Participating mechanism of a major contributing gene ysnE for auxin biosynthesis in Bacillus amyloliquefaciens SQR9
Jiahui Shao and Yucong Li contributed equally to this study.
Abstract
The phytohormone indole-3-acetic acid (IAA) has been demonstrated to contribute to the plant growth-promoting effect of rhizobacteria, but the IAA biosynthesis pathway in rhizobacteria remains unclear. The ysnE gene, encoding a putative tryptophan acetyltransferase, has been demonstrated to be involved in and strongly contribute to IAA production in Bacillus, but the mechanism is unknown. In this study, to investigate how ysnE participates in IAA biosynthesis in the plant growth-promoting rhizobacterium Bacillus amyloliquefaciens SQR9, differences in the produced IAA biosynthesis intermediates between wild-type SQR9 and ΔysnE were analyzed and compared, and the effects of different intermediate compounds on the production of IAA and the accumulation of other intermediates were also investigated. The results showed that the mutant ΔysnE produced more indole-3-lactic acid (ILA) and tryptamine (TAM) than the SQR9 wild-type strain (nearly 1.6- and 2.1-fold), while the production of tryptophol (TOL) was significantly decreased by 46%. When indole-3-pyruvic acid (IPA) served as the substrate, the concentration of ILA in the ΔysnE fermentation broth was much higher than that of the wild type, while IAA and TOL were significantly lower, and ΔysnE was lower than SQR9 in IAA and TOL with the addition of TAM. The TOL content in the ΔysnE fermentation broth was much lower than that in the wild-type SQR9 with the addition of ILA. We suggest that ysnE may be involved in the IPA and TAM pathways and play roles in indole acetaldehyde (IAAld) synthesis from IPA and TAM and in the conversion of ILA to TOL.
Abbreviations
-
- GNAT
-
- GCN5-related N-acetyltransferase
-
- IAA
-
- indole-3-acetic acid
-
- IAA1d
-
- indole acetaldehyde
-
- IAM
-
- indole-3-acetamide
-
- IAN
-
- indole-3-acetonitrile
-
- ILA
-
- indole-3-lactic acid
-
- IPA
-
- indole-3-pyruvic acid
-
- IPDC
-
- indole-3-pyruvate decarboxylase
-
- PGPR
-
- plant growth-promoting rhizobacteria
-
- TAM
-
- tryptamine
-
- TOL
-
- tryptophol
-
- TSO
-
- tryptophan side-chain oxidase
1 INTRODUCTION
Plant growth-promoting rhizobacteria (PGPRs) are a wide range of microorganisms that live in association with plant roots and enhance plant growth through a number of mechanisms [1-3]. Gram-positive bacteria, especially bacilli strains, are often used as commercialized PGPRs for their ability to form heat- and desiccation-resistant spores [4] and to produce a variety of secondary metabolites [5, 6]. Various factors, such as the volatiles 3-hydroxy-2-butanone (acetone), 2,3-butanediol, and phytase, and biocontrol agents released by Bacillus subtilis and Bacillus amyloliquefaciens, have been suggested to be involved in the growth-promotion effect of Bacillus, and these factors trigger plant growth directly or indirectly [7-10].
Phytohormone IAA is an important factor that promotes plant growth by changing the cell wall, increasing cell permeability through swelling of exposed plant cells, and inducing the production of another hormone, ethylene [11-13]. The production of IAA is widespread among plant-associated bacteria [14]. As more bacterial species have been analyzed, different IAA synthesis pathways in bacteria have been identified [15, 16], among which tryptophan has been confirmed to be the main precursor for IAA biosynthesis [16]. Tryptophan in root exudates is the natural source for rhizosphere microorganisms, which may stimulate IAA secretion within the rhizosphere and induce a physiological response in the host plant [17, 18]. Five different pathways using tryptophan as a precursor for IAA synthesis were identified in bacteria by the identification of different intermediates, which include indole-3-pyruvic acid (IPA), indole-3-acetamide (IAM), indole-3-acetonitrile (IAN), tryptamine (TAM), and tryptophan side-chain oxidase (TSO) pathways [16].
Although much work has been performed on the IAA synthesis pathway in various bacteria, little information about the genes and enzymes involved in IAA biosynthesis by Bacillus is available. In B. amyloliquefaciens FZB42, IAA production was increased fivefold in the presence of tryptophan [19]. The deletion of the putative nitrilase gene yhcX in the IAN pathway of FZB42 led to a 50% decrease in IAA production, and disruption of the putative tryptophan acetyltransferase gene ysnE resulted in a 72% decrease in IAA production [19]. In B. amyloliquefaciens SQR9, inactivation of the ysnE, dhaS (encoding indole-3-acetaldehyde dehydrogenase), yclC (encoding a UbiD family decarboxylase), and yhcX genes led to 86%, 77%, 55%, and 24% reductions in IAA secretion, respectively [20]. Coexpression of three genes, patB (encoding a conserved hypothetical protein predicted to be an aminotransferase), yclC, and dhaS, as an entire IPA pathway for IAA synthesis in SQR9 and B. subtilis 168 showed increased IAA production [20].
The ysnE gene plays an important role in IAA biosynthesis as its deletion decreased IAA production by 86% in B. amyloliquefaciens SQR9 [20], but the functional mechanism of this gene is unclear. The predicted product of ysnE belongs to the N-acetyltransferase superfamily of various enzymes that catalyze the transfer of an acyl group to a nitrogen atom on the acceptor molecule. In Azospirillum brasilense, the ysnE gene, which is located within the tryptophan biosynthesis gene cluster, has been considered to participate in tryptophan-dependent IAA synthesis [21]. In Bacillus strains, this gene is located far from the tryptophan biosynthesis gene cluster.
The PGPR strain B. amyloliquefaciens SQR9, which was isolated from the cucumber rhizosphere, showed impressive suppression of Fusarium oxysporum, the causative agent of Fusarium wilt of cucumber [22-24], and promoted plant growth by producing various factors [13]. Our previous study indicated that tryptophan is the main precursor for IAA synthesis in SQR9. When colonized by SQR9, cucumber secretes more tryptophan in its root exudates, and in return, SQR9 synthesizes more IAA in the rhizosphere, using root-secreted tryptophan to promote cucumber plant growth [13, 18]. In our previous study, the IPA, TAM, and IAN pathways, as well as the uncharacterized ysnE-involved pathway, were believed to participate in IAA biosynthesis of SQR9 [20]. The uncharacterized ysnE-involved IAA biosynthesis pathway appeared to be the most important route in SQR9 for IAA production as its deletion decreased IAA production by 86% [20]. In this study, the functional mechanism by which ysnE participates in IAA biosynthesis in SQR9 was explored by comparing IAA-related intermediates produced by wild-type SQR9 and ΔysnE, and we propose that the ysnE gene may function in both the IPA and TAM pathways and affect the synthesis of TOL.
2 MATERIALS AND METHODS
2.1 Strains and growth conditions
B. amyloliquefaciens SQR9 (China General Microbiology Culture Collection Center [CGMCC] accession no. 5808) was isolated from the rhizosphere of cucumber. The B. amyloliquefaciens SQR9 mutant strain ΔysnE was constructed in our previous study [20]. B. amyloliquefaciens SQR9 and ΔysnE were grown at 30°C in low-salt Luria–Bertani medium (peptone, 10 g·L−1; yeast extract, 5 g·L−1; NaCl, 5 g·L−1) solidified with 1.5% agar; when necessary, 5 μg·ml−1 chloramphenicol was added. For the detection of different IAA metabolism intermediates produced by wild-type SQR9 and ΔysnE, strains were cultured in 100 ml Landy medium supplied with 3 mM l-tryptophan and were grown for 24, 48, 72, and 96 h at 25°C and 90 rpm in the dark. To analyze the effect of different intermediate compounds on IAA and the production of other metabolic intermediates in SQR9 and ΔysnE, the strains were grown with 3, 1, and 0.5 mM authentic substances of IAA, IAN, TAM, IPA, TOL, and ILA (Sigma-Aldrich) for 96 h at 25°C and 90 rpm in the dark.
2.2 Extraction of IAA and IAA biosynthesis intermediates
After incubation, the density of the culture was measured spectrometrically at 600 nm, and the cell culture was adjusted to OD600 = 5.0 with sterilized water. Then, the SQR9 and ΔysnE cells were removed by centrifugation (5000g, 20 min, 4°C). Supernatants (100 ml) of each strain culture were adjusted to pH 2.5 with 1.0 M HCl and were extracted three times with ethyl acetate (1:3, v/v). The organic solvents were vacuum-dried at 37°C and dissolved in 3 ml methanol. The extracted samples were filtered through a 0.45-µm membrane before further analysis.
2.3 Thin-layer chromatography (TLC) analysis
Ten microliters of the mixed standard substances (TAM, IPA, IAM, IAA, and TRP, 1 mg·L−1 for each) and 20 μl samples extracted from 24, 48, 72, and 96 h cultured fermentations were applied on silica plates and chromatographed with chloroform/methanol/H2O (84:14:1, by vol.). The separated compounds were visualized by color development with Ehrich reagent [25].
2.4 Reverse-phase high-performance liquid chromatography (RP-HPLC)
Extracted samples were separated with RP-HPLC using a reversed-phase C18 analytical column (9.4 by 150 mm; Agilent Technologies). Gradient elution was performed by varying the proportion of solvent A (methanol) to solvent B (0.1% acetic acid), with a flow rate of 0.8 ml·min−1 at 220 nm using a UV detector. The mobile phase composition started at methanol–0.1% acetic acid (10/90), followed by a linear increase in solvent A to 35% in 40 min, and then the mobile phase composition was maintained for 20 min for the next run. IAA and related intermediates were quantified by integrating the areas under peaks with the standards of the authentic substances (IAA, IAN, TAM, IPA, IAM, TOL, and ILA) from Sigma-Aldrich.
2.5 Combined liquid chromatography–mass spectrometry (LC-MS) analysis
An Agilent 1100 triple quadrupole LC/MS apparatus (Agilent Technologies) was used to determine the differences in IAA-related intermediates between wild-type SQR9 and ΔysnE. The electrospray needle was operated at a spray voltage of 4.5 kV and a capillary temperature of 300°C. The mobile phase components were the same as those of HPLC. MS analysis was performed by electrospray ionization in positive ion mode, with the authentic substances serving as controls.
3 RESULTS
3.1 Analysis of IAA and biosynthesis intermediates during SQR9 growth
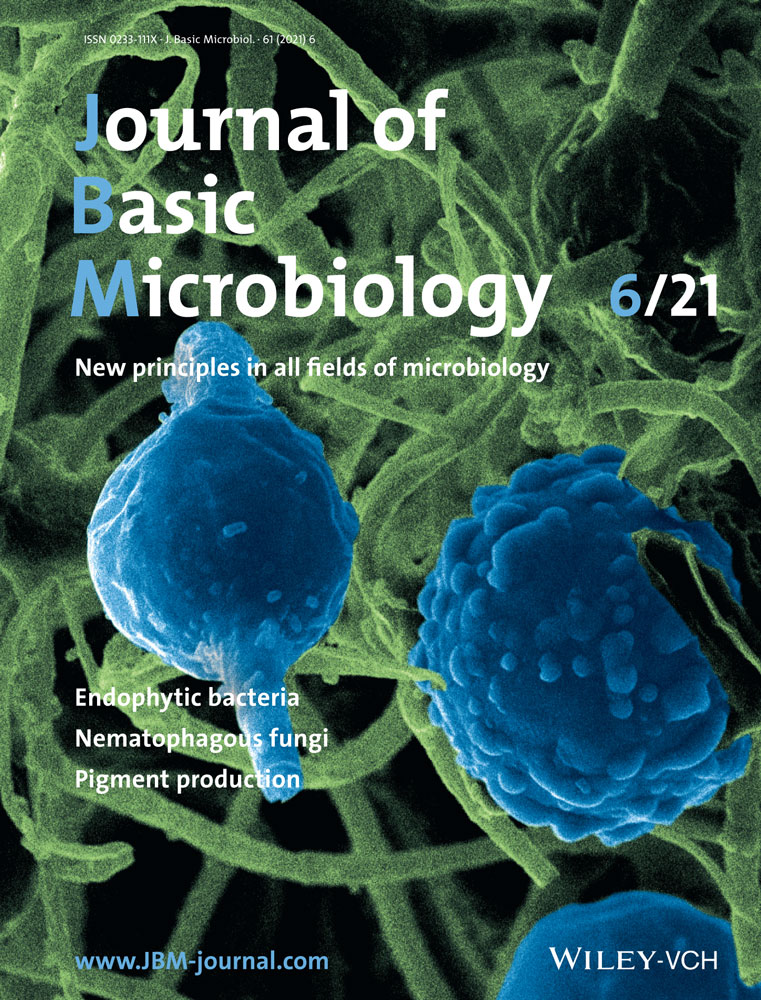
IAA synthesis in SQR9 depends on tryptophan as more IAA was produced with 3 mM l-tryptophan in the medium compared with the no l-tryptophan treatment (Figure 1a). TLC analysis of the extracted supernatants of both wild-type SQR9 and ΔysnE fermentation broth showed that the related intermediates during IAA biosynthesis, such as IAA, TAM, and IPA, began to accumulate at 48 h after incubation and reached a peak at 96 h (Figure 1b). Additionally, more intermediate compounds were produced by ΔysnE than wild-type SQR9, suggesting that the relevant IAA synthesis pathway was disrupted in the mutant (Figure 1b).
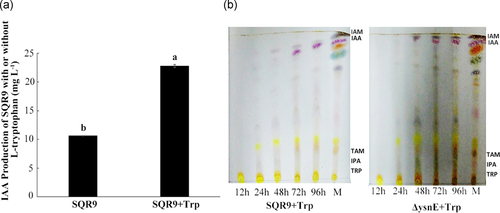
3.2 Differences in IAA-related intermediates between wild-type SQR9 and ΔysnE
To identify the role of the ysnE gene in the IAA synthesis pathway, IAA synthesis-related intermediates in the fermentation broth of both wild-type SQR9 and ΔysnE were analyzed and compared by HPLC and LC-MS. The results showed that the ILA and TAM concentrations were increased in the ΔysnE fermentation broth compared with SQR9, while the amount of TOL was significantly decreased by 46% (Figure 2a). IPA and indole-3-acetaldehyde (IAAld) are unstable and can be converted into ILA and TOL in fermentation broth [16], so we measured the concentrations of ILA and TOL to represent IPA and IAAld. In the LC-MS analysis, IPA was detected in the ΔysnE fermentation broth but not in the broth of SQR9 (Figure 2b). The HPLC and LC-MS results indicated that a large amount of IPA and TAM accumulated in the mutant strain and could not be converted into IAAld in the next metabolic reaction, leading to a decrease in TOL.
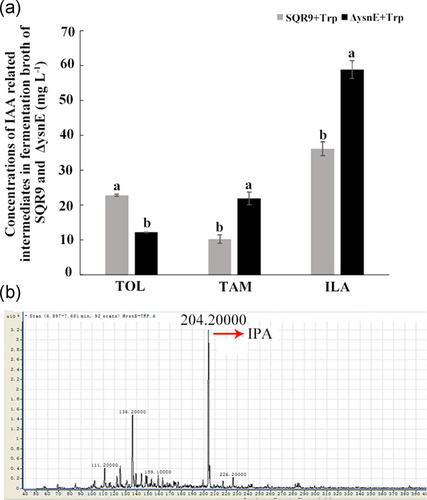
3.3 The effect of different IAA synthesis intermediates on the production of IAA and related intermediates
To investigate the function of the ysnE gene in IAA biosynthesis in SQR9, various intermediates in different potential IAA synthesis pathways were added to the growth medium of SQR9 and ΔysnE to measure the final IAA production and the accumulation of other intermediates. These intermediates were added at a suitable concentration that had no effect on the growth of either SQR9 or ΔysnE.
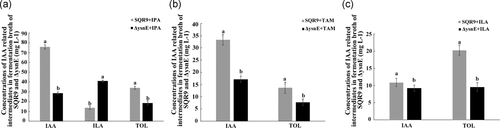
When 0.5 mM IPA was provided in the medium, the ILA content in the ΔysnE fermentation broth was much higher, while the IAA and TOL were significantly lower than those of the wild-type SQR9 (Figure 3a). The IAA and TOL contents in SQR9 were significantly higher than those of ΔysnE with the addition of 0.5 mM TAM (Figure 3b). Similarly, when 0.5 mM ILA was added to the medium, the TOL content in the ΔysnE fermentation broth was much lower than that of the wild-type SQR9, while IAA production was not different (Figure 3c). In all of these treatments, IAM and IAN in SQR9 and ΔysnE broth were also detected but showed no difference between these two strains (data not shown). We also added IAM and IAN as substrates to investigate their effects on IAA production and the accumulation of other intermediates in SQR9 and ΔysnE, but no differences were detected (data not shown). We speculated that the ysnE gene may be involved in the IPA and TAM pathways and may play roles in the conversions between indole pyruvate and indole acetaldehyde or tryptamine and indole acetaldehyde.
4 DISCUSSION
The PGPR strain B. amyloliquefaciens SQR9 showed impressive growth-promoting effects on cucumber and maize, and the phytohormone IAA has been suggested to contribute to its plant-growth-promoting effect [13]. The possible IAA synthetic pathways in SQR9 were investigated using a combination of chemical and genetic analysis. Pathways such as IAN, TAM, IPA, and the ysnE gene involved in an uncharacterized pathway, which is supposed to be the most important pathway, are suggested to exist in this strain [20]. In addition, an entire predicted IPA pathway, which consists of patB, yclC, and dhaS, was authenticated through homologous and heterologous expression [20]. In this study, the function of the ysnE gene in IAA biosynthesis was investigated.
The ysnE disruption mutant ΔysnE only produced 14% IAA of the wild type, but there is little information on this specific metabolic process [20]. In A. brasilense and B. amyloliquefaciens FZB42, this gene was suggested to encode a putative tryptophan acetyltransferase that belongs to the N-acetyltransferase superfamily of various enzymes and was supposed to participate in tryptophan-dependent IAA synthesis pathway [21, 26]. We suspected that the ysnE gene-participating IAA synthesis process was an important pathway in B. amyloliquefaciens strains, and intensive study on how it works in IAA biosynthesis will have great significance.
To investigate how the gene product of the ysnE gene functions in IAA synthesis in SQR9, the differences in IAA-related intermediates between wild-type SQR9 and ΔysnE and the effect of different IAA metabolism intermediates on IAA production were studied. In bacteria, some IAA-related intermediates can be converted into storage compounds; for example, the reduction of IPA and IAAld to ILA and TOL, respectively [15, 16]. The physiological function of these compounds remains unknown. These storage compounds may be converted into IAA when needed by the bacterium [15]. In our study, IPA and IAAld were not detected by using HPLC, although IPA was detected in LC-MS analysis. It is possible that IPA and IAAld are rapidly oxidized. The accumulation of their storage compounds ILA and TOL was detected by HPLC, so we measured ILA and TOL instead of IPA and IAAld in the quantification analysis. ILA and TAM accumulated in ΔysnE fermentation broth, while the amount of TOL was significantly decreased in ΔysnE compared with SQR9, suggesting that intermediate products, including IPyA and TAM, cannot be used for subsequent biochemical reactions to synthesize IAA. When IPA was supplied as a substrate, the ILA content in the ΔysnE fermentation broth was much higher, while IAA and TOL were much lower than that of the wild type. When TAM was supplied as a substrate, the concentration of IAA and TOL in SQR9 was also significantly higher than that of ΔysnE. It has been proposed that ILA is not only a storage form but might also act as a precursor of IAA through a pathway initially involving decarboxylation of lactic acid to produce TOL, and TOL could be oxidized to IAAld and IAA when needed [27]. In SQR9, when ILA was supplied as a substrate, the TOL content in the ΔysnE fermentation broth was much lower than that of the wild type, while IAA was almost unchanged. Therefore, we propose that the ysnE gene is involved in the synthesis of TOL and IAA by affecting the reaction from ILA to TOL and the conversion from IPA and TAM to IAAld in the IPA pathway and TAM pathway, respectively (Figure 4).
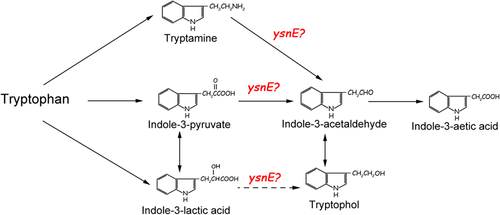
The IPA pathway is thought to exist in both plants and bacteria, and most beneficial bacteria produce IAA via this pathway [16]. The gene encoding the key enzyme indole-3-pyruvate decarboxylase (IPDC), a decarboxylase that converts IPA into IAAld, has been identified and characterized in A. brasilense, Enterobacter cloacae, and Pseudomonas putida [28, 29]. In these organisms, inactivation of this pathway resulted in lower IAA production, up to 90% reduction in A. brasilense, indicating the importance of the IPA pathway in auxin production [30]. However, the gene responsible for the decarboxylase activity of the IPA pathway remains elusive in Bacillus [15]. In our previous study, yclC (encoding a UbiD family decarboxylase), participating in our predicted IPA biosynthesis pathway in SQR9, was suggested to act as a decarboxylase, converting IPA into IAAld [20]. The TAM pathway has been suggested in Bacillus cereus and Azospirillum by the identification of tryptophan decarboxylase activity [31] and the conversion of exogenous tryptophan to IAA [25], but the genes involved in this pathway still need to be confirmed. The predicted product of the ysnE gene belongs to the GCN5-related N-acetyltransferase (GNAT) superfamily, which contains a large and diverse group of proteins. The GNAT superfamily plays roles in a wide range of cellular functions by catalyzing the transfer of acetyl groups from acetyl coenzyme A (Ac-CoA) to various acceptor substrates, ranging from small molecules to proteins, including aminoglycosides, serotonin, glucose-6-phosphate, and lysine of histone tails [32, 33], while how the N-acetyltransferase YsnE works in the conversion from IPA, TAM and ILA to IAAld and TOL is unknown. In the IAA biosynthetic network, different pathways may interact with one another, which poses difficulties in characterizing the working mechanism of enzymes [16]. To identify how YsnE participates in the IAA biosynthesis pathways in SQR9, the detection of other related indolic compounds and the analysis of the crystal structure of YsnE and its enzyme activity are required to uncover the metabolic function of this gene product.
ACKNOWLEDGMENTS
This study was financially supported by the Fundamental Research Funds for the Central Universities (KJQN201744), the National Natural Science Foundation of China (31601826), the General Financial Grant from the China Postdoctoral Science Foundation (2018M642268), and the Key R&D Program of Shandong Province (2019JZZY020614).
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.