Purification of thermostable α-galactosidase from Irpex lacteus and its use for hydrolysis of oligosaccharides
Abstract
A monomeric α-galactosidase (ILGI) from the mushroom Irpex lacteus was purified 94.19-fold to electrophoretic homogeneity. ILGI exhibited a specific activity of 18.36 U mg−1 and demonstrated a molecular mass of 60 kDa in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). ILGI was optimally active at 80 °C and pH 5.0, and it was stable over a temperature range of 4–70 °C and a wide pH range of 2.0–12.0. ILGI was completely inactivated by Ag+ and Hg2+ ions and N-bromosuccinimide (NBS). Moreover, ILGI exhibited good resistance to proteases. Galactose acted as a noncompetitive inhibitor with Ki and Kis of 3.34 and 0.29 mM, respectively. The α-galactosidase presented a broad substrate specificity, which included p-nitrophenyl α-D-galactopyranoside (pNPGal), melibiose, stachyose, and raffinose with Km values of 1.27, 3.24, 7.1, and 22.12 mM, correspondingly. ILGI exhibited efficient and complete hydrolysis to raffinose and stachyose. The aforementioned features of this enzyme suggest its potential value in food and feed industries.
Abbreviations
-
- DNS
-
- 3,5-dinitrosalicylic acid
-
- HPAEC-PAD
-
- high-performance anion-exchange chromatography with pulsed amperometric detection
-
- ILGI
-
- an α-galactosidase purified from Irpex lacteus
-
- I. lacteus
-
- Irpex lacteus
-
- NBS
-
- N-bromosuccinimide
-
- pNP
-
- p-nitrophenol
-
- pNPGal
-
- p-nitrophenyl α-D-galactopyranoside
-
- Q-Sepharose
-
- quaternary ammonium base-Sepharose
-
- RFOs
-
- raffinose family oligosaccharides
-
- SDS-PAGE
-
- sodium dodecyl sulfate-polyacrylamide gel electrophoresis
-
- TLC
-
- thin-layer chromatography
Introduction
Soy products are excellent food and feed sources with rich protein contents and balanced amino acid patterns 1. For humans, soymilk is an alternative protein source for the lactose-intolerant population 2. For animals, soybean cake meal is an important raw material in feed industries. However, soybeans contain raffinose family oligosaccharides (RFOs), including raffinose and stachyose 3. These oligosaccharides act as antinutritional factors and can cause flatulence because of the lack of α-galactosidase in the human and monogastric animal intestinal tracts, which restrict the application of soybean 4.
α-Galactosidases are a group of exoglycosidases catalyzing the cleavage of terminal α-1,6-linked galactosyl residues of α-galactosides, including RFOs, galacto (gluco)-mannans, and galactolipids 5. α-Galactosidases are involved in various important applications. In food and feed industries, α-galactosidases can be directly used as additives to remove oligosaccharides in bean products and enhance the nutritional value 6. In biomedicine, these enzymes are used to convert blood types 7 and treat Fabry disease 8. In the sugar industry, raffinose can be removed from beet molasses to increase the yield of sucrose 9. Furthermore, α-galactosidases can enhance the bleachability of softwood kraft pulp in paper production 10.
α-Galactosidases are widely distributed in microorganisms, plants, and mammals 11-13. According to their amino acid sequence similarities, these enzymes can be classified into glycoside hydrolase (GH) families 4, 27, 36, 57, 97, and 110 14. Fungal α-galactosidases offer considerable potential in industrial applications for their extracellular localization, optimal acidic pH, and broad stability profiles, as well as the convenience to scale up the enzyme production process 15.
Irpex lacteus (Polyporus tulipiferae) is a widespread fungus normally growing on dead wood and causing white rot 16. I. lacteus is an important edible and medicinal mushroom. It is easy to culture and scale up and contains various bioactive substances. This mushroom was reported to exert potential antitumor and antinephritis activities 17. Previous research also reported that two milk-clotting enzymes from I. lacteus can be used to make string cheese 18, 19.
In the present study, increased α-galactosidase activity was detected from I. lacteus in submerged culture, and the enzyme was found thermostable. Thus, a novel α-galactosidase from I. lacteus, denoted herein as ILGI, was purified and characterized. The hydrolytic properties of this enzyme, as well as its potential applications in food and feed industries are discussed in this paper.
Materials and methods
Materials
Bean pulp and soybean cake meal used for fermentation were purchased from Kang Mingwei Culture Media Company (Beijing, P. R. China). Cornstarch was purchased from a supermarket. Vernase and alcalase were purchased from AOBOX Biotechnology Company (Beijing, P. R. China). Quaternary ammonium base-Sepharose (Q-Sepharose), Superdex 75 HR 10/30, and AKTA Purifier were obtained from GE Healthcare, USA. Proteinase K and chymotrypsin were obtained from MERCK and Solarbio, respectively. Yeast extract, neutral protease, trypsin, p-nitrophenyl α-D-galactopyranoside (pNPGal), melibiose, raffinose, stachyose, locust bean gum, and guar gum were obtained from Sigma Chemical Company (St. Louis, MO). All other chemicals used were of analytical grade and commercially available unless otherwise noted.
Strain and cultivation
A mushroom strain isolated from Yunnan Province and identified as I. lacteus was utilized in this study to produce α-galactosidase. The fungus was stored in the China General Microbiological Culture Collection Center (CGMCC5.809) and precultured on potato dextrose agar at 28 °C. After 4 days, two 5 mm2 agar plugs were inoculated in 250 ml flasks with 50 ml of seed media (cornstarch, 20 g L−1; bean pulp, 7 g L−1; yeast extract, 5 g L−1; K2HPO4 · 3H2O, 1 g L−1; MgSO4 · 7H2O, 0.5 g L−1; 5 mm glass beads, approximately 20 pieces) and incubated at 26 °C and 200 rpm for 4 days. Large-scale fermentation was conducted in 60 Erlenmeyer flasks (250 ml), with each flask containing 50 ml of fermentation media. Each flask was inoculated with 3 ml of the seed media and placed in a rotary shaker at 200 rpm and 26 °C for 7 days. The culture filtrate were collected and used to purify α-galactosidase.
Enzyme activity assay
α-Galactosidase activity was assayed using pNPGal as substrate 20. The reaction system, which contained 20 µl of pNPGal (10 mM, pH 4.6) and 20 µl of suitably diluted enzyme, was incubated at 60 °C for 10 min and was stopped by adding 160 µl of Na2CO3 (0.5 M). The enzyme activity was determined from the released p-nitrophenol (pNP) amount, which was measured spectrophotometrically at 405 nm. One unit of α-galactosidase activity was defined as the amount of enzyme that induced the release of 1 µmol of pNP per minute. All experiments on enzyme properties were performed in triplicate.
Purification of ILGI
All purification procedures were conducted at room temperature unless otherwise indicated. The culture filtrate was dialyzed against deionized water at 4 °C and subsequently adjusted to pH 7.5. Afterward, the filtrate was loaded onto a column (5 × 20 cm) of Q-Sepharose, which was pre-equilibrated and then eluted with buffer A (10 mM Tris-HCl buffer, pH 7.5). α-Galactosidase activity was located in fraction Q1, which was eluted with buffer A containing 150 mM NaCl at a flow rate of 10 ml min−1. Fraction Q1 was dialyzed against deionized water and adjusted to pH 5.2. Subsequently, fraction Q1 was applied to a column (2.5 × 10 cm) of Q-Sepharose previously equilibrated with buffer B (10 mM NaOAc–HOAc buffer, pH 5.2). After the unadsorbed proteins were eluted, the column was also eluted with a linear gradient of 0–300 mM NaCl in buffer B at a flow rate of 2 ml min−1. The main fractions that exhibited enzyme activity (fraction Q2) were pooled and freeze-dried after dialysis against deionized water. Fraction Q2 was dissolved in 400 µl of buffer B and subjected to fast protein liquid chromatography on a gel-filtration column (Superdex 75 HR 10/30, GE-Healthcare, USA). The mobile phase was buffer B, which contained 0.1 M NaCl. Elution was performed at a flow rate of 0.5 ml min−1. The active fractions (SU1) were then pooled and freeze-dried. The gel-filtration step was repeated, and the purified enzyme (SU2) was collected. The purification process is illustrated using a frame diagram in Fig. 1.

Estimation of molecular mass
The molecular mass of ILGI was estimated by gel-filtration chromatography (Superdex 75 HR 10/30). Afterward, the purified enzyme was subjected to SDS-PAGE and stained with Coomassie Brilliant Blue R-250.
Determination of inner amino acid sequences
The purified protein sample was digested by trypsin and analyzed by MALDI-TOF/TOF. The m/z values obtained from the MALDI-TOF/TOF spectra that corresponded to the peptides of ILGI were matched to galactosidases through Mascot (www.matrixscience.com) and the National Center for Biotechnology (NCBI, http://blast.ncbi.nlm.nih.gov/Blast.cgi). The identified criteria were digestion with trypsin and one missed cleavage allowed; the rest were set by default. During the search, all peptide masses were assumed to be monoisotopic with a mass accuracy of ±0.2 Da.
pH and temperature profiles
The optimal pH of ILGI was assayed within the pH range of 2.0–10.0. The pH stability was determined by incubating 10 µl of the enzyme solution with 10 µl of the buffer at a pH range of 1.0–13.0 and room temperature for 2 h. After incubation, the mixture was used to determine the enzyme activity at 60 °C and pH 4.6, with pNPGal as substrate. The buffers used were 0.2 M KCl–HCl for pH 1.0–2.0, 0.2 M Na2HPO4-citric acid for pH 3.0–8.0, 0.2 M glycine-NaOH for pH 9.0–10.0, 0.2 M Na2HPO4–NaOH for pH 11.0–12.0, and 0.2 M KCl–NaOH for pH 13.0.
The optimal temperature was determined within the range of 4–100 °C at pH 4.6. Thermal stability was investigated by measuring the residual activity at 60 °C and pH 4.6 after incubation for 2 h at 4–90 °C. The trend of enzyme activity over time was examined at 60, 70, and 80 °C.
Metal ions, chemical modification reagents, simple sugars, and protease treatments
The purified ILGI was preincubated at room temperature for 2 h with various metal ions and EDTA (at 1.25, 2.5, 5, and 10 mM), simple sugars (glucose, sucrose, galactose, xylose, and fructose at 0.1 M), and other chemical modification reagents (at 0.1–1.0 mM). For protease treatments, vernase was dissolved in pH 4.0 buffer, neutral protease in pH 7.0 buffer, proteinase K in pH 7.5 buffer, trypsin and α-chymotrypsin in pH 8.0 buffer, and alcalase in pH 10.0 buffer. ILGI was preincubated with protease (10 mg ml−1) at 37 °C (except for vernase and alcalase, which were preincubated at 50 °C) for 1 h. Residual enzyme activity was determined using standard methods. The activity of α-galactosidase preincubated with solvent was considered to be 100%.
Substrate specificity and enzyme kinetics
All the substrates were dissolved in NaOAc–HOAc buffer (pH 4.6, 0.1 M). The substrate specificities of ILGI with synthetic compounds were determined using standard methods. The reaction systems comprised 20 µl of ILGI (0.053 U ml−1) and 20 µl of synthetic compounds (10 mM), including pNPGal, p-nitrophenyl β-D-galactopyranoside, and p-nitrophenyl α-D-glucopyranoside. For natural compounds, the reaction system contained 20 µl of ILGI (1.06 U ml−1) and 80 µl of substrates, including raffinose (50 mM), stachyose (50 mM), locust bean gum (1%), and guar gum (1%); the final enzymatic activity was 0.212 U ml−1. The mixtures were kept at 60 °C for 30 min. Reducing sugars released during hydrolysis were measured using 3,5-dinitrosalicylic acid (DNS) reagent as described by Miller 21. When melibiose was used as substrate, the amount of glucose released was measured with a glucose oxidase kit (Beijing BHKT Clinical Reagent Co., Ltd.). The reaction mixture containing 20 µl of α-galactosidase (1.06 U ml−1) and 80 µl of melibiose (50 mM) was kept at 60 °C for 10 min. One unit of enzyme activity was defined as the amount of enzyme that released 1 µmol of reducing sugar per minute.
The Km and Vmax values of the ILGI for the hydrolysis of pNPGal (0.2–1.0 mM), melibiose (2–18 mM), raffinose (2–18 mM), and stachyose (2–18 mM) were obtained at 60 °C and pH 4.6 by using the Lineweaver–Burk plot. When galactose was used as an inhibitor, the inhibition constants (Ki and Kis) were also calculated using the Lineweaver–Burk plot. Concentrations of pNPGal ranged from 0.2 to 3.0 mM. Galactose concentrations were 2.5, 5, and 10 mM.
Enzymatic hydrolysis of stachyose
A reaction mixture of 2.99 U ml−1 ILGI with 10 mM stachyose (pH 4.6) was incubated at 60 °C for 10 min, 20 min, 30 min, 1 h, 1.5 h, and 2 h and then boiled for 5 min to stop the reaction. The degradation rates were detected by the DNS reagent, and the hydrolysates were analyzed qualitatively by thin-layer chromatography (TLC). Approximately 3 µl of the reaction mixture was spotted onto a silica gel plate (GF254, Branch of Qingdao Haiyang Chemical Co., Ltd.) and developed in a solvent system that consisted of propanol:acetic acid:water at 1.0:1.5:0.1 (v/v/v). Subsequently, the silica gel plate was immersed into a chromogenic reagent consisting of diphenylamine (1 g), aniline (1 ml), acetone (50 ml), and 85% phosphoric acid (10 ml) and then heated at 99 °C until saccharides were spotted.
Hydrolysis of RFOs from soybean and soybean cake meal
Soybeans and soybean cake were powdered by a grinder and defatted with petroleum ether. Up to 10 g of the powder was added to 100 ml of water, and the mixture was ultrasonicated for 2 h at 90 °C. This mixture was centrifuged, and the supernatant was lyophilized. The lyophilized samples of soybean and soybean cake were resuspended in 30 and 20 ml of sodium acetate buffer (pH 4.6), respectively. The solutions were incubated with 1 U ml−1 of purified α-galactosidase at 60 °C for 5 min, 15 min, 30 min, 1 h, and 2 h. Samples were boiled for 5 min, centrifuged, and then filtrated with 3 K membrane to remove proteins. After these treatments, the samples were analyzed by high-performance anion exchange chromatography with pulsed amperometric detection (HPAEC-PAD, Dionex ion chromatography system ICS-5000).
HPAEC-PAD analysis was performed using a Dionex CarboPac PA200 analytical column (3 × 250 mm). Up to 10 μl of the sample was applied at a column temperature of 30 °C, and a gradient sodium hydroxide solution was used as mobile phase at a flow rate of 0.45 ml min−1.
Results
Purification of ILGI
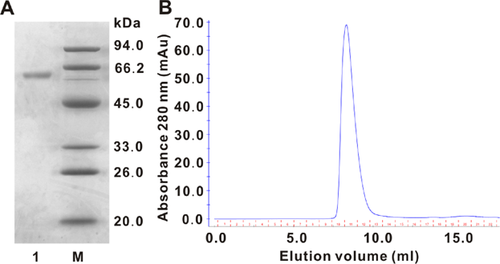
The culture filtrate of I. lacteus with α-galactosidase activity was subjected to ion-exchange chromatography and gel-filtration chromatography to homogeneity (Table 1). The enzyme was purified 64.4-fold compared with the culture filtrate with a recovery rate of 1.97%. The specific activity against pNPGal was 0.3166 U mg protein−1. The molecular mass of ILGI was estimated to be 60 kDa according to the results of SDS-PAGE (Fig. 2A) and gel filtration (Fig. 2B).
| Chromatographic fraction | Total protein (mg) | Total activity (U) | Specific activity (U mg−1) | Recovery of activity (%) | Purification fold |
|---|---|---|---|---|---|
| Culture filtrate | 5823.68 | 1141.45 | 0.2 | 100 | 1 |
| Q1 | 438.68 | 787.6 | 1.8 | 69 | 9.16 |
| Q2 | 70.95 | 582.14 | 8.2 | 51 | 41.86 |
| SU1 | 9.92 | 122.1 | 12.3 | 10.7 | 62.79 |
| SU2 | 1.4 | 25.7 | 18.36 | 2.25 | 94.19 |
Afterward, the purified α-galactosidase was digested by trypsin and subjected to high-quality MALDI-TOF peptide mass spectrum. Three inner peptides from ILGI (KFGIYSSAGTYTCGGRF, KLPGFHCAMSRI, and RIIDFAAPVGQKA) were sequenced. These peptides were used for homology searching by BLAST, which exhibited the highest similarity to the GH 27 family α-galactosidase from Trametes versicolor. The first fragment contained a conserved amino acid “Y” (from the sequence “KFGIYSSAGT”) of the GH 27 family in the active site. These results suggest that the protein is a novel GH-27 α-galactosidase.
Biochemical properties of ILGI
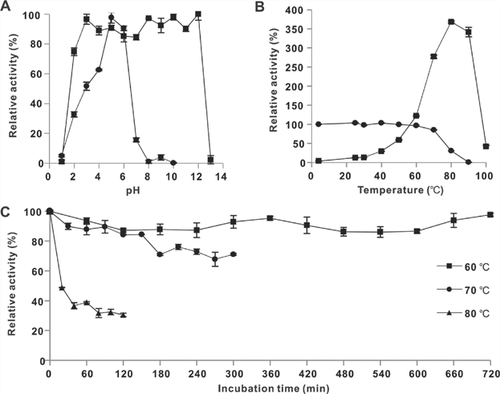
ILGI exhibited the maximum activity at pH 5.0 (Fig. 3A) and was fairly stable under a wide pH range of 2.0–12.0 (Fig. 3A), retaining over 75% enzyme activity after 2 h of incubation. The optimal temperature for the enzyme activity was 80 °C (Fig. 3B). ILGI demonstrated excellent thermal stability, i.e., it was stable from 4 to 70 °C (Fig. 3B). It retained 98% activity at 60 °C for 12 h and 82 and 71% activities after exposure to 70 °C for 2 and 5 h, respectively (Fig. 3C). After treatment at 80 °C for 2 h, ILGI still retained 30% activity (Fig. 3C).
As shown in Table 2, ILGI activity was completely inhibited by Hg+, Ag+, and high Fe3+ concentrations (10 mM). Cu2+ was a partial inhibitor at high concentration (at 10 mM, 75% enzyme activity was retained). K+ demonstrated slight inhibition at 10 mM. Ca2+, Mg2+, Cd2+, Mn2+, Zn2+, Fe2+, Pb2+, Al3+, and EDTA showed negligible effects on enzyme activity. For the chemical modification reagents, the enzyme activity was significantly inhibited after incubation with 0.1 mM NBS, and no reduction in the activity was detected after treatment with 2,4,6-trinitrophenol (TNBS) and diethyl pyrocarbonate (DEPC) (Fig. 4A). ILGI retained high activity when incubated with 100 mM sucrose (99.8%), fructose (106%), and xylose (86.6%). By contrast, ILGI was partially inhibited by 100 mM glucose (60.7%) and strongly inhibited by 100 mM galactose (17%) (Fig. 4B).
| Relative α-galactosidase activity (%) | ||||
|---|---|---|---|---|
| Metal ions and EDTA | 1.25 mM | 2.5 mM | 5 mM | 10 mM |
| K+ | 103 ± 2 | 102 ± 3 | 96 ± 0.8 | 93 ± 3 |
| Ag+ | 2 ± 1 | 3 ± 1 | 2 ± 1 | 9 ± 7 |
| Ca2+ | 106 ± 4 | 101 ± 2 | 101 ± 1 | 101 ± 1 |
| Cu2+ | 93 ± 2 | 91 ± 4 | 81 ± 3 | 76 ± 5 |
| Mg2+ | 101 ± 2 | 100 ± 4 | 99 ± 3 | 98 ± 2 |
| Cd2+ | 96 ± 2 | 96 ± 5 | 96 ± 5 | 106 ± 2 |
| Mn2+ | 89 ± 6 | 95 ± 7 | 107 ± 3 | 119 ± 4 |
| Hg2+ | 3 ± 2 | 1 ± 0.36 | 1 ± 0.53 | 1 ± 0.35 |
| Pb2+ | 94 ± 7 | 97 ± 2 | 96 ± 4 | 100 ± 3 |
| Zn2+ | 93 ± 1 | 92 ± 5 | 99 ± 3 | 108 ± 4 |
| Fe2+ | 100 ± 1 | 94 ± 5 | 114 ± 9 | 93 ± 6 |
| Al3+ | 92 ± 7 | 101 ± 8 | 97 ± 3 | 93 ± 1 |
| Fe3+ | 60 ± 3 | 43 ± 2 | 28 ± 2 | 8 ± 5 |
| EDTA | 105 ± 5 | 102 ± 7 | 100 ± 4 | 98 ± 4 |
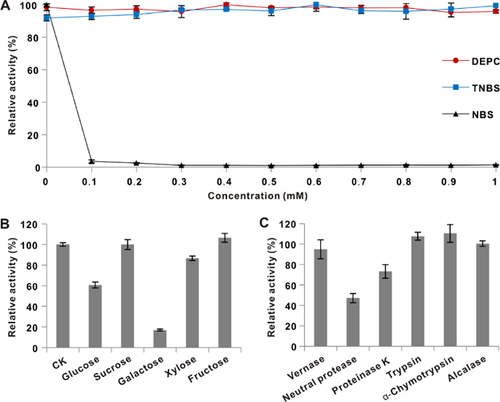
ILGI exhibited strong resistance to vernase, proteinase K, trypsin, α-chymotrypsin, and alcalase by retaining 94.8, 73.1, 107.6, 110.5, and 100.5% activities, respectively, after 1 h of incubation. However, ILGI was partially inhibited by neutral protease, retaining 47.1% activity (Fig. 4C).
Substrate specificity and determination of kinetic parameters
The substrate specificity of ILGI was tested, and the results are listed in Table 3. ILGI displayed the highest efficiency to pNPGal but did not present detectable activity against p-nitrophenyl β-D-galactopyranoside and p-nitrophenyl α-D-glucopyranoside. ILGI exhibited higher activity toward melibiose, raffinose, and stachyose than toward pNPGal (100%) but cannot act efficiently toward locust bean gum and guar gum.
| Substrates | Relative activity (%) | Enzyme activity (U ml−1) |
|---|---|---|
| pNPGal | 100 ± 5 | 1.06 |
| p-Nitrophenyl β-D-galactopyranoside | nda | 0.007 |
| p-Nitrophenyl α-D-glucopyranoside | nd | 0.003 |
| Melibiose | 58 ± 4 | 0.62 |
| Raffinose | 56 ± 3 | 0.60 |
| Stachyose | 64 ± 2 | 0.68 |
| Locust bean gum | 9.8 ± 1 | 0.10 |
| Guar gum | nd | 0.014 |
- a nd, not detected.
With regard to the kinetic properties of ILGI (Table 4), it showed the lowest Km toward pNPGal, followed in increasing order by melibiose, stachyose, and raffinose. The rate of hydrolysis (Vmax) of raffinose was the highest, followed in decreasing order by stachyose, pNPGal, and melibiose. Although ILGI showed a higher catalytic constant (kcat) for raffinose, the catalytic efficiency (kcat/Km) for raffinose was the lowest. By contrast, the kcat and kcat/Km values for pNPGal were the highest.
| Substrates | Km (mM) | Vmax (mM min−1) | kcat (s−1) | kcat/Km (mM−1 s−1) |
|---|---|---|---|---|
| pNPGal | 1.27 | 0.12 | 44 | 17.48 |
| Melibiose | 3.24 | 0.11 | 5 | 1.63 |
| Raffinose | 22.15 | 0.31 | 15 | 0.66 |
| Stachyose | 7.1 | 0.22 | 10 | 1.44 |
Galactose was found to be a noncompetitive (uncompetitive and noncompetitive mixed) inhibitor of ILGI when pNPGal was used as assay substrate (Fig. 5). The inhibition constants (Ki and Kis) were determined by Dixon plot to be 3.34 and 0.29 mM, correspondingly.

Enzymatic hydrolysis of stachyose and TLC analysis
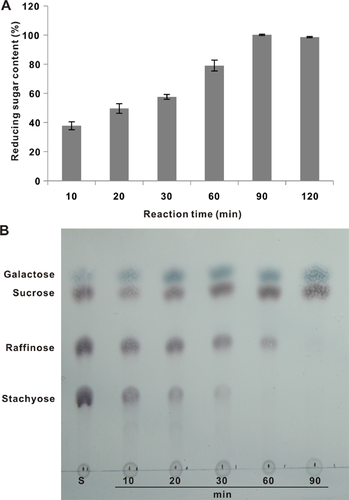
The change in the hydrolysis of stachyose over time is shown in Fig. 6. The reducing sugar content gradually increased within 1.5 h and then remained constant; this finding demonstrated that stachyose was completely hydrolyzed (Fig. 6A). TLC analysis indicated that stachyose was hydrolyzed into raffinose, galactose, and sucrose within 1 h and was completely hydrolyzed after 1.5 h, forming galactose and sucrose (Fig. 6B).
Hydrolysis of RFOs from soybean and soybean cake meal
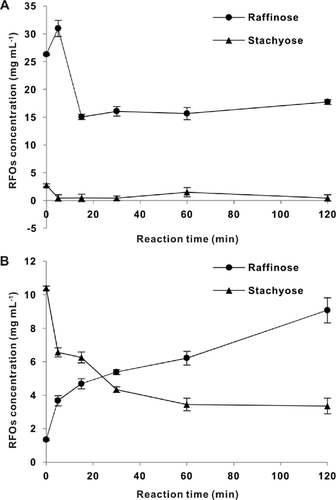
HPAEC results showed that 52.6 and 31.2 mg of raffinose and stachyose, respectively, were contained in 1 g each of soybean and soybean cake. As shown in Fig. 7, the raffinose content of soybean cake meal (Fig. 7A) extract declined from a high concentration of 26 mg ml−1 (52 mM) to 15 mg ml−1 (30 mM) after enzyme treatment for 30 min, while the stachyose content of soy milk (Fig. 7B) declined from 10 mg ml−1 (16 mM) to 4 mg ml−1 (6 mM). The raffinose content initially increased because of stachyose hydrolysis.
Discussion
Numerous α-galactosidases have been purified and characterized for their potential biochemical and industrial application values. In the present study, a novel α-galactosidase was purified from the mycelial fermentation of the edible mushroom I. lacteus. The obtained enzyme is a monomeric protein with a molecular mass of 60 kDa. The molecular mass of α-galactosidases formed by microorganisms is significantly different. The α-galactosidases from Streptomyces griseoloalbus 22, Aspergillus sp. MK14 23, and Talaromyces emersonii 24 are monomeric proteins with molecular masses of 35, 59, and 70 kDa, respectively. Multimeric α-galactosidases from different microorganisms such as Lactobacillus helveticus (homodimer, 188 kDa), Rhizopus sp. (trimer, 210 kDa), and Penicillium sp. (tetramer, 330 kDa) have also been reported 11, 25, 26.
ILGI exhibits an optimal pH and temperature of 5.0 and 80 °C, indicating its suitability in applications under acidic and high-temperature conditions. ILGI also demonstrates excellent pH and thermal stabilities. These features suggest that ILGI can work well with low activity losses during frequent relatively harsh conditions of industrial processing 27. The efficiency of hydrolysis can increase at high temperatures, and the risk of contamination by mesophilic organisms can be reduced. The pH and thermal stabilities of ILGI are better than those of most α-galactosidases reported. A thermostable α-galactosidase from Aspergillus parasiticus is stable within the pH range of 5.0–8.0 and loses 50% activity after incubation at 60 °C for 5 h 28. The half-life for the inactivation of S. griseoloalbus α-galactosidase at 70 °C is 30 min, and ILGI is active over the pH range of 3.0–9.0 22. Two α-galactosidases have been isolated from the thermophilic Neosartorya fischeri P1. One of these is stable from pH 2.0 to pH 12.0 and loses 50% activity after incubation at 60 °C for 1 h; the other retains 50% activity after 15 min at 70 °C and is stable at pH 3.0–11.0 29.
Similar to most known α-galactosidases, ILGI is inhibited by Cu2+, Fe3+, Ag+, Hg2+, and NBS 22, 30. Generally, Ag+ and Hg2+ extremely inhibit α-galactosidase activity because these metal ions may attack cysteine and interfere with the substrate binding in the catalytic site; cysteine is one of three free cysteine residues in the catalytic pocket 31. NBS can also severely inactivate α-galactosidases, indicating that one or more tryptophan residues are located in or near the active site 32. Other reagents, namely, EDTA, DEPC, and TNBS, do not show any considerable effects on enzyme activity, demonstrating that ILGI is not a metalloenzyme, and the key parts (i.e., active and catalytic sites) of ILGI do not contain histidine or arginine residues 32.
ILGI is noncompetitively (uncompetitively and noncompetitively mixed) inhibited by galactose; a similar result has been reported by Viana et al. 33. However, galactose has been reported to be a competitive inhibitor against many α-galactosidases. Two α-galactosidases from S. griseoloalbus are competitively inhibited by galactose with Ki values of 23.4 and 13.1 mM 22. The same situations occurred in Aspergillus terreus and Cypripedium arietinum α-galactosidases, with Ki values of 0.76 and 1 mM, respectively 7, 34.
ILGI activity is slightly activated by incubation with trypsin and α-chymotrypsin. Some reported α-galactosidases exhibit a similar property. Cao et al. 35 found a recombinant α-galactosidase from Rhizopus sp. that can be activated by treatment with trypsin, α-chymotrypsin, and collagenase. A protease-resistant α-galactosidase from Rhizomucor miehei was determined to be activated in the presence of proteinase K, trypsin, and subtilisin 30. The relative activity of an α-galactosidase from Sphingomonas sp. was increased by treatment with trypsin and proteinase K 36. Further research is still required to explain the mechanism for the increased enzymatic activity after protease digestion. Protease resistance is significant in different industries because α-galactosidases are commonly used as additives in conjunction with proteases.
ILGI exhibits the highest activities on pNPGal but does not display any activity on other synthetic substrates containing β-linkages or glucose residues. This phenomenon indicates that both galactose and α-linked residues are essential for enzymatic activity. Similar results can be observed with α-galactosidase from Debaryomyces hansenii 33. Natural oligosaccharides, such as melibiose, raffinose, and stachyose, are susceptible substrates for ILGI. Furthermore, ILGI exhibits slight hydrolysis in locust bean gum but hardly acts on guar gum. α-Galactosidases were divided into two groups by Dey et al. 37 on the basis of their substrate specificity. α-Galactosidases from GH family 27 are typically classified into one group and exhibit enzymatic activities on short oligosaccharides and polymeric substrates 22, 29. By contrast, GH family 36 α-galactosidases present high specificity toward small oligosaccharides 5, 30.
With regard to their kinetic parameters, most α-galactosidases demonstrate better binding affinity (lowest Km) toward pNPGal than toward oligosaccharides 7, 30, 34, 38. A higher catalytic efficiency (kcat/Km) may indicate that ILGI can hydrolyze the substrates at a faster rate 30.
Many of the studied α-galactosidase suffered from a slow or incomplete hydrolysis toward RFOs, which hindered the industrial application. Chen et al. 5 achieved 100% hydrolysis within 8 h by incubating stachyose with 10 U ml−1 of α-galactosidase. Incomplete hydrolysis by α-galactosidase was reported by Cao et al. 35 after a 24 h reaction; 20.65% of stachyose was degraded after incubation with α-galactosidases from Rhizopus sp. F78 ACCC 30795. As discussed in sections Enzymatic Hydrolysis of Stachyose and TLC Analysis and Hydrolysis of RFOs from Soybean and Soybean Cake Meal, ILGI completely hydrolyzed stachyose and showed rapid and efficient hydrolysis toward high concentration of RFOs from soymilk and soybean cake meal. The incomplete hydrolysis may be ascribed to the high concentration of RFOs and low enzymatic activity, as well as the inhibition of galactose. Many studies have reported the transglycosylation of α-galactosidases 23, 28. As presented in section Hydrolysis of RFOs From Soybean and Soybean Cake Meal, the raffinose content slightly increased at the end of the reaction. This increase may be caused by transglycosylation and needs further research. Consistent with previous experiments, 60 °C was selected as the reaction temperature (sections Biochemical Properties of ILGI and Substrate Specificity and Determination of Kinetic Parameters). However, 70 °C may also be a good choice in industrial applications because ILGI demonstrates increased activity and considerable stability at this temperature.
In particular, I. lacteus is an appropriate source for enzyme production because it is an edible mushroom that grows rapidly. The crude enzyme from I. lacteus is safe and can be directly used as food and feed additives. Generally, crude enzymes are cheap and easy to produce. We estimated the cost of small-scale production of ILGI (crude enzyme) to be similar to that of commercial products. For large-scale production, the cost will be lower. In addition to the culture filtrate, the mycelium of I. lacteus can be used as feed nutritional additives.
In conclusion, ILGI exhibits distinctive features such as thermal and pH stabilities, protease resistance, and effective degradation on RFOs. In future studies, high levels of ILGI can be cloned and expressed in bacteria or yeast for purification 5. Fermentation conditions can also be optimized because I. lacteus itself is appropriate to be used as food and feed additives 17. The post-processing techniques of its enzyme, such as immobilization, are also important to enhance the enzymatic properties 1, 2. These findings suggest that ILGI is a good choice as a promising product with potential applications in industry and research.
Acknowledgments
This work was financially supported by China Agriculture Research System (CARS24) and Special Fund for Agro-scientific Research in the Public Interest (No. 201303080).
Conflict of interest
The authors have declared no conflict of interest.




