefly and Renilla luciferase activities. The results were normalized regarding transfection efficiency and are expressed as the ratio of luciferase to pGL4.74 activity (denoted as “relative activity”). NP cells were transfected with a plasmid encoding green fluorescent protein to check the transfection efficiency, which was 60–70% in NP cells. Luciferase activities and relative ratios were quantified using a Turner Designs Luminometer Model TD-20/20 instrument (Promega).
Statistical Analysis
All experiments were used for same isolated cells. Moreover, all experiments were performed in triplicates and each experiment was done at least twice independently, and data are presented as the mean ± SD. Student's t-test or one-way ANOVA was used to investigate significant differences in expression levels between the study groups. While the t-test is used to compare the means between two groups, one-way ANOVA is used to compare means between three or more groups. Values of p < 0.05 were considered significant.
RESULTS
Expression of C/EBP Subtypes in IVD Cells
The expression of C/EBP family members in mature rat cells was studied using real-time PCR. We measured the mRNA expression of the C/EBP subtypes C/EBP α, β, δ, ϵ, γ, and ζ in rat NP and AF cells (Fig. 1A). C/EBP α, β, and δ mRNA expression was significantly higher in rat AF cells than it was in rat NP cells. Moreover, the expression of the C/EBP β mRNA was significantly higher than that of the mRNA of other C/EBP family members, both in rat NP and AF cells.
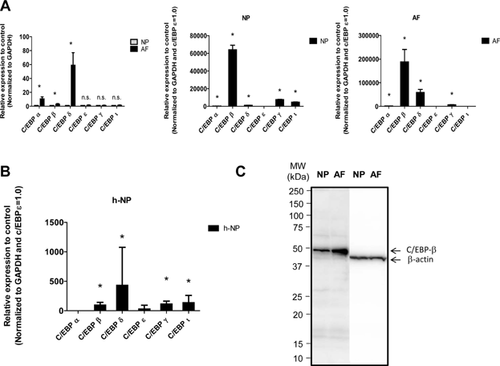
Next, we measured the mRNA levels of the members of the C/EBP family in the human samples. Real-time PCR showed a higher level of the C/EBP δ mRNA compared with the C/EBP β mRNA in the human samples. Different expression patterns of the C/EBP δ and C/EBP β mRNAs were observed between rat and human NP cells (Fig. 1B). In addition, expression of C/EBP β in IVD cultured cells was studied using western blot analysis. Figure 1C indicates that both NP and AF cells expressed a prominent 45 kDa C/EBP β band. Moreover, the expression level of C/EBP β in AF cells was higher than that observed in NP cells.
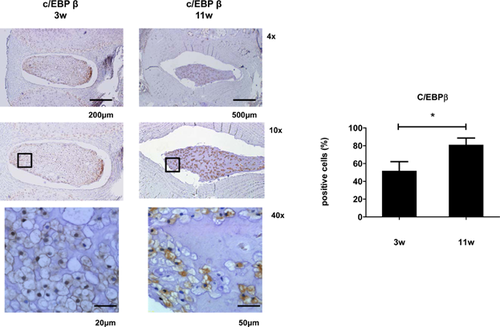
To determine whether altered C/EBP β expression contributes to the age-related increase in rat NP cells, we measured C/EBP β expression levels in IVDs from sagittal sections of skeletally young (3 weeks) and mature (11 weeks) rat discs. The percentage of cells that were immunopositive for C/EBP β in the rat NP increased significantly from 3 to 11 weeks. The percentage of cells in the rat NP that were immunopositive for C/EBP β also increased significantly with age. C/EBP β was robustly expressed in cells of the NP and weakly expressed in cells of the AF and cartilaginous end plate in mature rat discs. In all cases, staining was localized to the cytosol to a greater extent compared with the nucleus (Fig. 2).
C/EBP β Induced TNF-α Expression in NP Cells
To explore the premise that C/EBP β associated with IVD degeneration regulated TNF-α expression, we first assessed whether TNF-α expression is modulated by C/EBP β in rat NP cells. We investigated the regulation of TNF-α expression to determine whether a relation exists between TNF-α and the C/EBP β of NP cells by analyzing the 0.83 kb promoter sequence of rat TNF-α. Sequence analysis shows that the TNF-α promoter contains two C/EBPβ-binding motifs (198-CCAACAACTG-207, 613-AACAGTTGCG-622) (data not shown). We cotransfected NP cells with a plasmid expressing C/EBP β (LAP) and mutated plasmid (LIP), the results showed an increase in the activity of the TNF-α promoter 24 h after LAP expression vector treatment, whereas TNF-α promoter activity was not affected by cotransfection with LIP expression vector (Fig. 3A). These results suggest that C/EBP β regulates the expression of TNF-α at the transcriptional level. To confirm the reporter assay data, we cotransfected rat NP cells with a plasmid expressing LAP and performed a real-time PCR analysis of TNF-α mRNA expression. This analysis demonstrated that cotransfection with LAP expression vector increased the expression of the TNF-α mRNA (3.66 ± 0.70) (Fig. 3B). A densitometric analysis of western blots also confirmed that in rat NP cells, the accumulation of the TNF-α protein was increased significantly after cotransfection with LAP expression vector compared with what was observed in unstimulated control cells, whereas the expression of the TNF-α protein was not affected by cotransfection with LIP expression vector (Fig. 3C).
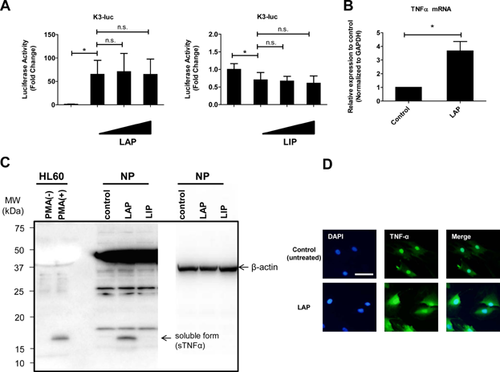
Induction of TNF-α by cotransfection with LAP expression vector was also demonstrated by immunofluorescence staining. As shown in Figure 3D, TNF-α expression was weak in the basal state, but was apparent in the cytosol and nuclear envelope after transfection with LAP expression vector. On the other hands, we have examined whether C/EBP β exposure increased expression of C/EBP β gene and protein. The results showed that there was not changed the expression of C/EBP β gene and protein compared to the control cells (Fig. S1).
TNF-α Was Activated by C/EBP β Via the MAPK Pathways in NP Cells
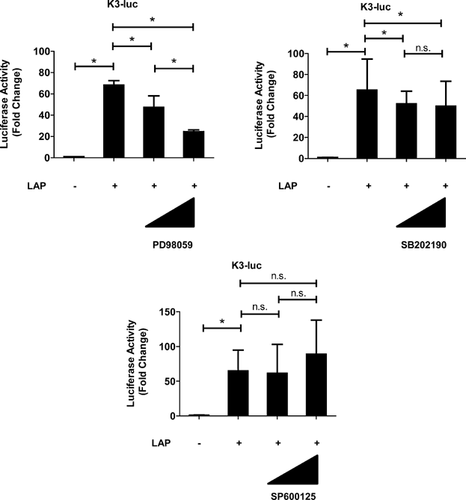
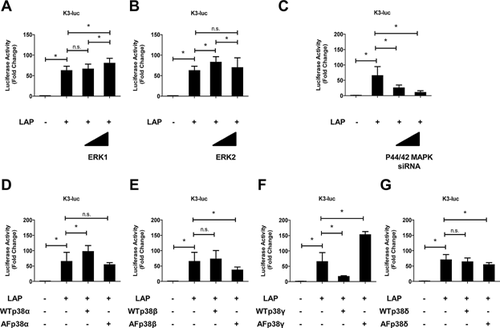
The role of C/EBP β in the TNF-α promoter in mediating the MAPK pathway was investigated in rat NP cells. To identify which MAPK pathways are necessary for C/EBP β-induced activation of TNF-α, the ERK1/2 pathway was selectively blocked with PD98059, the p38 pathway was blocked with SB202190, and the JNK pathway was blocked with SP600125. The C/EBP β-induced TNF-α promoter activity in the presence of MAPK inhibitors was measured to confirm that C/EBP β-dependent transient activation of the MAPK pathways was required for TNF-α activation. Figure 4 shows that cotransfection with LAP expression vector significantly increased the activity of the TNF-α promoter; this activity was suppressed completely by the MAPK inhibitors PD98059 and SB202190 but not by SP600125. Rat NP cells were transfected with LAP expression vector and treated with inhibitors of ERK (PD98059) and p38 (SB202190) signaling individually. Suppression of the TNF-α mRNA and TNF-α protein was also partially blocked by the individual inhibitors (Fig. 5A and B). To investigate the mechanism of MAPK pathway (ERK1/2 and p38)-mediated regulation of TNF-α expression, we transfected rat NP cells with LAP expression vector together with WT and AF expression plasmids, and measured TNF-α promoter activity. TNF-α promoter activity was induced after transfection with a high dose of WT-ERK1. However, no induction of TNF-α promoter activity was observed after transfection with a high dose of WT-ERK2 (Fig. 6A). In a further approach, we transfected rat NP cells with an LAP expression vector together with a control or p44/42MAPK siRNA at 24 h prior to the experiments. Treatment with the p44/42 MAPK siRNA significantly reduced LAP-induced TNF-α promoter activity compared with the control siRNA. Subsequently, rat NP cells were cotransfected with LAP expression vector and WT and AF-p38 plasmids. The results of both the gain- and loss-of-function analyses showed that cotransfection with p38γ regulated TNF-α promoter activity.

DISCUSSION
This study was the first to demonstrate that NP cells expressed C/EBP β, and that the expression of TNF-α is regulated by C/EBP β via the MAPK pathways. We also demonstrated that the transcription factor C/EBP β might be associated with degenerative IVD disease.
Some groups have reported that C/EBP β is critical for chondrocyte hypertrophy and plays an important role in cellular senescence in the aging articular cartilage.22, 27 Therefore, we hypothesized the presence of relationship between C/EBP β and TNF-α in the process of degeneration of IVDs.
Inflammation and cytokine release from IVD play an important direct role in IVD degeneration. Prior studies showed that TNF-α, which is one of the pro-inflammatory catabolic cytokines, was implicated in disc pathology associated with IVD degeneration.11 The administration of TNF-α to bovine discs resulted in catabolic changes that included an increase in the expression of nerve growth factors and aggrecan degradation, which is similar to the degeneration process observed in humans. Furthermore, TNF-α acts as an initiating factor that triggers a cascade of inflammatory cell signaling, which accelerated matrix degradation.28, 29 Expression of TNF-α leads to a catabolic shift within IVD tissue whereby AF and NP protease activities become increased and may lead to degeneration.28, 30 However, the upstream and downstream regulatory elements of TNF-α in IVD cells remain unknown, and the molecular mediators present in the IVD are poorly understood. Based on this background, we predicted that blocking TNF-α and its upstream signaling activity using therapeutic reagents would lead to inhibition of the process of degeneration of IVDs.
Expression studies showed that C/EBP β is expressed in both human and rat NP cells. Given the ability of normal NP cells to produce several cytokines, it was not surprising to find that NP cells produced baseline levels of C/EBP β. The higher level of expression observed in the rat NP tissue at 11 weeks compared with that detected at 3 weeks probably reflects the IVD degeneration state. We also conclude that C/EBP β is one of the factors involved in the constitutive regulation of TNF-α. Based on previous work and these data regarding the regulation of TNF-α in IVD cells, we hypothesized that C/EBP β is one of the most important regulators of genes, transcription, and protein activity in IVD cells. Although the underlying mechanism remains unknown, some evidence indicates that C/EBP β-dependent TNF-α expression in NP cells may be regulated by MAPK signaling. In addition, we thought that C/EBP β involved in the trigger in the early stages of IVD degeneration.
MAPKs are a family of serine/threonine protein kinases that play essential roles by transducing environmental stress signals into altered gene expression. There are numerous MAPKs, which are grouped into distinct families: The ERKs, the JNKs, and the p38 MAPKs (p38α, p38β, p38γ, and p38δ). Aiming to elucidate further the role of C/EBP β in TNF-α promoter regulation, we assessed whether MAPK signaling is required for the C/EBP β-dependent induction of TNF-α in rat NP cells. We first evaluated the activation of these signaling pathways after treatment with MAPK inhibitors. The results showed the presence of a significant suppression of C/EBP β-mediated induction of TNF-α promoter activity after treatment with the ERK inhibitor PD98059 and the p38 inhibitor SB202190, but not the JNK inhibitor SP600125. Similarly, a pronounced decrease in the C/EBP β-mediated increase in the levels of the TNF-α gene and protein was observed in the presence of ERK and p38 pathway inhibitors. Our promoter studies showed that this regulation occurred at the transcript, gene and protein levels. Subsequently, we gained mechanistic insights into this regulation from gain- and loss-of-function transfection studies. We found that the MAPK isoforms that were responsible for regulating TNF-α promoter activity were likely to be ERK1/2 and/or p38γ, because cotransfection with LAP expression vector significantly increased the activity of the TNF-α promoter, and WT- or AF- ERK1/2 and p38γ had opposite effects on TNF-α-dependent transcription via the activation of C/EBP β (Gain and loss of function analyses). However, we cannot rule out the possibility that p38α, p38β or p38δ also plays a critical role in TNF-α expression in NP cells. Noteworthy, the activity of the C/EBP β is responsive to TNF-α in NP cells, and these pathways have been shown to modulate MAPKs signaling.
Moreover, there is an evidence implicating that TNF-α-dependent Wnt signaling in NP cells may be regulated by NF-κβ signaling, although the details of the transduction pathway are not known.31 Therefore, future studies will have to examine more closely the role of the components of the various MAPKs signaling and their cross-talk signaling in the regulation of Wnt signaling in NP cells, and will be needed to examine the relationship between Wnt signaling and C/EBP β in IVD cells (Fig. S2). Modulation of these signaling pathways using small molecular inhibitors may provide a new approach to treat IVD degeneration.
A major limitation of this study was that rat NP cells were isolated from normal IVDs. As these discs are mostly notochordal in origin, their characteristics and underlying mechanisms are not transferable to the human condition, which may affect the interpretation of the results. Although the disappearance of notochordal cells precedes the onset of IVD degeneration, it remains unclear whether the disappearance of these cells is involved in the initiation of IVD degeneration. This uncertainty is caused largely by a poor understanding of the function of notochordal cells in normal, healthy IVDs during development and in young adults. However, it is ethically difficult to collect IVDs from infants. In addition, we wanted to assess, firstly, whether C/EBP β promotes TNF-α activation in normal NP cells. For these reasons, we used NP cells from the discs of healthy rats.
In conclusion, prior studies showed that TNF-α was implicated in disc pathology associated with IVD degeneration.29, 32, 33 Based on this background, we found that the expression of TNF-α was regulated by C/EBP β through the MAPK pathways. Moreover, C/EBP β is expressed in both human and rat NP cells. The higher level of expression observed in the rat NP tissue at 11 weeks compared with that detected at 3 weeks probably reflects the IVD degeneration state. Thus, we thought that C/EBP β involved in the trigger in the early stages of IVD degeneration. We believe that our findings may open a new avenue toward the understanding of the cellular and molecular mechanisms of IVD cells, and toward the exploration of new approaches for preventing, minimizing, or completely relieving IVD degeneration.
AUTHORS' CONTRIBUTIONS
AH participated in the design of the study, performed experiments, analyzed data and wrote the article. SH isolated the cells, and performed experiments and the statistical analyses. DS participated in the design, and performed experiments and performed the statistical analysis. JM participated in the design and coordination and helped to draft the article. All authors read and approved the final article.
ACKNOWLEDGMENTS
We greatly appreciate the helpful advice of Dr. Tadayuki Sato and the technical assistance of Noboru Kawabe and Masatoshi Ito.




