Expression of growth differentiation factor 6 in the human developing fetal spine retreats from vertebral ossifying regions and is restricted to cartilaginous tissues
ABSTRACT
During embryogenesis vertebral segmentation is initiated by sclerotomal cell migration and condensation around the notochord, forming anlagen of vertebral bodies and intervertebral discs. The factors that govern the segmentation are not clear. Previous research demonstrated that mutations in growth differentiation factor 6 resulted in congenital vertebral fusion, suggesting this factor plays a role in development of vertebral column. In this study, we detected expression and localization of growth differentiation factor 6 in human fetal spinal column, especially in the period of early ossification of vertebrae and the developing intervertebral discs. The extracellular matrix proteins were also examined. Results showed that high levels of growth differentiation factor 6 were expressed in the nucleus pulposus of intervertebral discs and the hypertrophic chondrocytes adjacent to the ossification centre in vertebral bodies, where strong expression of proteoglycan and collagens was also detected. As fetal age increased, the expression of growth differentiation factor 6 was decreased correspondingly with the progress of ossification in vertebral bodies and restricted to cartilaginous regions. This expression pattern and the genetic link to vertebral fusion suggest that growth differentiation factor 6 may play an important role in suppression of ossification to ensure proper vertebral segmentation during spinal development. © 2015 Orthopaedic Research Society. Published by Wiley Periodicals, Inc. J Orthop Res 34:279–289, 2016.
The spinal column of vertebrates is initially developed by forming a vertebral axis from the epithelial somite and notochord, which undergoes chondrogenesis, segmentation and ossification to outline an alternating structure of vertebral bodies (VB) and intervertebral discs (IVD). Vertebral segmentation is initiated by sclerotomal cell migration and condensation around the notochord to form the less condensed anlagen of VB and condensed regions. As the VB ossify, the condensed segment between the ossifying centres forms the IVD, consisting of a central nucleus pulposus (NP, nucleus), and surrounding rings of annulus fibrosis (AF, annulus) that are separated from the VB by cartilaginous endplates (EP). The NP is formed from the primitive notochordal cells and the AF is derived from the embryonic mesenchyme.1, 2 The formation of VB and IVD is one of the crucial transition processes during vertebral column development. This complex transition is achieved predominately by the interplay of secreted growth factors and signal molecule networks including Notch, Wnt, Sonic Hedgehog (SHH), bone morphogenetic proteins (BMPs), and BMP antagonist noggin signalling pathways.3, 4 Transcription factors L-Sox5 and Sox6 are also critical regulators in vertebral column formation.5 Interference in the signal pathways will result in failure of correct vertebral development. A variety of congenital vertebral anomalies are known to be caused by segmentation defects.6 Although the genetic etiology and molecular mechanisms are not fully understood, mutational inactivation or malfunction of important signaling molecules has been demonstrated in congenital vertebral malformation including bone, growth plate and disc.7, 8
Growth differentiation factor 6 (GDF6), also known as cartilage derived morphogenetic protein 2 (CDMP2), is a member of the BMP family (BMP13).9 GDF6 functions as a regulatory protein associated with the growth and differentiation of developing embryos.10 GDF6 expression has been detected in both fetal, and post-natal cartilaginous tissues, developing long bones, tendons, eye, and neural tissues in human, murine, and bovine species.10-12 In human embryos, strong expression of GDF6 was found restricted to the hypertrophic chondrocytes of ossifying long bone centers.11 GDF6 is also expressed in normal and osteoarthritic human articular cartilage tissues.13, 14 To date, expression of GDF6 in the developing human vertebrae and IVD has not been established.
Mutations in the GDF6 gene, which have been identified in individuals with familial or sporadic Klippel–Feil syndrome (KFS), are associated with vertebral segmentation defects during fetal development, manifested as bony fusions in vertebrae, and other joints.15, 16 Fusion of ankle, and wrist joints were reported in GDF6 knockout mice,17 where expression is normally detected in stripes of cells, perhaps delineating the boundary between bone and cartilage tissues. We have previously shown that GDF6 retards the development of bone cells derived from mesenchymal progenitors in vitro18 and promotes the production of IVD extracellular matrix in an ovine annular stab injury model in vivo.19 While the factors that govern vertebral column development are not clear, GDF6 may play an important role in segmentation of the vertebrae. This study aimed to identify the expression pattern of GDF6 in the developing human fetal spine relative to key extracellular matrix protein expression, vertebral ossification, and morphological structures.
MATERIALS AND METHODS
Tissue Collection
Human fetal spine tissue was studied following approval from the Human Research Ethics Committee of the University of New South Wales. Fetal spine specimens between 8 and 19 weeks of gestation (n = 12) were obtained by informed consent and provided by the Prince of Wales Hospital, Randwick, Sydney. No genetic abnormalities were reported for these specimens. Tissue was collected fresh and immediately fixed in 10% neutral buffered formalin for 24 h followed by sectioning in the sagittal plane and embedding in paraffin.
Histological Staining
Paraffin embedded blocks of tissue were cut into serial sections (4 µm) and mounted on slides (Thermo Fisher, Australia). Histological analysis included hemotoxylin–eosin staining to illustrate tissue morphology and alcian blue staining to visualize proteoglycan and extracellular matrix proteins. For visualization of collagen, unstained sections were viewed under polarized light using a Leica microscope (DM6000 B; Leica Microsystems, Wetzlar, Germany) with a digital imaging workstation.
Antibodies
Polyclonal antibodies specific for collagen I (goat) and the collagen II (goat) were obtained from Santa Cruz (Biotechnology Inc, CA). Aggrecan was detected using a mouse monoclonal antibody obtained from AbD Serotec (Raleigh, NC). GDF6 expression was detected using a monoclonal antibody (ProMab Biotechnologies Inc, Richmond, CA) raised against the purified active domain of an Escherichia coli-derived protein preparation (Peprotech Inc, NJ). The specificity of this antibody for GDF6 was determined by ELISA and western blot.
Immunohistochemical Staining
Slides were deparaffinized in xylene and rehydrated through a graded ethanol series. For GDF6, antigen retrieval was performed with 0.5% proteinase-K solution (Roche Diagnostics, Manheim, Germany) for 10 min at 37°C. The slides were scavenged with 3% (v/v) H2O2 and blocked with 10% skim milk powder in tris buffered saline. The slides were then probed with primary antibodies against collagen I (1:50), collagen II (1:200), aggrecan (1:100), or GDF6 (1:150) for 1 h at room temperature. After washing, the slides were treated with Multilink solution prior to incubation in a streptavidin-conjugated peroxidase solution (DAKO, Glostrup, Denmark). The sections were then visualized with 3, 3-diaminobenzidine hydrochloride (DAKO, Glostrup, Denmark) solution and counterstained with hematoxylin. Negative controls were treated under the same condition except with the omission of the primary antibody.
In Situ Hybridization
Preparation of Probes
A 450 bp cDNA fragment spanning start site of the protein coding region of GDF6 was sub-cloned into the pGEM3z vector system at the EcoRI site and the orientation with respect to the RNA polymerase start site was determined using restriction enzyme digestion. The plasmid constructs were linearized using HindIII or SacI and transcribed using T7 (sense) or SP6 RNA polymerase (antisense), respectively. Finally, the transcripts were labeled with digoxigenin (DIG) using a DIG-RNA labeling kit as specified by the manufacturer (Roche Diagnostics, Manheim, Germany).
Hybridization
In situ hybridization was performed as we have previously described.20 Briefly, paraffin-embedded spinal tissue sections were deparaffinized, rehydrated, and fixed with 4% (w/v) paraformaldehyde in PBS. Thereafter, the sections were treated with proteinase-K (2 μg/ml). The sections were then hybridized with the DIG-labeled RNA probes at 55°C for 12–15 h. Following hybridization, the sections were washed and the probes were detected with anti-digoxigenin Fab fragment antibody (1:250) conjugated to alkaline phosphatase (Roche Diagnostics, Manheim, Germany) for 2 h at room temperature. Finally, the sections were treated with NBT/BCIP (5-bromo-4-chloro-3-iodolyl-phosphate/nitroblue tetrazolium chloride) and counterstained with 1% (w/v) neutral red (Sigma–Aldrich, St. Louis, MO). Controls included hybridization with a sense probe, omission of either probes or omission of the anti-digoxigenin antibody.
RESULTS
Histological Structure of the Developing Human Spinal Column
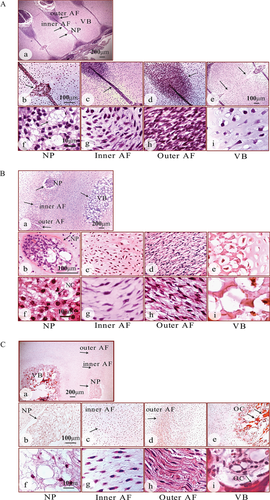
The gestational age of each specimen is shown in Table 1. At 8–11 weeks of gestation, the IVD had a distinct nucleus and annulus (Fig. 1A–a, arrows). The primitive nucleus showed a typical notochordal cell (NC) morphology (Fig. 1A–b, f, arrows) as previously described.21 Densely packed collagen fibers were visible in the outer annulus, but with an incompletely organized arrangement (Fig. 1A–d, and h, arrow). The cells of the inner annulus were more loosely packed and less organised (Fig. 1A–c, and g, arrow). The endochondral ossification of the vertebral bodies was yet to occur and contained a mucous streak in the center (Fig. 1A–e, and i, arrow). By 12–15 weeks, the disc structure became clearly defined (Fig. 1B–a, arrows). The expanded nucleus was highly cellular containing heavily vacuolated notochordal cells (Fig. 1B–b, and f, arrows). The annulus merged with an outer region of concentric lamellae (Fig. 1B–d, and h) and a less organised inner region (Fig. 1B–c, and g). The vertebral bodies showed small ossifying centers, surrounded by hypertrophic chondrocytes (Fig. 1B–a, e, and i). By 16–19 weeks, the fetal spines had more defined disc structures (Fig. 1C, arrows). A further enlarged nucleus contained the cell clusters embedded in an abundant mucoid matrix (Fig. 1C–a, b, and f). The inner annulus contained chondrocyte—like cells (Fig. 1C–c, and g, arrow), whilst the lamellar structure of the outer annulus appeared more organized (Fig. 1C–d, and h, arrow). The expanded ossifying centers of the vertebral bodies showed areas of bone deposition (Fig. 1C–e, and i, arrows).
| Age group | Gestational age (weeks) | Number of samples |
|---|---|---|
| 8–11 weeks n = 4 | 8 | 2 |
| 11 | 2 | |
| 12–15 weeks n = 5 | 12 | 1 |
| 13 | 1 | |
| 14 | 1 | |
| 15 | 2 | |
| 16–19 weeks n = 3 | 17 | 1 |
| 18 | 1 | |
| 19 | 1 |
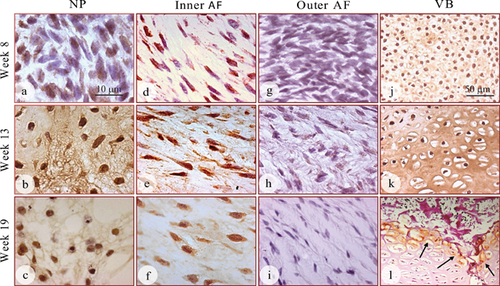
GDF6 Expression in the Developing Human Spinal Column
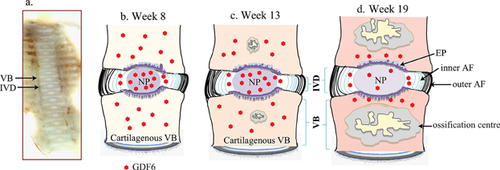
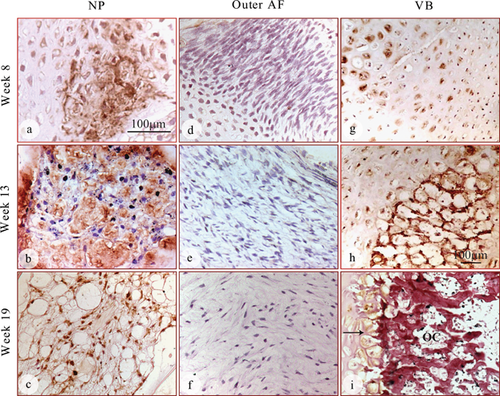
GDF6 protein was expressed in the developing IVD of all fetal spines examined by immunohistochemical staining and its expression was detected both inside the cells and in the extracellular matrix (Fig. 2). The expression of GDF6 in the nucleus appeared to intensify over time, with the 13 and 19 week specimens showing stronger staining than that in specimens at 8 weeks (Fig. 2a, b, and c). GDF6 expression was detectable in the cells of the inner annulus (Fig. 2d, e, and f), but could not be detected in the outer annulus at any of the time points (Fig. 2 g, h, and i). The developing vertebral bodies showed strong expression of GDF6 throughout the cartilaginous region, the most intense expression detected in both the cells and the extracellular matrix at the earlier time points of gestation (weeks 8 and 13, Fig. 2j, and k). During weeks 16–19, GDF6 expression in the vertebral bodies was only found in the surrounding hypertrophic chondrocytes (Fig. 2l, arrows) and diminished as the ossifying center expanded and developed into fully formed bone (Fig. 2l). A schematic diagram was made to show the organization of the spinal column and associated GDF6 expression at each stage of development (Fig. 3).
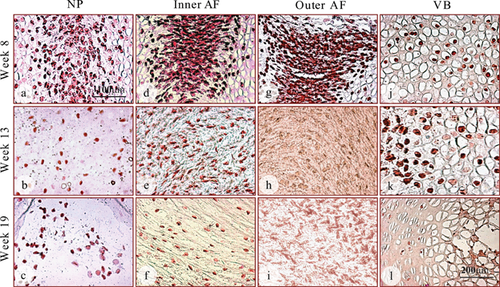
The distribution of GDF6 detected by immunohistochemistry was further confirmed using in situ hybridization with an antisense mRNA transcript probe (Fig. 4). GDF6 mRNA expression was detected inside cells of the nucleus, inner annulus, and vertebrae of all fetal spines examined. Surprisingly, GDF6 mRNA expression was also detected in the outer annulus at the earliest time point (week 8; Fig. 4g), where no GDF6 protein had been detected (Fig. 2g). The discrepancy is possibly due to the GDF6 protein level in the outer annulus being below the detectable level of the antibody. At later time points, however, GDF6 mRNA expression vanished in the outer annulus (Fig. 4h, and i), mirroring the lack of GDF6 immuno-staining in these tissues (Fig. 2h, and i). In the vertebral bodies, GDF6 mRNA expression was not detected in the ossifying centers, but was restricted to the peripheral hypertrophic chondrocytes (Fig. 4j, k, and l), especially observed in the tissue at 19 weeks (Fig. 4l). As negative controls, GDF6 mRNA expression was not detected in any of the fetal spines when “sense” mRNA transcripts were used or omission of probes (data not shown).
Expression of Collagens in the Developing Human Spinal Column
In order to further understand how GDF6 expression in the developing spine integrates with other molecular components, the distribution, and expression of collagens were evaluated using polarized light microscopy and immunohistochemical localization of collagen I and II (Fig. 5–6). Unevenly distributed bright collagen fibers were clearly visible throughout the cartilaginous vertebral rudiment and developing disc from weeks 8 to 19 when viewed under polarized light (Fig. 5a–c, arrows). Bright collagen bundles appeared to have a random arrangement in the nucleus (Fig. 5a–f). Well organized and parallel collagen lamellae were apparent in the annulus with less light intensity at the earlier time point (week 8, Fig. 5g), increasing in intensity by 13 and 19 weeks of gestation (Fig. 5h, and i). The collagen fibers were dense with an apparently uneven distribution and irregular organization through the spine vertebral rudiment (Fig. 5a–c and j–l). Fibers were at their most dense in the center of the vertebral bodies, with reduced density of collagen at the outer edges of the vertebral bodies (Fig. 5a–c and j–l).
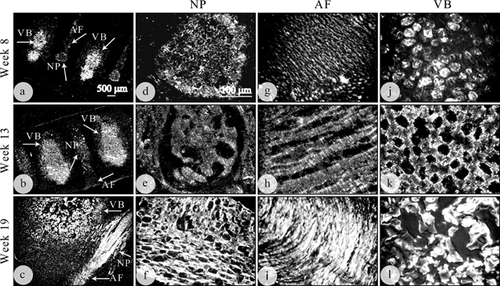
Collagen II was specifically expressed in a decreasing gradient from the center of the developing disc outwards, with strongest staining in the nucleus (Fig. 6a–c), weak staining in the inner annulus (data not shown) and undetectable staining in the outer annulus (Fig. 6d, e, and f). The human fetal spine is largely cartilaginous in the early weeks of development. Strong expression of collagen II was visible in the cartilaginous vertebral bodies at the earlier time points (8–13 weeks; Fig. 6g, and h) prior to ossification at the later stages, where expression was limited to the hypertrophic chondrocytes surrounding the ossifying vertebral bodies (week 19; Fig. 6i, arrow). Collagen I was detectable in all areas of the developing spine, but expressed most strongly in the outer annulus at all time points, in a gradient fashion across the inner annulus to the outer annulus (data not shown).
Proteoglycan Production in the Developing Human Spinal Column
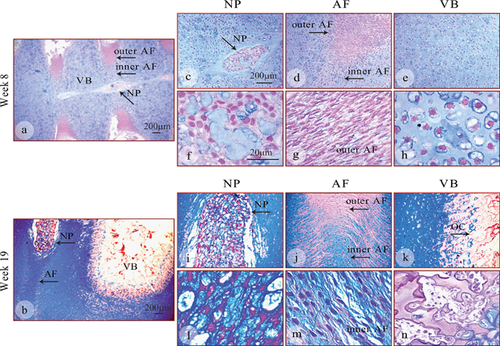
The proteoglycans (PGs) was detected using histological (alcian blue, Fig. 7) and, immunohistochemical (specifically aggrecan) (Fig. 8) staining. In the early specimens (8 weeks, Fig. 7a and c–h), the vertebral bodies (Fig. 7e and h) were primarily cartilaginous, and showed strong coloration, indicating proteoglycan rich extracellular matrix. The outer annulus was almost entirely negative at 8 weeks (Fig. 7a, d, and g, arrows), whilst PGs were a major component of the nucleus (Fig. 7a, c, and f, arrows) and inner annulus (Fig. 7d, arrow) at this stage of development. In 19 week specimen (Fig. 7b and i–n), strong blue staining was detected in the nucleus (Fig. 7b, i, and l, arrows), with an increased number of blue staining nuclei showing the presence of an increased number of PG-producing cells. Similarly, in the annulus (Fig. 7b, j, and m, arrows), PGs could be readily detected at 19 weeks and were strongly expressed in the inner annulus, decreasing towards the outer annulus (Fig. 7b, and j). At the edge of the ossifying vertebral bodies, blue PG staining was visible near the hypertrophic chondrocytes which rapidly produce extracellular matrix (Fig. 7b and k). The innermost part of the ossifying center (Fig. 7k and n, arrow) was pink (negative staining), indicating deposition of bone matrix by osteoblasts and the absence of PG synthesis.
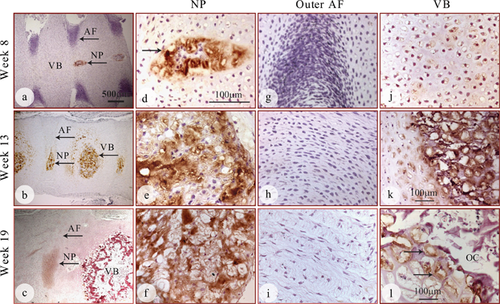
Immunohistochemical staining showed strong positive expression of aggrecan in both the cells and extracellular matrix in the nucleus (Fig. 8a–f, arrows) while the outer annulus was largely negative at all stages (Fig. 8a–c and g–I, arrows). In the vertebral bodies, aggrecan expression was weak in both the cells and the extracellular matrix at week 8 (Fig. 8j), but increased at week 13 (Fig. 8k, arrow) and decreased at week 19 (Fig 8l). Specifically, aggrecan expression was only found in chondrocytes surrounding the vertebral bodies at 19 weeks of gestation (Fig. 8l, arrows). The innermost part of the ossifying center, where bone deposition occurred, was largely devoid of aggrecan expression (Fig. 8 c and l).
DISCUSSION
The identification of mutations in the GDF6 gene in Klippel–Feil syndrome characterized by the bony fusion of cervical vertebrae suggests an important role of GDF6 in vertebral segmentation.15 The normal GDF6 expression pattern has not yet been established in the developing spine, although expression was detected in the adult human IVD22 and in hypertrophic chondrocytes of developing long bones in human fetal tissue.11 In the present study, the expression pattern of GDF6 in the developing human fetal spine was identified for the first time, especially in the early period of vertebral ossification and IVD formation. GDF6 expression was present in cartilaginous tissue, but absent in the endochondral ossification center that was surrounded by GDF6-expresssing hypertrophic chondrocytes. At the cellular level, GDF6 was expressed strongly in notochordal cells, suggesting its involvement in notochord mediated signal networks. Correspondingly, the expression of key extracellular matrix proteins, characteristic of chondrocytic cells, at both protein, and mRNA levels, was found to coincide with GDF6 expression.
In this study, fetal tissues were selected from week 8 to week 19 of gestation in order to observe the relationship of GDF6 expression pattern to vertebral ossification as well as to IVD development. Fetal tissue at week 8 is at the end of the embryonic period and the cartilaginous vertebral column has just formed, to be followed by the establishment of the primary ossification center. Our results showed that the highest GDF6 expression was present in the earliest samples in vertebral development. GDF6 expression was strongest at 8–13 weeks in developing vertebral bodies and the primitive nucleus pulposus. At this stage, vertebral bodies are predominately cartilaginous, and the ossification center is forming. At 16–19 weeks the formation of ossifying centers, which is obvious in the vertebral bodies at the later gestational time points, was accompanied by diminished expression of GDF6 and ongoing expression was detected mainly in the cartilaginous area. Actually, the GDF6 expression retreated gradually from the ossification center to the surrounding hypertrophic chondrocytes, where it could be observed as a boundary line of GDF6 expression by immunohistochemical staining. It is not clear why GDF6 expression was sharply decreased in chondrocytic cells at the rear of the boundary. It may be that GDF6 is a critical factor for holding the boundary between ossifying and cartilaginous tissues to prevent overgrowth of bone. Diminishing GDF6 expression in the ossifying centers suggests that absence of GDF6 is indispensable to endochondral ossification. Our findings, from a different angle, were supported by previous reports that the expression of GDF6 may retard or inhibit osteogenic differentiation.18, 23 In GDF6 knockout mice, the absence of GDF6 caused a “loss of boundary” manifesting as improper ossification at the border between the parietal and frontal bones of the skull, leading to a failure of correct coronal suture development.23
In the developing IVD, the GDF6 expression level was unevenly distributed, being strong in the cartilaginous nucleus pulposus, weak in the fibro-cartilaginous inner annulus fibrosus, and clearly absent in the fibrous outer annulus. GDF6 expression was not only found inside the cells, but was also detected in the extracellular matrix, indicating the expected secretion of GDF6 into the surrounding matrix. While a number of systemic and local factors have been identified playing important roles in specific stages of endochondral ossification,24 the present study suggests that GDF6 may be one of the critical signaling molecules in the network regulating vertebral column development.
The influence of GDF6 on expression of, for example, L-Sox5, Sox6, or SHH are not yet known. GDF6 is found in the notochord and can influence extracellular matrix production so could conceivably regulate L-Sox5/Sox6 expression, as has been demonstrated for key chondrogenic transcription factor Sox9.16, 25 The expression pattern of GDF6 also implies that it may be involved in determining the site and formation of bone tissue boundaries. Initially established by the interactions between the Notch, Wnt, and SHH signaling pathways, the underlying mechanisms of tissue boundary delineation remain poorly understood.3, 26, 27 The involvement of connective tissue growth factor (CTGF/CCN2) in coordination of chondrogenesis during skeletal development and its association with hypertrophic zones may suggest potential interactions with GDF6. CTGF/CCN2 promotes extracellular matrix protein production26 and shows activity in preventing bone formation by negative regulation of BMP-2 signaling during osteoblast differentiation.27 Hypothetically, CTGF/CCN2 may be involved in or positively regulate GDF6 activity during IVD development. This study showed the presence of large notochordal cells in the central region of the primitive disc at 8–13 weeks of development, with a gradual reduction of notochordal cell numbers at 17–19 weeks, consistent with previous reports.28 The notochordal cells expressed GDF6 protein and mRNA and secreted a matrix composed of proteoglycans and type II collagen. While there remains some uncertainty, the notochordal cells do play a central role in initiating nucleus formation and vertebral segmentation.29, 30 Notochordal cells can upregulate proteoglycan production in disc chondrocytes31 and protect nucleus cells from IL-1ß induced cellular apoptosis.32 In the human spine, notochordal cells gradually disappear within a decade of life and are replaced by chondrocyte-like cells.33 Their disappearance is suggested to contribute to IVD matrix changes and the decline in the cellularity of the disc during aging. The localization of GDF6 in notochordal cells raises the possibility of a role for GDF6 in their regulation and maintenance, or even their commitment to the chondrogenic lineage.
GDF6 expression was closely associated with the distribution of characteristic chondrocytic markers collagen II and proteoglycans including aggrecan during disc development. Collagen I protein was also distributed widely in the developing disc, but not in regions of high GDF6 expression. Rather, strong collagen I expression was localized to the outermost region of the annulus, which lacked GDF6 expression. These data are consistent with previous studies showing that GDF6 can promote extracellular matrix synthesis typical of tendon, cartilage, or the IVD in vivo19, 34and in vitro35-37 The co-localization of GDF6 and extracellular matrix molecule expression is in keeping with a potential role of GDF6 in promoting and maintaining chondrocytic morphogenesis during human IVD development and homeostasis. The current study was exclusively focused on the GDF6 expression pattern in the early stage of vertebral column development, with methodology limited to histological analyses. Although our results can be interpreted qualitatively, there are a lack of quantifiable data and relatively small size of samples. These limitations are mainly due to the difficulties in obtaining human fetal specimens and strict ethical guidelines.
In summary, this study evaluated the expression pattern of GDF6 in the developing human spine relative to vertebral ossification and extracellular matrix synthesis, highlighting the potential role of GDF6 in the delineation of tissue boundaries and phenotype development of intervertebral discs. The findings in this study support the proposal that GDF6 has an important regulatory role in ossification vs chondrogenesis during vertebral segmentation and spine development. Our data will reinforce previous research findings from our and other laboratories to demonstrate that GDF6 has more chondrogenic characteristics than other clinically used BMP members. Further studies are warranted on the functional involvement of GDF6 in fetal vertebral column development to understand better the molecular basis underpinning vertebral or other bone and joint congenital malformations.
AUTHOR' CONTRIBUTIONS
Study design: AW, LW, DB and AD. Study conduct: AW and SP. Data collection: AW, ZF, TG, SP and DB. Data analysis: AW, LW, BS and TG. Data interpretation: AW, BS, LW and DB. Drafting manuscript: AW, BS, LW and AD. AD takes responsibility for the integrity of the data analysis. All authors have read and approved the final submitted manuscript.
ACKNOWLEDGMENTS
The authors would like to thank Spine Service, St. George Hospital, and Orthopedic Research Institute, Sydney, Australia for support and funding of this study.




