Static and dynamic compression application and removal on the intervertebral discs of growing rats
ABSTRACT
Fusionless implants are used to correct pediatric progressive spinal deformities, most of them spanning the intervertebral disc. This study aimed at investigating the effects of in vivo static versus dynamic compression application and removal on discs of growing rats. A microloading device applied compression. 48 immature rats (28 d.o.) were divided into two groups (43d, 53d). Each group included four subgroups: control (no surgery), sham (device installed without loading), static (0.2 MPa) and dynamic compressions (0.2 MPa ± 30% with 0.1 Hz). In 43d subgroups, compression was applied for 15 days. In 53d subgroups, compression was followed by 10 days without loading. Disc heights, nucleus/annulus volumetric proportions and nucleus proteoglycan contents were analyzed using one-way ANOVA and post-hoc Tukey comparisons (p < 0.05). Disc heights of 43d and 53d static and dynamic loading rats were lower than shams (p < 0.05). Volumetric proportions remained similar. At 43d, nucleus proteoglycan contents increased in both static and dynamic loading rats. However, at 53d, static loading rats had lower proteoglycan content than dynamic loading rats (p < 0.05). Disc structure is altered following static compression removal, but nucleus proteoglycan content remaining elevated in dynamic group. Dynamic fusionless implants would better preserve disc integrity. © 2015 Orthopaedic Research Society. Published by Wiley Periodicals, Inc. J Orthop Res 34:290–298, 2016.
New fusionless treatments have recently been developed to treat young patients with mild to moderate spinal deformations such as adolescent idiopathic scoliosis.1 Innovative fusionless devices are very promising options as they apply appropriate mechanical forces to correct spinal curves while maintaining growth potential in young patients. Moreover, this minimally invasive approach should avoid intervertebral disc fusion, hence preserving spinal mobility,2, 3 as opposed to currently used invasive surgical instrumentations.4 However, compression-based fusionless treatments such as vertebral body staples and tethers connect adjacent vertebral bodies and step across the intervertebral disc in between.5-7 Instrumented vertebrae and intervertebral discs are, therefore, submitted to asymmetric compressive loading, which could also cause intervertebral disc degeneration.
It is currently not known whether fusionless treatments correcting the scoliotic spine could alter intervertebral disc integrity in these young patients,4 triggering degeneration in intervertebral discs spanned by fusionless implants either during treatment or after implant removal. To validate the clinical use of fusionless devices, scientific data are required to document potentially adverse short and long-term effects on the intervertebral disc biological integrity and function.
The intervertebral disc is a soft connective tissue composed of a nucleus pulposus (NP) in the center, surrounded by an annulus fibrosis (AF). Its roles consist in transmitting mechanicals loads between each vertebra level, while allowing movement and flexibility of the spine.8, 9 The rat tail model is well suited to study the effects of mechanical loading on the intervertebral disc as its biological structure mimics the ones in pediatric spines.8, 10 Moreover, rat caudal vertebrae and intervertebral discs are easily accessible for surgical manipulations to investigate the mechanobiological responses of both soft and hard tissues. This animal model has essentially been developed to understand intervertebral disc degeneration mechanisms with age.11, 12 Therefore, abundant literature data provide findings on older rats or mice, submitted to different magnitude and frequency loadings,10 some of them well beyond physiological forces.12, 13 These studies have found alterations in disc metabolism, such as proteoglycan loss and morphological changes such as height reduction. When disc is immobilized, detrimental degenerative effects have also been observed in the disc.14, 15 However, when loadings remained within physiological ranges, namely from 0.1 to 1.0 MPa and frequencies between 0.01 and 1.0 Hz, gene expression were maintained similarly to unloaded groups.15 Some in vivo studies have investigated the effects of physiological mechanical compression on skeletally immature rat discs. Stokes et al.1, 4 showed that intervertebral disc height was reduced along with an increased stiffness in growing rats submitted to a sustained compression of 0.1 MPa. Their study could, however, not conclude about a potential metabolic-related cause and assumed that reduced mobility due to device implantation could be a major factor responsible for the observed intervertebral disc height reduction.1
The objectives of this study are to evaluate the effects of application and subsequent removal of static versus dynamic compression within physiological ranges on intervertebral discs geometry and volumetric proportions (NP versus AF) using an in vivo growing rat-tail model. It was hypothesized that: (i) both static and dynamic compressive loading reduced intervertebral disc height combined with volumetric (NP versus AF) changes only in static compressive loading, and that (ii) subsequently removing compression maintained intervertebral disc integrity in dynamic loading while keeping or increasing structure and composition changes in static loading.
MATERIALS AND METHODS
In Vivo Experimental Setup
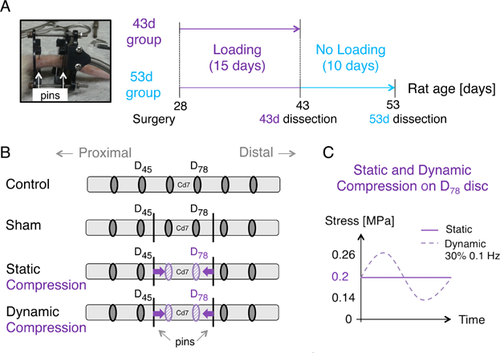
The study protocol was approved by the Institutional Animal Care Committee. Forty-eight male Sprague–Dawley rats were divided into two groups (43d and 53d) corresponding to their age at euthanasia (in days), each containing four subgroups: control (n = 6), sham (n = 6), static (n = 6), and dynamic (n = 6; Fig. 1A through C, Table 1). All rats were received at 21 days old and had 1-week acclimatization. Control rats received no surgical treatment. Sham, static and dynamic loading rats were operated at 28 days old. Sham rats of both 43d and 53d had a micro-loading device implanted but no loading applied. Static loading rats of 43d group had 15 days of sustained loading at 0.2 MPa before euthanasia. Static loading rats of 53d group had 15 days of sustained loading at 0.2 MPa followed by 10 days without compression. Similarly to the static loading subgroup, dynamic loading rats were loaded at 0.2 MPa ± 30% at a frequency of 0.1 Hz in 43d group and loading was removed for 10 days in 53d group. Loading duration and rat age were selected according to the rat pubertal growth spurt16, 17 similarly to other studies on bone growth modulation.18-20 Loading level intensity remained within physiological ranges and was enough to reduce without stopping bone growth.20, 21
| 43d Group | 53d Group | |||
|---|---|---|---|---|
| Number of Rats | Loading Application (15 days) | Number of Rats | Loading Application (15 days) + Loading Removal (10 days) | |
| Control | n = 6 | N/A | n = 6 | N/A |
| Sham | n = 6 | 0 MPa | n = 6 | 0 MPa + 0 MPa |
| Static | n = 6 | 0.2 MPa | n = 6 | 0.2 MPa + 0 MPa |
| Dynamic | n = 6 | 0.2 MPa ± 30% at 0.1 Hz | n = 6 | 0.2 MPa ± 30% at 0.1 Hz + 0 MPa |
Compressive Loading Application
The device was implanted on the 6th and 8th caudal vertebrae (Cd6 and Cd8), to load the 7th caudal vertebra (Cd7) and the two adjacent intervertebral discs, referred to as D67 and D78 (Fig. 1B). The surgical procedure was similar to other published studies,19, 20 with a loading device well tolerated by the animals. Compression was transmitted through the inflation and/or deflation of a latex bladder (DipTech Systems Inc, Kent, OH) controlled by an air-compressed system, as previously described.19, 20 The micro-compression device was calibrated with a micro-mechanical loading machine (MACH-1, Biomomentum, Laval, Canada; 17N force range and 0.026N resolution) prior to its use in vivo to apply a finely controlled force. The force was increased and adjusted daily according to the growth of Cd7 transverse area, modeled as a circular surface.18
Tissue Processing
After euthanasia using guillotine on the anesthetized rat, intervertebral discs (D45, D78) were collected. D45 was used as an inter-animal control disc, while D78, the distal loaded disc, was analyzed. Intervertebral discs were fixed in formalin (Anachemia, Montréal, Canada), dehydrated, clarified, and infiltrated before embedding in methylmetacrylate (MMA; Fisher Scientific Canada, Nepean, Canada). Due to tissue processing problems, one loaded disc D78 in the 43d dynamic group as well as one control disc D45 in the 53d dynamic group were removed from the study.
Intervertebral Disc Height
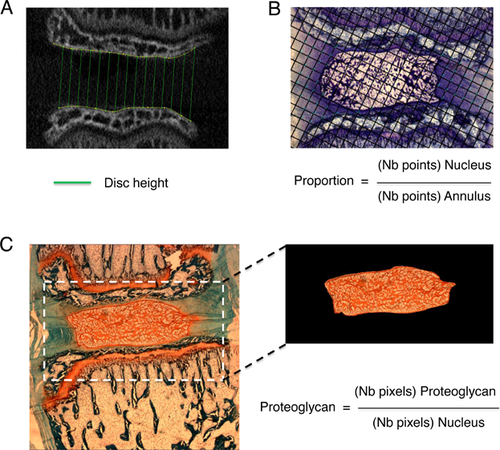
MMA embedded samples (caudal vertebrae and discs) were imaged using a microCT (SkyScan 1176, Bruker, Kontich, Belgium) with a 0.5 mm aluminum filter suitable for bone quality assessment, a 50 kV source and an image pixel size of 8.74 µm. Tri-dimensional reconstruction (Skyscan N Recon software) was performed in the area of interest, that is, selection of the distal end of the proximal vertebra (up to the growth plate) and the proximal end of the distal vertebra (up to the growth plate) centered on the disc invisible on X-rays (Fig. 2A). Dataset was further transferred to ImageJ (National Institutes of Health) to select six images around the center of both adjacent vertebrae and the intervertebral disc height for D45 and D78 was calculated using a semi-automated custom-made Matlab program.19, 20 The program modeled the distal edge of Cd7 as well as the proximal edge of Cd8 as splines and calculated the distance between both splines to estimate disc height (Fig. 2A). Moreover, intervertebral disc D45 height was used to normalize D78 height to correct potential individual growth variability within the same group.
Intervertebral Disc Histology
Caudal segment samples, including vertebrae and discs, were cut in 6 µm sections using a microtome (Leica SM2500) in the longitudinal bone axis of the 5th and 7th caudal vertebrae. Slides were organized in series of six slides until disc mid-section was reached. Then, the first mid-section slide was stained with 0.2% toluidine blue and used for volumetric proportion measurements. The second slide was stained with Safranin-O and Fast-Green for proteoglycan content.
Stereological Analyses—Volumetric Proportions
Three to six toluidine-blue stained images/disc were analyzed. Images were taken with an optical microscope (Leica DMR with Retiga Qimaging Camera) at 2.5× magnification from selected sections showing maximal nucleus (disc mid-section). Quantification was done using the point counting stereological method22-25 using a grid produced on Gimp software, randomly positioned on the images with dimensions insuring sufficient intersection points (i.e., 50–300 points) in each tissue (nucleus and annulus). Quantification was done using ImageJ cell counter plugin (Fig .2B).
Proteoglycan Content of the Nucleus
Series around disc mid-section were analyzed. Safranin-O stained images were taken using the optic microscope at 2.5× magnification. The number of red stained pixels was quantified with ImageJ by adjusting saturation and brightness thresholds for each image. Proteoglycan content in the nucleus was calculated as the ratio of the number of red-stained pixels in the nucleus divided by the total number of pixels representing the nucleus (Fig. 2C).
Statistical Analyses
One-way ANOVA for repeated measures was performed to identify differences between mean values of each parameter from each rat subgroup, for each intervertebral disc (inter-animal control disc D45, and loaded disc D78) separately for both 43d and 53d groups. All subgroups were further compared using post-hoc Tukey's comparisons with a significance level of p < 0.05.
RESULTS
Rats weighed 88.4 g (±7.2) on the day of surgery (28 d.o.) in 43d groups and 85.3 g (±12.2) in 53d groups. Their weights reached 223.5 g (±18.8) in 43d groups and 317.3 g (±42.6) in 53d groups. Significant body weight reduction was reported only between 43d shams compared with controls (p = 0.019).
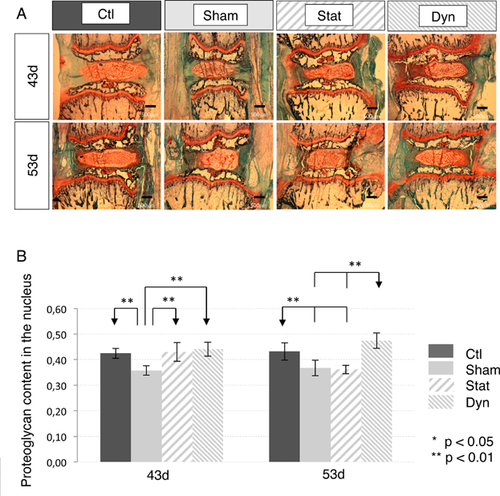
Static and Dynamic Loading Rats Showed Similar Nucleus Proteoglycan Increase at 43d, But at 53d, It Decreased Among Static Loading Rats and Remained High Among Dynamic Loading Rats
In 43d groups, both static and dynamic loadings showed significantly higher proteoglycan contents compared to shams (Fig. 3A and B, Table 2). Both loaded subgroups had similar levels compared to controls. However, in 53d groups, following loading removal, the level of proteoglycans of the static subgroup was similar to the sham level, therefore significantly lower than dynamics (Fig. 3A and B, Table 2). Dynamic proteoglycan content, on the other hand, remained elevated with no statistical differences with controls (Fig. 3A and B, Table 2).
| Nucleus Proteoglycan Content | Nucleus/Annulus Proportion | |||
|---|---|---|---|---|
| Intervertebral Discs D45 and D78 | D45 | D78 | D45 | D78 |
| 43d group | ||||
| Control | 0.458 ± 0.039 | 0.424 ± 0.019 | 0.521 ± 0.086 | 0.505 ± 0.082 |
| Sham | 0.414 ± 0.040 | 0.357 ± 0.018‡ | 0.469 ± 0.076 | 0.483 ± 0.034 |
| Static | 0.436 ± 0.040 | 0.430 ± 0.036** | 0.477 ± 0.039 | 0.480 ± 0.064 |
| Dynamic (X78) | 0.426 ± 0.033 | 0.441 ± 0.027** | 0.499 ± 0.047 | 0.432 ± 0.064 |
| 53d group | ||||
| Control | 0.496 ± 0.060 | 0.432 ± 0.034 | 0.540 ± 0.046 | 0.521 ± 0.076 |
| Sham | 0.452 ± 0.038 | 0.368 ± 0.031‡ | 0.493 ± 0.053 | 0.492 ± 0.038 |
| Static | 0.464 ± 0.054 | 0.361 ± 0.016‡ | 0.527 ± 0.027 | 0.468 ± 0.038 |
| Dynamic (X45) | 0.467 ± 0.045 | 0.474 ± 0.030## | 0.483 ± 0.047 | 0.440 ± 0.074 |
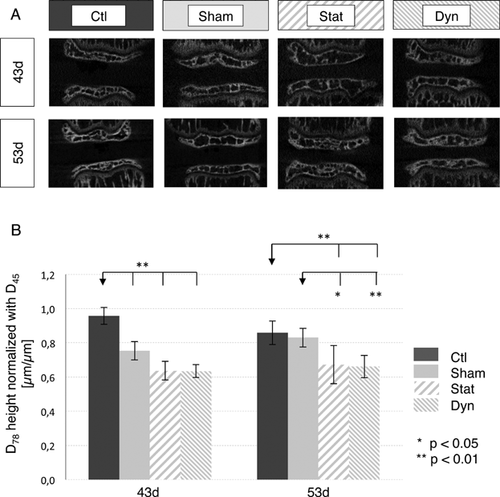
Static and Dynamic Loading Rats Resulted in Similar Intervertebral Disc Height Reduction in Both 2 and 53d Groups
Intervertebral disc height ratios of D78/D45 were reduced similarly in both static and dynamic subgroups from 43d and 53d groups (Fig. 4A and B). Immediately following loading (43d), the reduction in height ratio was not considered significant, although p values were close to 0.05 when comparing static (p = 0.056) and dynamic loading rats (p = 0.052) with shams. However, in 53d groups, disc height ratio was significantly lower in both static (p = 0.011) and dynamic loading rats (p = 0.006) compared to shams.
Static and Dynamic Loading Rats Showed Similar Volumetric Proportions
Nucleus versus annulus volumetric proportions did not change neither between groups nor subgroups. They remained around 50% when nucleus is maximal in its mid-section (Table 2).
Static and Dynamic Loading Discs Had Similar Qualitative Aspects of Their Annulus Fibers
Safranin-O stained sections showed aligned fibers in the annulus and single chondrocytes or chondrocytes in small isogenous groups (clusters of chondrocytes) aligned along the fibers direction in all subgroups of both 43d and 53d groups (Fig. 3A). No visible sign of annulus fiber disruption was observed between loaded subgroups, shams and controls in neither 43d nor 53d groups.
A Sham Effect Was Observed in Nucleus Proteoglycan Content and in Intervertebral Disc Height in the 43d Group
When comparing control and sham rats, a sham effect was noted in nucleus proteoglycan content for both 43d and 53d groups, where sham proteoglycan content was significantly reduced compared to controls (p < 0.01; Fig. 3B). Moreover, in the 43d groups, intervertebral disc height ratio was significantly lower in shams compared to controls (Fig. 4B, Table 3). However, intervertebral disc height ratio was restored in shams of the 53d group (Fig. 4B, Table 3).
| 43d Group | 53d Group | |||
|---|---|---|---|---|
| Group/disc heights (μm) | D45 | D78 | D45 | D78 |
| Control (n = 6) | 1,237.7 ± 209.9 | 1,184.1 ± 95.4 | 1,361.3 ± 145.6 | 1,167.9 ± 93.5 |
| Sham (n = 6) | 1,108.6 ± 132.2 | 835.2 ± 91.1‡ | 1,245.6 ± 115.1 | 1,033.9 ± 67.8* |
| Static (n = 6) | 1,250.6 ± 71.6 | 796.0 ± 96.2‡ | 1,338.1 ± 128.4 | 898.9 ± 148.6‡ |
| Dynamic (n = 6) | 1,216.7 ± 195.7 | 772.3 ± 68.9‡ | 1,205.3 ± 78.7† | 796.3 ± 78.0‡,** |
Little Differences Were Obtained Between Intra-Animal Intervertebral Disc Controls D45
No significant differences were observed in intervertebral control disc D45 composition, namely volumetric proportion and proteoglycan content of the nucleus (Table 2). However, intervertebral disc height was reduced in 53d dynamic subgroup compared to controls (Table 3).
DISCUSSION
This study investigated the effects of applying and removing two compression regimens (static, dynamic) within physiological ranges on the intervertebral disc volumetric and nucleus proteoglycan proportions of young immature rats. The hypothesis stated that intervertebral disc geometry and nucleus proteoglycan proportion would be modified following both static and dynamic compressions. However, compression removal would differentiate static and dynamic regimens, with the assumption that dynamic could be a force loading type that would better preserve soft tissues integrity. Our results showed that static and dynamic compression removal lead to differences in nucleus proteoglycan content. Static loading rats had a significantly decreased nucleus proteoglycan contents compared to dynamic loading rats. This could be an early sign of intervertebral disc degeneration,9 suggesting that long-term static (and sham) treatment would be more detrimental to the intervertebral disc compared to dynamic treatment. However, in both static and dynamic subgroups, intervertebral disc height remained lower even after compression removal. It seems, therefore, that treatments on the intervertebral disc, even within physiological ranges, can cause long-term changes in its structure and nucleus proteoglycan content.
Differences Between Static and Dynamic Compression Became Apparent Following Loading Removal
In this study, proteoglycan assessment was performed using stoichiometric-binding Safranin-O staining26 along with an imaging algorithm. Safranin-O staining was shown to be accurate as long as there is no severe proteoglycan loss,26 which corresponded to this study as only early signs of degeneration were eventually expected. Dimethylmethylene blue assay for glycosaminoglycanes quantification would have required fresh tissue samples27 and would not have enabled proteoglycan localization. Proteoglycan content in the nucleus was found similar between static and dynamic loading rats following compression. A decreased proteoglycan content has previously been observed in the nucleus when static compression was well beyond physiological levels13, 15, 28 and when dynamic frequency was too elevated11 on older mature animals as models of disc degeneration. Iatridis et al.15 mentioned compressive loading thresholds at 1.0 MPa, and at a frequency below 0.01 Hz or above 1.0 Hz. We intentionally used low stress levels (0.2 MPa) to remain within physiological ranges; the dynamic frequency of 0.1 Hz used in this study is within the stimulus range showed to maintain intervertebral disc metabolism.15 Also, we studied young rats to model immature spine mechanobiology similarly to Stokes et al.1 as opposed to most literature data obtained from older animals for disc degeneration purposes.8, 10-12, 29 Similar force ranges selected in Iatridis et al.29 on older rats, resulted in a glycosaminoglycan increase in the intervertebral disc composition after static compression applied in vivo on a rat tail model with two loaded discs for up to 56 days. Ching et al. compared in vivo static versus dynamic compressions at three different frequencies (0.5, 1.5, 2.5 Hz) using mature rats for 1 h/day for a total duration of 14 days. Their protocol would simulate loading application and removal for several cycles with a removal time interval 24 times higher than loading time. In the study by Ching et al.11 on older rats, it was observed that total proteoglycan significantly decreased in static group compared to lowest frequency (0.5 Hz) dynamic group, but when frequency was increased, proteoglycan levels dropped to the static levels. In our study, we used a frequency of 0.1 Hz, which is five times lower than the frequency employed in the study by Ching et al.11 Indeed, dynamic compression in our study (magnitude of 0.2 MPa combined with 0.1 Hz of low frequency loading) shows proteoglycan increase compared with shams. Our results support the hypothesis of a compression threshold level in static as well as dynamic compression to maintain proteoglycan content in the nucleus.
Following loading removal, obtained results discriminated static from dynamic loading conditions. Proteoglycan levels decreased in the static subgroup while remaining elevated in the dynamic one. Proteoglycan decrease is one of the early signs of intervertebral disc degeneration as proteoglycans are responsible for nucleus osmotic properties to resist compression.9 Static compression might cause large proteoglycan cleavage or chemical modifications of their G1 domain which affect the integrity and turnover of intervertebral disc proteoglycans as reported in older subjects by non-enzymatic glycation.30 Maintaining static compressive stress might force proteoglycans to remain within the nucleus, therefore, limiting their loss. When loading is removed, modified, and smaller size proteoglycans could migrate out of the nucleus.31 In the dynamic case, proteoglycan levels remained unchanged after loading removal, suggesting that proteoglycans integrity is preserved, similarly to the controls. Balance between proteoglycan anabolism and catabolism seemed to be maintained in control and dynamic groups, but it could possibly be established with different metabolic rates. Identical proteoglycan content might as well mean higher turnover and metabolism combining proteoglycan loss and upregulation of proteoglycan production.15 Dynamic loading with appropriate magnitude and frequency could reproduce physiological conditions and enable fluid movements through the structure, which might contribute to maintaining the overall structural balance. Further analyses such as dimethylmethylene blue assays on fresh samples of additional rat cohorts could complement the results to investigate glycosaminoglycan levels.26 Therefore, other studies would be required to investigate and analyze different proteoglycan types in order to understand pathways of proteoglycan transformations when submitted to different physiological loading types.
Static and Dynamic Intervertebral Disc Height Remained Reduced Even After Loading Removal
Intervertebral disc height reduction following compressive loading is well documented in the literature, in immature animals as well.32 In normal healthy intervertebral discs, diurnal variations of intervertebral disc height are observed depending on compression magnitude related to daily activity and position. Due to intervertebral disc viscoelastic properties therefore to its time-dependent response to loading, disc undergoes creep, that is, its height gradually decreases under compression until reaching an equilibrium deformation, and it is normally restored following a period of rest. However, in our study, height was not restored following loading removal, with a possible explanation associated with our experimental design. Fixations and rods were maintained in place after compression removal to support the structural device. Although rods were kept loose to minimize their effect and ensure no additional compression, this setup could have added rigidity to the structure. This setup group could be seen as an intermediate case between shams found in the literature instrumented with fixations only, and immobilized groups with both fixations and rods.29 Immobilization was found to cause significant changes on intervertebral disc geometry, especially its height.14, 29 Therefore, our sham effect might be a result of partial immobilization, reduced motion, and increased segment rigidity. Moreover as no significant differences were found between shams, static and dynamic loading removal, device design might be the origin of remaining intervertebral disc reduction compared to controls.
Sham Effect and Limitations Are Associated to the Used Rat Model
Apart from intervertebral disc height at 43d, a sham effect was also observed in terms of proteoglycan content in both 43d and 53d groups. Sham effects were previously reported in the literature, and explained by device implantation and procedure that cause added stress.14, 29 As aforementioned, our sham group is similar to a partially immobilized group. Consequently, slow proteoglycan turnover and fluid movements could explain the reduction of intervertebral disc proteoglycans.
Other limitations of this study include nucleus cell phenotype differences between rats and humans. In rats, notochordal cells in the nucleus are found in a higher proportion compared to humans, where chondrocyte-type cells prevail. Notochordal cells accelerate intervertebral disc remodeling and recovery.1, 33 Therefore, rat intervertebral disc results are optimistic for knowledge transfer to humans. Human intervertebral discs should have lower metabolic rate and different mechanically induced remodeling thresholds. Another limitation includes loading removal duration chosen at 10 days to enable intervertebral disc cell recovery. More substantial changes might have been observed with a longer duration of loading removal. Monitoring weekly in vivo changes on the intervertebral disc would have required other technologies or added greater amount of animals to the study.
CONCLUSION
Results from this study support a better preservation of intervertebral disc structure in response to dynamic compression. Following loading removal, proteoglycans levels decreased in the static group while remaining elevated in the dynamic group. Careful control in both static and dynamic compressions is critical to maintain proteoglycan content in the nucleus, therefore preventing early signs of disc degeneration. These results are essential to gain knowledge to prevent possible adverse effects of fusionless implants on intervertebral discs composing immature spines. In particular, fusionless implants are interesting treatment options for young scoliotic patients if they successfully maintain disc health following compressive loading that enable spinal corrections. Thus, these results support the development of fusionless implants applying dynamic compression.
AUTHORS' CONTRIBUTIONS
Study design: ALM, EM, and IV. Study conduct: ALM, GG, EM, and IL. Data interpretation: ALM, GG, FM, and IV. Substantial revisions: GG, IL, FM, and IV. All authors have read and approved the final submitted version of the manuscript.
ACKNOWLEDGMENTS
The authors would like to warmly thank laboratory team members, Souad Rhalmi, Charlotte Zaouter, Saadallah Bouhanik, and all the animal care technicians for their precious contributions and technical advices. This work was funded by the Natural Sciences and Engineering Research Council of Canada (NSERC; I.V.), the Canada Research Chair in Mechanobiology of the Pediatric Musculoskeletal System (I.V.), as well as the NSERC/CREATE program (ALM) and the Network for Oral and Bone Health Research (RSBO; ALM). G. Grimard is a consultant and owns stock options at Emovi, Inc.




