Cosmos white rot: First characterization, physiology, host range, disease resistance, and chemical control
Abstract
A new disease of Cosmos sulphureus Cav. causing external and internal stem discoloration, premature death, and wilting was observed in 27.8% of plants with an average disease severity rating of 4.4 in Gazipur, Bangladesh. Morphological, pathological, and molecular analyses identified the isolated fungus as Sclerotinia sclerotiorum (Lib) de Bary, the causative agent of white rot disease. The optimum growth and sclerotium formation of S. sclerotiorum occurred at 20°C and pH 5.0, while glucose, peptone, yeast extract, casein, and ascorbic acid were the appropriate nutrient sources. Furthermore, mycelial growth and sclerotial development were favored in media containing potassium, magnesium, calcium, and sodium. As many as 20 plant species of 10 families; Calendula officinalisi, Chrysanthemum indicum, Catharanthus roseus, Solanum tuberosum, S. lycopersicum, S. melongena, Capsicum annum, Lablab purpureus, Phaseolus vulgari, Lens culinaris, Vigna radiata, Vigna mungo, Daucus carota, Raphanus sativus, Brassica juncea, Punica granatum, Spinacia oleracea, Ipomoea batatas, Ipomoea aquatica, and Elaeocarpus serratus were identified as the new hosts of the pathogen in Bangladesh. None of the C. sulphureus and Cosmos bipinnatus germplasms screened were genetically resistant to the pathogen. Among the tested fungicides, Autostin 50 WDG (carbendazim) and Rovral (Dicarboxamide) were most inhibitory to the fungus, while Autostin 50 WDG provided an efficient control of the pathogen in vivo up to 15 days after spray. The acquired results on characterization, physiology, host range, resistance, and fungicidal control of the pathogen could be valuable for effectively managing cosmos white rot in the field.
Abbreviations
-
- BSMRAU
-
- Bangabandhu Sheikh Mujibur Rahman Agricultural University
-
- CAZymes
-
- carbohydrate-active enzymes
-
- CB
-
- Cosmos bipinnatus
-
- CS
-
- Cosmos sulphureus
-
- CSP
-
- Cosmos Sclerotinia Pathogen
-
- HR
-
- highly resistant
-
- HRT
-
- Department of Horticulture
-
- HS
-
- highly susceptible
-
- ITS
-
- internal transcribed spacer
-
- LSD
-
- least significant difference test
-
- MBC
-
- methyl-2-benzimidazole carbamate
-
- MR
-
- moderately resistant
-
- MS
-
- moderately susceptible
-
- PCR
-
- polymerase chain reaction
-
- PDA
-
- Potato dextrose agar
-
- R
-
- resistant
-
- S
-
- susceptible (S)
1 INTRODUCTION
Cosmos came from the Greek word “Kosmos,” which means adorn, ornament, and beautiful. The medium-sized flowering herbaceous plant is commonly called the garden cosmos or Mexican aster (family Asteraceae). It originated in Latin America and later spread throughout Europe, Asia, and Africa [1]. More than 20 annual and perennial species of Cosmos exist. However, two annual species Cosmos bipinnatus (Mexican aster) and Cosmos sulphureus (yellow cosmos) are primarily grown in most ornamental gardens across the globe [2]. In Bangladesh, C. sulphureus is commonly used in the winter garden, while C. bipinnatus is mainly grown during fall. C. sulphureus is shorter, having a more compact floret than C. bipinnatus. Between these two species, C. sulphureus has numerous popular cultivars in the international horticultural trade, resulting in its comprehensive and intentional spread by humans [3]. The species name “sulphureus” is in reference to the orange–yellow colors of the flower. In parts of Africa, Asia, North and Central America, and the Pacific Islands, it is recorded as an environmental weed and occasionally an invasive plant [3]. In Asia, C. sulphureus was first introduced as an ornamental plant [4]. Under favorable environmental conditions, it produces flowers in winter and seeds in the spring [4]. Besides its ornamental value, it has diverse traditional uses, such as food, for health benefits, as a natural dye, or even as a natural bioherbicide [4, 5]. Therefore, it could be considered an economically important ornamental flower worldwide.
The exact time (year) of introducing commercial cultivation of the cosmos in Bangladesh is unknown. However, cosmos plants have become essential elements in winter home yards, institutional gardens, and roadsides decoration presently. Its cultivation usually starts in October–November and remains in the garden until February–March. According to reports from various countries, diseases such as wilts, powdery mildew, Botrytis blight, canker, aster yellows, and white smut are reported to attack the cosmos reports from various countries [6]. However, cosmos diseases remain unreported in Bangladesh, except for the cucumber mosaic virus [7]. In 2013–2014, a new disease with specific symptoms of stem damage and premature plant death of C. bipinnatus was observed sporadically in different parts such as Jessore, Dhaka, Bogura of Bangladesh (approximately to the author's knowledge). However, the disease appeared in severe forms with substantial plant damage in different C. sulphureus winter gardens in Gazipur district in January 2018. Since then, the disease has become a constant presence with severe prevalence in C. sulphureus gardens during January–February within this geographic area. Therefore, an initiative was taken to identify and characterize the causal organism and possible remedies for the disease. All the visible signs and symptoms of the disease indicate the possibility of the suspected pathogen as one of the sclerotia forming species such as Sclerotinia sclerotiorum.
S. sclerotiorum is a filamentous phytopathogenic Ascomycete fungus with broad ecological distributions, infecting more than 500 plants such as beans, okra, potato, sunflower, soybean, marigold, pea, jackfruit, and different flower species [8-14]. The crop losses to the disease can range up to 50% [11]. The pathogen prefers cool, wet environments and can survive as sclerotia in the soil for several years after being introduced to a field [12]. The sclerotia can either directly germinate to form mycelium or produce apothecia and ascospores, which can be dispersed, land on a host plant, and cause massive infection to its hosts [13]. As a result, the management of S. sclerotiorum rot becomes very challenging.
As the disease is new to the cosmos in Bangladesh, data regarding the accurate identification of the pathogen, its physiology, resistance level in cosmos cultivars, host range, and potential fungicides have been lacking. Thus, the present study was undertaken to isolate and identify the S. sclerotiorum causing white rot in the cosmos and examine the suitable conditions and nutritional requirements for its optimum growth and sclerotia formation. Further research was conducted to determine the host range, explore effective sources of resistance, and select the effective fungicides for controlling the pathogen.
2 MATERIALS AND METHODS
2.1 Disease survey, symptoms study, and sample collection
A survey was conducted for the newly observed cosmos disease in Bangabandhu Sheikh Mujibur Rahman Agricultural Unviersity (BSMRAU), Gazipur, and its vicinity areas from 27 January to 8 February 2018. Four hundred and sixty-four cosmos plants from 10 cosmos gardens were randomly surveyed for suspected disease symptoms. Cosmos plants were mostly flowering to the late growth stage. Disease incidence was assessed as the percentage of symptomatic plants compared to the total number of surveyed plants. Severity was calculated following a scale of 0–5, where 0 = no symptoms, 1 = up to 10%, 2 = 11%–35%, 3 = 36%–65%, 4 = 66%–90%, and 5 = 91%–100% of the foliage affected by the pathogen [15]. The sclerotia developed inside the diseased tissues were collected by splitting open the stems and assessed for morphological characteristics. Diseased samples from 10 spots were collected and transported to the laboratory.
2.2 Isolation and preservation of the pathogen
Following preliminary washing with tap water, stems were dried on sterile blotter paper and then cut into 1–2 cm pieces. Dark-colored sclerotia were also collected from the infected stem. Infected tissue pieces and sclerotia were surface-sterilized in 70% ethanol for 2 min, then rinsed in sterile distilled water three times and dried on blotter paper. Later, tissue pieces and sclerotia were plated in Petri dishes containing potato dextrose agar (PDA) and incubated at laboratory temperature (25 ± 2°C) in the dark. Emerging mycelial colonies were transferred to new PDA plates. Ten pure isolates were obtained by hyphal tip isolation and marked as Isolate CSP1 (Cosmos Sclerotinia Pathogen 1) to CSP10. Pure isolates were cultured on PDA slants and preserved at 4°C.
2.3 Cultural and morphological characterization of the pathogen
The pathogen was initially identified based on the emerging colony, and morphological characters and their similarities with the previous reference works in the same laboratory [9, 10, 14, 15]. The pure mycelial isolates were transferred onto PDA plates and cultured at 25 ± 2°C in the dark for a week to study the colony characteristics. Permanent slides were prepared from the colony and examined under a light microscope to observe the fungal structures. These plates continued to incubate for 15 days to study sclerotial characteristics.
2.4 In planta pathogenicity test
Since all isolates of suspected pathogen appeared identical in colony morphology, a pathogenicity test was conducted with three randomly selected isolates (CSP1, CSP3, and CSP8) on the cosmos variety C. sulfurous. Seeds were surface-sterilized in 70% ethanol solution as described above and sown in seed trays (25.4 × 12 cm) filled with sterile soil. After germination, 2-week-old seedlings were transferred to earthen pots (12 × 12 cm) filled with sterile soil and grown in a net house under natural light and temperature conditions. Five-day-old actively growing fungal plates were used for inoculation. Three 4-week-old plants were inoculated with each isolate separately, and three plants were included as a control. Plants were wounded at the stem bark with a sterile blade above 10 cm of the soil surface (one wound per seedling) and inoculated with a 5 mm PDA plug. The inoculated stems were sealed with moistened cheesecloth. The seedlings were then covered with polybags and incubated in a dark, humid chamber for 48 h at 22 ± 1°C. After incubation, seedlings were shifted to the net house again. Seedlings were monitored daily for 20 days until the disease was developed. The suspected pathogen was reisolated from symptomatic tissues in three independent experiments to satisfy Koch's postulates.
2.5 Characterization of apothecia and ascospores of the pathogen
The isolated fungus was investigated to be a phytopathogenic member of the Sclerotinia genus by its ability to produce discal apothecia and eight binucleate ascospores. Fifty sclerotia were collected from three culture plates (CSP1, CSP3, and CSP8) to induce and study the apothecia. All sclerotia were surface sterilized with 1% NaOCl for 2 min, rinsed with sterilized distilled water two times, and air-dried. Five 9 cm sterile Petri dishes were filled with wet sterile sand. Surface sterilized sclerotia were placed inside the sand (10 sclerotia/plates) and incubated at 4°C in the dark for 6 weeks. The Petri dishes having germinating sclerotia were transferred to another incubator at 20°C under scattered fluorescent irradiation (260 μmol/m2/s) until apothecial discs were developed [15]. The morphological features of apothecia and ascospores were examined under a light microscope (40× and 100×). Fifteen apothecia were closely studied for morphological traits.
2.6 Molecular identification of S. sclerotiorum isolate CSP1 and CSP8
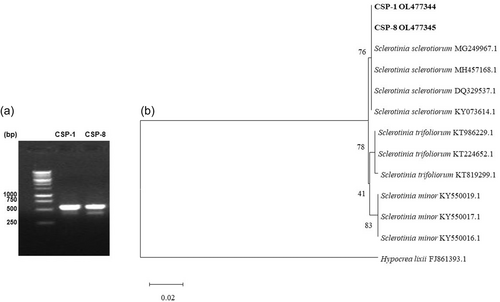
On the basis of the pathogenicity test results, one highly pathogenic isolate (CSP1) and one less virulent strain (CSP8) were chosen for molecular identification. Total genomic DNA was extracted from the mycelia of S. sclerotiorum isolate CSP1 and CSP8 as described by Islam et al. [15]. Polymerase chain reaction (PCR) was conducted with forward primer ITS-1 (5ʹ-TCCGTAGGTGAACCTGCG G-3ʹ) and reverse primer ITS-4 (5ʹ-TCCTCCGCTTATT GATATGC-3ʹ) to amplify rDNA-ITS (Internal Transcribed Spacer) regions of the fungal isolates. Two complete sequences were obtained for each ITS region, and the BLAST search program was used to search for nucleotide sequence homology in GenBank. Highly homologous sequences were aligned using Clustal-X version 2.0.11 and manually adjusted as required. Neighbor-joining trees were generated using the MEGA X version. Bootstrap replication (1000 replications) was used as statistical support for the nodes in the phylogenetic trees. Based on maximum sequence homology percentage, query coverage, and the lowest E-value, ITS sequence data of four archived isolates of S. sclerotiorum retrieved from GenBank were selected for phylogenetic analysis. Additionally, sequences of three isolates of S. minor and S. trifoliorum were included as references, with Hypocrea lixii serving as an outgroup taxon (GenBank accession no. FJ 861393.1).
2.7 Mycelial growth and sclerotia formation of S. sclerotiorum at different temperature and pH regimes
To determine the optimum growing temperature and pH of the isolated pathogen, the effect of various temperature and pH regimes on mycelial growth and sclerotia formation of S. sclerotiorum isolate CSP1 (most virulent isolate was used) was evaluated in three independent trials. PDA media were prepared and inoculated at the center with a 5 mm diameter mycelium plug excised from a 5-day-old S. sclerotiorum culture. The culture plates were incubated at 5, 10, 15, 20, 25, 30, and 35°C. Likewise, the effect of pH on growth and sclerotia formation of S. sclerotiorum isolate CSP1 was evaluated by growing the fungus at acidic, neutral, and alkaline pH. PDA media were prepared in 9-cm-diameter Petri plates and adjusted their pH to 5.0, 7.0, and 9.0 using 0.1 N HCl and NaOH. Petri plates were inoculated from a 5-day-old S. sclerotiorum culture and incubated at 20°C in the dark. There were three replicates for each temperature and pH range separately, and each replicate consisted of three Petri plates. The mycelial growth of the fungus was measured at 24, 48, 72, and 96 h postincubation. The growth of the cultures was continued in the incubator for 15 days to obtain the maximum yield of sclerotia. After 15 days, the number and weight of sclerotia were measured.
2.8 Nutritional requirements for growth and sclerotia formation of S. sclerotiorum
2.8.1 Effect of different carbon and nitrogen sources
Mycelium growth of S. sclerotiorum was monitored by growing it on different carbon and nitrogen sources. Four carbon sources such as sucrose, mannitol, dextrose, and soluble starch; and seven nitrogen sources such as peptone, glycine, arginine, tyrosine, caesine, albumin, and tryptophan were used to assess the nutritional requirement separately. The minimal medium (nitrogen source 4.0 g/L, carbon source 20 g/L, MgSO4 0.5 g/L, KH2SO4 1.0 g/L, and agar 20 g/L) was prepared by supplementing with the selected carbon and nitrogen sources. When carbon source minimal media were prepared, peptone was used as the unique nitrogen source. Similarly, glucose was used as the unique carbon source in different nitrogen source minimal media. The initial pH was adjusted to 5 as per 2.7. There were three replications per carbon and nitrogen source, and each replication consisted of three 9-cm-diameter Petri plates. The mycelial plug inoculation, culture plate incubation, and data collection were conducted as per 2.7.
2.8.2 Effect of minerals and vitamins
Two different minimal media were prepared for testing the effect of mineral and vitamin sources separately. The first minimal medium (pepton 4.0 g/L, glucose 20 g/L, mineral 0.5–1.0 g/L, KH2SO4 1.0 g/L, and agar 20 g/L) was prepared by incorporating FeSO4, molybdenum, ZnSO4, CuSO4, or MgSO4 at the rate of 0.3 g/L or KH2PO4, NaCl, CaCl2, or MnSO4 at the rate of 1.0 g/L as the mineral source. For testing the effect of vitamin sources, a second minimal medium (pepton 4.0 g, glucose 20 g, MgSO4 0.5 g, KH2SO4 1.0 g, vitamin 0.05 g, and agar 20 g per liter) was prepared by supplementing vitamin with either riboflavin, ascorbic acids, thiamine, biotin or nicotinic acid (0.05 g). The replication, mycelial plug inoculation, incubation, and data collection were conducted as per 2.8.1.
2.9 Determination of host range of S. sclerotiorum isolate CSP1
By screening against the most virulent strain, the broader host range of the fungus was determined. Twenty-five seasonal and perennial horticultural plants which had not been reported as hosts of the pathogen previously from Bangladesh were inoculated with S. sclerotiorum isolate CSP1. Seeds of seasonal plants were collected from the Horticultural Research Center, Bangladesh Agricultural Research Institute, Gazipur, Bangladesh, and sown in plastic pots (12 × 12 cm) filled with sterile field soil. Five plants were grown for each genotype, allocating one plant per pot in a net house under natural light and temperature conditions. When plants were at 4-week-old, one fully expanded leaflet from each plant was cut and rinsed with sterile distilled water. The leaflets were dried between the paper towels and placed on water-soaked three-layered blotter papers in 9-cm-diameter Petri Dishes. For perennial plants, fully expanded, healthy-looking leaves were collected from the established plants in the Horticultural Garden of BSMRAU. Five leaves were prepared for each host in the same way as described for seasonal plants. A 3 mm mycelial plug of the mentioned 5-day-old isolate was placed on each leaf. The inoculated leaves were kept in the dark at 20°C for 48 h. Additional leaves were inoculated with autoclaved PDA plugs as controls. Then inoculated leaves were transferred to a growth chamber at a photoperiod of 14 h/10 h light/dark and 21°C. Five days after the pathogen challenge, disease severity was estimated by measuring the percent area diseased on the leaves.
2.10 Screening of cosmos genotypes for resistance against S. sclerotiorum isolate CSP1
A study was conducted to determine the resistance of the available cosmos genotypes in Bangladesh against the disease using a detached leaf assay. Seven genotypes were screened, four from C. sulphureus and three from C. bipinnatus. Seeds collected from the Department of Horticulture, BSMRAU, Gazipur, Bangladesh, were surface-sterilized in 1% NaOCl solution and sown in seed trays (25.4 × 12 cm) filled with sterile field soil. Two weeks after germination, seedlings were transferred to earthen pots (12 × 12 cm) filled with sterile field soil and grown for 4–5 weeks under natural light and temperature conditions in a net house. There were nine plants for each genotype. One fully expanded leaflet was cut from each plant, rinsed with sterile distilled water, dried between the paper towels, and placed on three-layered water-soaked blotter papers in 9-cm-diameter Petri Dishes. A mycelial plug of 3-mm diameter excised from the edge of the fresh culture of S. sclerotiorum CSP1 was placed on the center of the midrib of each leaflet. The inoculated leaves were kept in the dark at 20°C for 48 h. The same number of leaves inoculated with autoclaved PDA plugs served as controls. Then inoculated leaves were transferred to a growth chamber at a photoperiod of 14 h/10 h light/dark and 21°C. Five days after the pathogen challenge, disease severity was estimated by measuring the percent area diseased on the leaves. Based on disease severity, each genotype was further categorized as highly resistant (HR)—0.00%–0.99%, resistant (R)—1.00%–9.99%, moderately resistant (MR)—10.00%–19.99%, moderately susceptible (MS)—20.00%–29.99%, susceptible (S)—30.00%–39.99%, and highly susceptible (HS)— ≥40.00%.
2.11 In vitro chemical control of S. sclerotiorum CSP1
| Trade name | Active ingredient | Fungicide group | Mode of action | Supplier |
|---|---|---|---|---|
| Autostin 50 WDG | Carbendazim | Benzimidazole (systemic) | Interfere the DNA biosynthesis during fungal cell division, arrest cell cycle, and induce apoptosis | Auto Crop Care Ltd., Dhaka, Bangladesh |
| Amistar Top | Azoxystrobin + Difenoconazole | Quinone outside Inhibitors + Triazole (systemic) | Inhibits spore germination at the early stage of fungal development | Syngenta Bangladesh Ltd., Dhaka, Bangladesh |
| Dithane M-45 | Mancozeb | Dithiocarbamate (contact) | Inhibits metal-dependent and sulfhydryl enzyme systems in fungi | Bayer Crop Science Ltd., Dhaka, Bangladesh |
| Rovral | Iprodione | Dicarboxamide (contact) | Inhibits DNA and RNA synthesis in the germinating fungal spore, inhibit mycelium growth | Bayer Crop Science Ltd., Dhaka, Bangladesh |
| Ridomil Gold MZ | Metalaxyl + Mancozeb | Acylalanine (systemic) | Interfere DNA synthesis, inhibit mycelial growth, and spore/haustoria formation | Syngenta Bangladesh Ltd., Dhaka, Bangladesh |
2.12 In planta chemical control of S. sclerotiorum CSP1
2.13 Experiment design and data analysis
The experimental design was completely randomized, consisting of three to five replications. The experiment was conducted at least three times. The statistics 10 program package was used for statistical analysis, and treatments were compared via analysis of variance ANOVA using the least significant difference test (LSD) at a 5% (p ≤ 0.05) probability level. Data were transformed using the arcsine transformation method when it was necessary.
3 RESULTS
3.1 Symptom study and detection of the disease
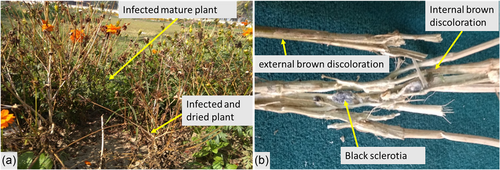
Out of 464 plants examined, 129 were recorded with the suspected disease symptom. In severely infected plants, leaves were dark brown and dried. Some of the infected plants were at the mature stage but yet to be senesced, whereas some of them died prematurely and showed a typical wilting symptom (Figure 1a). The disease incidence was 27.80%, with an average disease severity rating of 4.46. Both internal and external large brown to tan areas with necrotic tissues were observed in the infected stems (Figure 1b). White cottony mycelia and large black sclerotia were also detected along the stem pith when splitting the infected stem longitudinally. The sclerotia were round to irregular in shape and measured 3.0–9.8 × 1.0–6.0 mm in size (Figure 1b).
3.2 Isolation, morphological characterization, and preservation of the pathogen
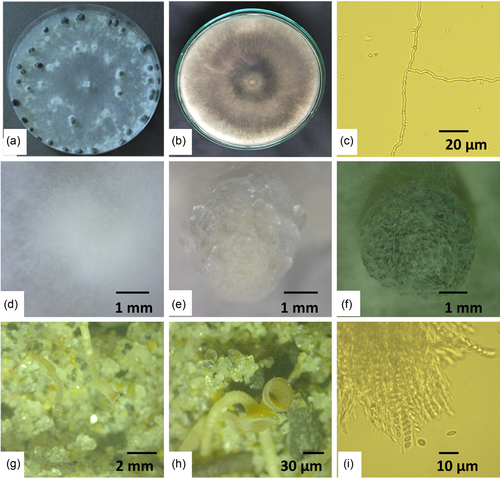
On PDA plates, all 10 isolates produced identical mycelial colonies that were fast-growing with white aerial thin mycelia but relatively thick at the colony margin (Figure 2a). Globose to cylindrical sclerotia were produced at the growing margins of all colonies after 10 days, forming concentric rings and radiating lines (Figure 1a). The reverse colony showed a dark gray color (Figure 2b). The hyphae were hyaline, branched, and multinucleate under the microscope (Figure 2c). No conidia or conidiophores were observed. As is typical with sclerotia-producing fungus, there were three distinct phases of sclerotial development. The initiation stage occurred with whitish aggregation of mycelia at 5 days post culture (Figure 2d); the development stage turned the whitish aggregates into beige color at 7–10 days post culture (Figure 2e), and the maturation stage took place after 10 days of incubation when the sclerotia turned from white to dark color (Figure 2f). Sclerotia had a black outer rind and a white inner cortex and developed in various numbers, forms, and sizes. The number and weight of sclerotia produced per PDA plate ranged from 6 to 40 and 6.38 to 50.81 mg, respectively. The mean length and width of 50 sclerotia were 5.4 and 4.6 mm, respectively. Individual sclerotium measured up to 9 mm long and 6 mm wide. After 4 weeks of incubation, sclerotia germinated and gave rise to white gray stripes, forming receptacles and producing apothecia in 5–6 weeks (Figure 2g,h). A single sclerotium produced up to four apothecia. Initially, apothecia were white to gray, hollow with a thick rim (Figure 2g), and then progressively differentially into a saucer shape or a cup or funnel shape at full maturity (Figure 2h). The mature apothecia had a diameter of 4–6 mm and a stripe length of 8–19 mm (Figure 2h). A thin slice of apothecia was also observed under a microscope for detailed study. The hymenial layer was full of paraphyses and asci (Figure 2i). Asci were cylindrical with a tapered base. Each ascus had eight ascospores (Figure 2i). Ascospores were uniseriate, hyaline, one-celled, smooth, ellipsoid, and uniform in size (Figure 2i).
3.3 In planta pathogenicity tests
Pathogenicity tests were performed on three of the 10 isolates, CSP1, CSP3, and CSP8 of the suspected pathogen. In the pathogenicity assays, all three isolates caused an infection on inoculated plants. Isolate CSP1 appeared more virulent than CSP3 and CSP8, causing infection rapidly in a greater proportion. The disease started with water-soaked and light tan lesions around the inoculation point by 72–96 h postinoculation, depending on the isolates. Later the lesions progressed both up and downward in the stem and created a distinct necrotic lesion within 5–7 days of inoculation. Eleven to fifteen days after inoculation, all inoculated plants wilted and finally died. Whitish mycelium and prominent sclerotia of S. sclerotiorum were visible inside the infected and dead stems. Whitish mycelia and sclerotia also emerged from collapsed tissues when kept in vitro moist chambers in the laboratory. Mycelia were transferred to the PDA plates, and new colonies were produced, which appeared identical to those recovered from field-infected plants. No disease symptoms were recorded in noninoculated control plants.
3.4 Molecular identification of the pathogen
The length of ITS sequences of two isolates (CSP1 and CSP8) obtained in this study was 572 bp (Figure 3a). The homology search through the GenBank DNA database revealed a 100% sequence identity with ITS rDNA sequences of several S. sclerotiorum isolates. A phylogenetic tree constructed with isolates of S. sclerotiorum, S. minor, S. trifoliorum, and H. lixii showed that the fungal isolates under study grouped with strains of S. sclerotiorum with strong bootstrap support (Figure 3b). Thus, the candidate fungus was identified as strains of S. sclerotiorum. The nucleotide sequences were deposited to GenBank under accession no. OL477344 and OL477345. There was, however, no distinction between CSP1 and CSP8 as indicated by ITS sequences; hence, the virulent isolate CSP1 was utilized to further characterize fungal physiology, nutritional requirements, host range, disease resistance, and fungicidal control.
3.5 Effect of temperature and pH on mycelial growth and sclerotia formation of S. sclerotiorum
The mycelial growth of S. sclerotiorum CSP1 was significantly affected by the temperature change from 5°C to 35°C. At 5°C, the fungus showed very sparse growth, acquiring only 9 mm radial growth 96 h after incubation. When the temperature increased to 10°C and 15°C, mycelial growth occurred faster, showing two- and threefolds higher growth than 5°C, respectively, 96 h after incubation (Figure 4a). However, increasing the temperature to 20°C resulted in the highest mycelial growth, producing a 40 mm colony diameter 96 h after incubation. Furthermore, an increase in temperature to 25 and 30°C reduced the growth by nearly half (21 mm) and ninefolds, respectively. However, at 35°C, the fungus growth was stopped entirely, and no growth was recorded even 96 h after incubation (Figure 4a).
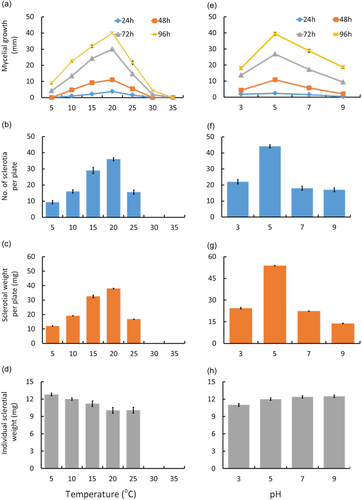
Additionally, the fungus produced sclerotia in a wide range of temperatures. However, sclerotia formation was significantly affected by incubation temperature. The maximum number of sclerotia (36/plate) was formed at 20°C followed by 15°C (33/plate) (Figure 4b). Similarly, the total sclerotial weight per plate (38 mg) was maximum at 20°C (Figure 4c). Temperatures higher or lower than 20°C decreased the sclerotial yield of the fungus, although the reduction was more noticeable at higher temperatures. For this reason, the total sclerotial number and weight sharply declined at 25°C, while no sclerotium was formed when the incubation temperature was at or above 30°C (Figure 4b,c). Yet, individual sclerotial weight was the highest in plates incubated at 5°C (12.8 mg), followed by those at 10°C (12 mg), 15°C (11.2 mg), 25°C (10.7 mg), and 20°C (10.5 mg) (Figure 4d).
The highest mycelial growth was observed at pH 5.0 (Figure 4e). The growth of mycelium declined when pH became higher or lower than 5.0. The maximum reduction in the growth of the pathogen was observed at pH 9.0, while the lowest reduction was at pH 7 (Figure 4e). Similarly, the sclerotia number (44.2) and sclerotial weight (53.8 mg) per plate were the highest at pH 5.0 followed by pH 3.0 (22, 24.4 mg), pH 7.0 (18, 22.4 mg), and pH 9.0 (17, 13.8 mg) (Figure 4f,g). On the other hand, the maximum individual sclerotial weight was observed at pH 7.0 (12.4 mg), indicating that the sclerotial size was greater at pH 7.0 than at pH 5.0 (Figure 4h).
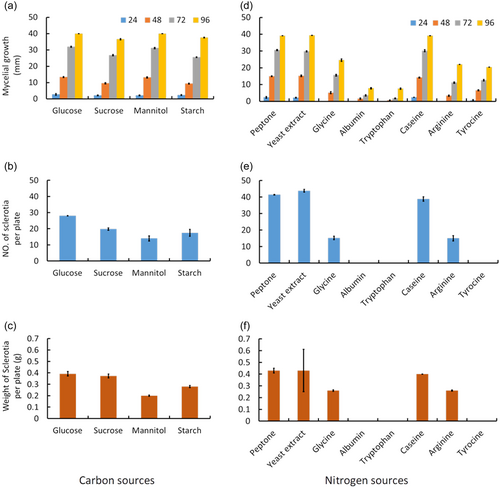
3.6 Effect of different carbon and nitrogen sources
As it was observed 96 h after culture, the highest mycelial growth of S. sclerotiorum CSP1 was recorded in glucose and mannitol (40 mm), followed by sucrose (36.6 mm) and insoluble starch (37.7 mm) (Figure 5a). However, the sclerotial number (28) and sclerotial weight (0.39 g) were recorded highest in glucose media (Figure 5b,c), while the lowest sclerotial number (14) and weight (0.2 g) were observed in mannitol media (Figure 5b,c). Similarly, different nitrogen sources significantly affected the growth and sclerotia formation of the pathogen. Peptone, yeast extract, and casein were the best nitrogen sources for maximum mycelial growth (39.2 to 39.4 mm) of S. sclerotiorum CSP1 (Figure 5d). Tryptophan and albumin gave the lowest mycelial growth (7.6 mm) after 96 h of incubation (Figure 5d). The yeast extract recorded the highest number (43.8) and weight (0.43 g) of sclerotia (Figure 5e,f). The sclerotial formation was inhibited entirely in tyrosine, tryptophan, and albumin media (Figure 5e,f).
3.7 Effect of different vitamin and mineral sources
All the five tested vitamins positively affected mycelial growth and exerted a statistically similar effect on the radial growth of S. sclerotiorum CSP1 96 h after culture (Figure 6a). However, they differed significantly among themselves in their effects on sclerotia formation. A significantly highest number of sclerotia was produced in minimal media amended with ascorbic (35.0) acid, followed by biotin (31.4) (Figure 6b). On the other hand, the lowest number of sclerotia was produced in media amended with thiamine (28.0), which was statistically similar to those produced by Riboflavin (29.0) and nicotinic acid (29.0) (Figure 6b). Similarly, the highest sclerotial weight (0.4 g) was attained in media containing ascorbic acid, and it was statistically similar to those produced by media containing biotin (0.39 g) (Figure 6c). Sclerotial weight was statistically lower in media containing other vitamins (Figure 6c).
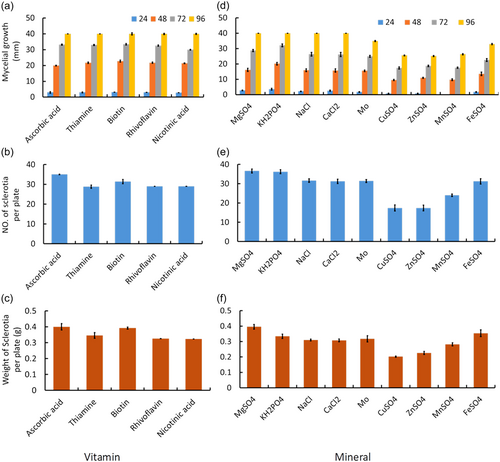
The present experimental results also revealed that significantly highest mycelial growth (40 mm) of S. sclerotiorum was estimated in media containing KH2PO4, MgSO4, NaCl, and CaCl2 96 h after culture (Figure 6d). On the other hand, the mycelial growth of this pathogen was slightly inhibited by CuSO4, ZnSO4, and MnSO4 (26.4 mm) (Figure 6d). The sclerotia formation was supported by all minerals, while the highest number of sclerotia was observed in media containing MgSO4 (36.60) and KH2PO4 (36.20) (Figure 6e). Media containing CuSO4, and ZnSO4, produced the lowest number (17) of sclerotia (Figure 6e). The sclerotial weight was maximum (0.40 g) in media containing MgSO4. However, the minimum sclerotial weight (0.20 g) was recorded in the CuSO4 rich medium, showing twofolds less sclerotial weight than MgSO4 (Figure 6f).
3.8 Determination of host range of S. sclerotiorum isolate CSP1
Regarding pathogenicity tests on various host plants, individual plant species demonstrated differences in susceptibility to infection by S. sclerotiorum isolate CSP1. As many as 20 plant species of 10 families: Calendula officinalisi, Chrysanthemum indicum (Asteraceae), Catharanthus roseus (Apocynaceae) Daucus carota (Apiaceae), Raphanus sativus, Brassica juncea (Brassicaceae), Punica granatum (Lythraceae), Solanum tuberosum, Solanum lycopersicum, Solanum melongena, Capsicum annum (Solanaceae), Lablab purpureus, Phaseolus vulgari, Lens culinaris, Vigna radiata, Vigna mungo (Fabaceae) Spinacia oleracea (Amaranthaceae), Ipomoea batatas, Ipomoea aquatica (Convolvulaceae), and Elaeocarpus serratus (Elaecarpaceae) were severely infected by the pathogen showing a considerable level of disease severity (21%–95%) (Table 2). Cestrum nocturnum (Solanaceae) and Clitoria ternatea (Fabaceae) were also infected by the isolate of the pathogen but developed only a low level of disease (4%–6%). On the other hand, no infection (0%) was caused by the pathogen on three plant species, and thereby, no disease (0.0%) was observed on Ixora coccinea, (Rubiaceae), Quisqualis indica (Combretaceae), and Nerium oleander (Apocynaceae) (Table 2).
| SL | Common name | Scientific name | Family | Disease severity (%)a |
|---|---|---|---|---|
| 1. | Calendula | Calendula officinalisi | Asteraceae | 48.75 ± 3.60 |
| 2. | Chrysanthemum | Chrysanthemum indicum | Asteraceae | 50.25 ± 3.44 |
| 3. | Rose Periwinkle | Catharanthus roseus | Apocynaceae | 28.00 ± 5.37 |
| 4. | Night jessamine | Cestrum nocturnum | Solanaceae | 4.25 ± 1.96 |
| 5. | Jungle flame | Ixora coccinea | Rubiaceae | 0.0 ± 0.00 |
| 6. | Rangoon creeper | Quisqualis indica | Combretaceae | 0.0 ± 0.00 |
| 7. | Butterfly pea | Clitoria ternatea | Fabaceae | 6.25 ± 1.25 |
| 8. | Oleander | Nerium oleande | Apocynaceae | 0.00 ± 0.00 |
| 9. | Carrot (cv. New Kuroda) | Daucus carota | Apiaceae | 40.25 ± 1.18 |
| 10. | Radish (cv. BARI Mula-1) | Raphanus sativus | Brassicaceae | 86.20 ± 2.14 |
| 11. | Mustard (cv. SS-75) | Brassica juncea | Brassicaceae | 76.25 ± 3.15 |
| 12. | Pomegranate | Punica granatum | Lythraceae | 70.75 ± 1.03 |
| 13. | Potato (cv. BARI Alu-72) | Solanum tuberosum | Solanaceae | 47.50 ± 1.50 |
| 14. | Tomato (cv. BARI Tomato-2) | Solanum lycopersicum | Solanaceae | 95.25 ± 3.15 |
| 15. | Brinjal (cv. Tarapuri) | Solanum melongena | Solanaceae | 49.25 ± 1.06 |
| 16. | Chilli (cv. BARI Morich-2) | Capsicum annum | Solanaceae | 65.00 ± 9.57 |
| 17. | Hyacinth Bean (cv. BARI Sheem-7) | Lablab purpureus | Fabaceae | 69.00 ± 1.14 |
| 18. | Bush bean (cv. BARI Jhar Sheem-2) | Phaseolus vulgari | Fabaceae | 52.25 ± 2.50 |
| 19. | Lentil (cv. BARI Mosur-1) | Lens culinaris | Fabaceae | 91.25 ± 4.15 |
| 20. | Mung bean (cv. BARI Mug-2) | Vigna radiata | Fabaceae | 90.75 ± 1.77 |
| 21. | Black gram (cv. BARI Mash-3) | Vigna mungo | Fabaceae | 89.64 ± 4.74 |
| 22. | Malabar Spinach (cv. BARI Puishak-2) | Spinacia oleracea | Amaranthaceae | 31.50 ± 1.09 |
| 23. | Sweet potato (cv. BARI Misti Alu-4) | Ipomoea batatas | Convolvulaceae | 59.25 ± 1.06 |
| 24. | Water spinach (cv. BARI Gima Kolmi-1) | Ipomoea aquatica | Convolvulaceae | 57.50 ± 1.31 |
| 25. | Indian olive | Elaeocarpus serratus | Elaecarpaceae | 21.50 ± 1.54 |
- a Values are mean ± SE. Disease severity was assessed following inoculation with Sclerotinia sclerotiorum isolate CSP-1on five detached leaves, one from each of five plants (each of two trials) (see text).
3.9 Screening of cosmos genotypes against S. sclerotiorum isolate CSP1
Water-soaked and light tan lesions developed at the point of inoculation 48 h after pathogen inoculation. Chlorosis and necrosis expanded into the entire leaf area within 4 days of inoculation. Leaves from all seven genotypes collapsed within 7 days of inoculation, and black sclerotia were formed within 10 days. The disease severity ranged between 44.25% and 100%, where the lowest and highest were recorded in CS1 (C. sulphureus 1) and CB2 (C. bipinnatus 2), respectively. Although individual genotypes exhibited differences in susceptibility to infection by S. sclerotiorum isolate CSP1, all were categorized as highly susceptible based on the disease severity scale (Table 3).
| Characteristics | Growth characteristics | ||||||
|---|---|---|---|---|---|---|---|
| Genotype | CS1 | CS2 | CS3 | CS4 | CB1 | CB2 | CB3 |
| Scientific name | C. sulphureus | C. sulphureus | C. sulphureus | C. sulphureus | C. bipinnatus | C. bipinnatus | C. bipinnatus |
| Sourcea | HRT, BSMRAU | HRT, BSMRAU | HRT, BSMRAU | HRT, BSMRAU | HRT, BSMRAU | HRT, BSMRAU | HRT, BSMRAU |
| Flower color | Dark orange | Light orange | Dark yellow | Light yellow | Light pink | White | Dark pink |
| Plant sizeb | Small | Small | Small | Small | Large | Large | Large |
| Flowering time (days) | 50–60 | 50–60 | 50–60 | 50–60 | 60–90 | 60–90 | 60–90 |
| Foliage | Opposite and pinnately divided | Opposite and pinnately divided | Opposite and pinnately divided | Opposite and pinnately divided | Densely populated | Densely populated | Densely populated |
| Disease severity (%)c | 44.25 ± 1.83 | 51.25 ± 3.17 | 86.25 ± 2.29 | 99.25 ± 2.48 | 99.00 ± 1.50 | 100.00 ± 0.00 | 92.34 ± 4.27 |
| Types of reactiond | HS | HS | HS | HS | HS | HS | HS |
- a Department of Horticulture, Bangabandhu Sheikh Mujibur Rahman Agricultural University (BSMRAU).
- b Plant size categories based on plant height are small (15–60 cm) and large (90–200 cm).
- c Values are mean ± SE. Disease severity was assessed following inoculation with Sclerotinia sclerotiorum isolate CSP-1on five detached leaves, one from each of five plants (each of two trials) (see text).
- d Reaction: HR, highly resistant; HS, highly susceptible; MR, moderately resistant; MS, moderately susceptible; R, resistant; S, susceptible. HR = 0.0%–0.99%, R ≤ 1.00–9.99, MR = 10.00%–19.99%, MS = 20.00%–29.99%, S = 30.00–39.99 and HS = ≥ 40.0% disease severity.
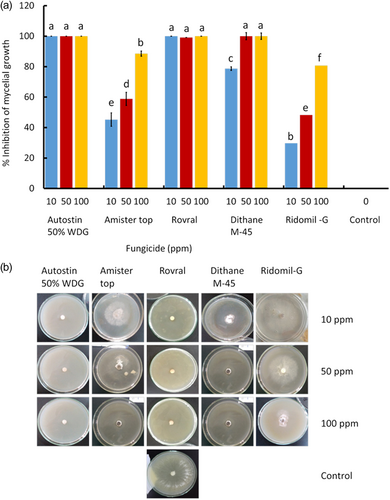
3.10 In vitro chemical control of S. sclerotiorum CSP1
In vitro efficacy of three popular systemic (Autostin 50 WDG, Amistar Top, and Ridomil Gold MZ) and two nonsystemic (Dithane M-45 and Rovral) fungicides were evaluated against the pathogen. Among the tested five fungicides, Autostin 50 WDG and Rovral completely inhibit (100% inhibition) the mycelial growth of S. sclerotiorum CSP1 at 100, 50, and 10 ppm of PDA (Figure 7a,b). On the other hand, Dithane M-45 completely inhibited mycelial growth at 100 and 50 ppm concentrations (Figure 7a,b). Amister top and Ridomil gold were only effective at 100 ppm, showing 88.5% and 80.12% inhibition of mycelial growth, respectively (Figure 7a,b).
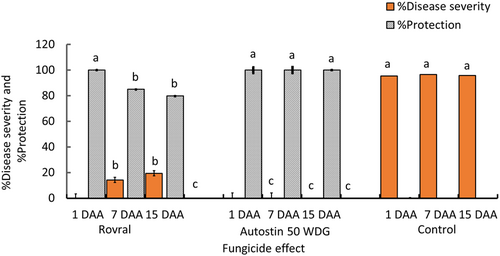
3.11 In planta chemical control of S. sclerotiorum CSP1
Although the disease severity in the control plant was 95.37%, no symptoms were observed in the Autostin 50 WDG, and Rovral-treated leaves 1 day after spray (Figure 8). Autostin 50 WDG continued to protect the cosmos plants till 7 and 15 days after application, and no disease was observed in the treated plants during these periods. It means that the residual effect of Autostin was persistent and protected plants for a longer duration. On the other hand, the residual effect of Rovral faded away to some extent with time, and the disease severity in the treated leaves was 14.25% and 19.45% at 7 and 15 days after application, respectively. This shows that Rovral can also provide significant protection against the disease 15 days after application, although the efficacy was slightly lower than Autostin (Figure 8).
4 DISCUSSION
This study identified and characterized a new disease in C. sulphureus field in Gazipur, Bangladesh. The disease has been occurring regularly in cosmos gardens since 2018, with significant plant losses. The disease exhibited a range of characteristic symptoms that have common analogies with Sclerotinia disease [9, 10, 14]. From the infected crop, a single fungus was consistently isolated that showed unique cultural and morphological characteristics similar to those of S. sclerotiorum, for example, fast-growing white aerial thin mycelia, hyaline, branched, and multinucleated hyphae, no conidia or conidiophore, globose to cylindrical sclerotia at the growing margins and production of apothecia, asci, and ascospores [10, 14, 15]. Sequence comparisons and phylogenetic analyses of ITS regions revealed them as the fungal strains of S. sclerotiorum. On these grounds, the new disease of C. sulphureus was recognized as an S. sclerotiorum disease. In Bangladesh, two annual cosmos species are grown, C. bipinnatus and C. sulphureus. A recent study has identified C. bipinnatus, as a new host crop of S. sclerotiorum in Bangladesh [17], while a strain infecting C. sulphureus was never reported. Therefore, the present study documented the first occurrence of S. sclerotiorum on C. sulphureus to our knowledge in Bangladesh. The disease caused by S. sclerotiorum in various host plants is usually known as white mold, stem rot/blight, watery soft rot, or cottony rot [18]. However, in the cosmos plants, the disease has been designated as 'Cosmos white rot' in several previous reports [19]. The S. sclerotiorum isolates collected in the present study did not differ in cultural or morphological traits but showed variation in virulence characteristics. This could be due to the isolates from the same host species in a limited infected area. Additional investigations involving isolates from diverse locations may reveal the morphological and genetic variability in the S. sclerotiorum-infecting cosmos in Bangladesh.
Physiological factors such as temperature, pH, and nutrients could either alone or in combination influence the mycelial growth, development, and viability of sclerotia [20]. In the present study, the temperature at 20°C and slightly acidic pH (5) benefitted maximum mycelial growth and sclerotial development of S. sclerotiorum CSP1. Growth and sclerotial production were severely restricted above 25°C (to a lesser extent at temperatures below 20°C) and at pH 9.0. These observations were supported by previous studies [10, 21, 22]. The development of Sclerotinia diseases on various crops has been associated with cool temperatures and low pH [23]. Temperature and pH affect oxalic production by the fungus. Oxalic acid is essential for the pathogenesis of S. sclerotiorum and sclerotia formation [24]. Cotton et al. [25] proved that oxalic acid production results from the discharge of polygalacturonases and reduced pH. On the other hand, Uloth et al. [26] demonstrated that oxalate production by S. sclerotiorum UWA 10S2 declined from a maximum level of 101 mg g−1 mycelium to 24 mg g−1 mycelium when the temperature increased from 20°C to 25°C. These indicate that the growth of S. sclerotiorum, its oxalic acid production, and the success of infection of hosts are all strongly influenced by temperature and pH.
Nutrient assimilation is vital for S. sclerotiorum to exist, proliferate, and remain within a host. Carbons, nitrogens, minerals, and vitamins serve various fundamental functions in the fungal cells by providing the essential compounds to create cell components and acting as the principal energy source [27]. Therefore, the nature of nutrient compounds often influences the mycelial growth and sclerotial development of the fungus [20]. However, there is limited available account of the nutrient requirements of S. sclerotiorum infecting various host plants. Glucose (as carbon source), peptone, yeast extract, and casein (as nitrogen source), along with different vitamins and minerals, facilitated maximum mycelial growth and sclerotial development of S. sclerotiorum. Previously d-glucose, along with saccharose and d-dextrose, proved to be the best carbon sources for the growth of S. sclerotiorum isolated from different hosts in various studies [10, 28, 29]. Whereas casein hydrolysate, l-proline, dl-asparagine, l-arginine, l-alanine, ammonium sulfate, potassium nitrate, sodium nitrate was reported as good nitrogen sources for S. sclerotiorum [28, 30]. These observations conclude that several carbons, nitrogen, minerals, and vitamins can serve as good nutrient sources for the selected pathogen. Therefore, physiological studies could enhance the opportunity to integrate a number of adaptation strategies to control the pathogen [20].
In Bangladesh, S. sclerotiorum appears as an emerging phytopathogen that gradually infects an increasing number of hosts [10, 15]. In the present study, the fungus was highly pathogenic to 20 hosts from the Asteraceae (C. officinalisi, C. indicum), Apocynaceae (C. roseus), Solanaceae (S. tuberosum, S. lycopersicum, S. melongena, C. annum), Fabaceae (L. purpureus, P. vulgari, L. culinaris, V. radiata, V. mungo), Apiaceae (D. carota), Brassicaceae (R. sativus, B. juncea), Lythraceae (P. granatum), Amaranthaceae (S. oleracea), Convolvulaceae (I. batatas, I. aquatica), and Elaecarpaceae (E. serratus). These plants were never reported as hosts of S. sclerotiorum in Bangladesh and are considered potentially new hosts. This finding confirms that the host range of fungus is quickly expanding across the globe [8]. Among necrotrophic fungi, S. sclerotiorum is notable for its extensive host range. The fungus is believed to utilize a range of pathogenicity factors that enable it to be a successful pathogen for many agriculturally and economically important crops. S. sclerotiorum secrets an assortment of carbohydrate-active enzymes (CAZymes) to degrade plant cell walls, facilitates plant tissue penetration by appressoria, and activates various genes required for infection and colonization [31, 32]. Understanding the underlying basis of the broad host range of the fungus could help locate potential targets for fungicide actions and improve fungicides for controlling S. sclerotiorum.
S. sclerotiorum is one of the aggressive pathogens that is difficult to control. An integrated management tactic combining various methods is advised to reduce their risk of losses from the disease [33]. Host resistance is the first line of desirable approaches to control the disease. Cosmos genotype selection with resistance to S. sclerotiorum can serve as future resources for breeding efforts to improve resistance to white mold. In the present study, individual cosmos genotypes differed in susceptibility, but none of the C. sulphureus and C. bipinnatus genotypes appeared resistant to the pathogen. This agrees with the observation of Kandel et al. [34], who reported that currently, no cultivars are genetically resistant to S. sclerotiorum. Similarly, Islam et al. [27] recently reported that the large majority (70.93%) of genotypes, including tested released cultivars, were susceptible to S. sclerotiorum. As S. sclerotiorum is a broad host necrotrophy, resistance to the pathogen is rare, requiring the involvement of many genes and pathways for complete resistance [35]. New tools, including molecular markers, are desirable to identify small differences in the phenotypes due to minor resistant genes.
Fungicide comprises a vital component in the integrated management of the disease [36], as it inhibits the growth, resistance structures, fruiting body, and spores of S. sclerotiorum and the disease development in the field [37]. In the current investigation, the efficacy of five different fungicides was evaluated against S. sclerotiorum rot of cosmos. Among them, two specific fungicides of Carbendazim (Autostin 50 WDG) and the dicarboxamide group (Rovral) effectively inhibited both in vitro and in planta development of the pathogen. This result was supported by several previous investigations [37, 38]. As stated by Prova et al. [10], carbendazim (Bavistin) and dicarboxamide (Rovral) were among the most effective fungicides against S. sclerotiorum. Likewise, according to Wang et al. [39], carbendazim (MBC-methyl 2-benzimidazole carbamate) and dimethachlon (dicarboxamide) are considered the major chemicals to reduce the incidence of S. sclerotiorum. Carbendazim provided a more extended period of protection against S. sclerotiorum than dicarboxamide. One reason for the shorter length of protection that the Rovral fungicide provided might be its protectant and contact nature. As with any contact fungicide, residues of Rovral might continue to be eroded via drying and vaporization, or as the plant tissues expand, the new tissue might be left unprotected. For these reasons, Rovral may need to be reapplied more often (a shorter spray interval) than Autostin 50 WDG. This is the first report on the efficacy of fungicides for managing S. sclerotiorum rot of cosmos to the authors' knowledge. Further multi-year and multi-location trials are needed before recommending controlling the white-rot disease of cosmos.
The morphological, molecular, and pathological data collected in the present study identified the new disease in C. sulphureus as white rot caused by S. sclerotiorum. As environmental conditions are conducive to disease development during the winter season, the potential of S. sclerotiorum to become a destructive pathogen of C. sulphureus is high. Appropriate characterization of the pathogen, its biology, host range, host resistance, and fungicidal control may provide valuable information about the epidemiology of the disease and aid in the implementation of effective disease management techniques.
ACKNOWLEDGMENTS
We express our gratitude to the Ministry of Science and Technology, Bangladesh, for supporting the research project. This study was funded by the Ministry of Science and Technology, Bangladesh.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data supporting the findings of this study are available from the corresponding author upon reasonable request.




