Recent advances in human viruses imaging studies
Abstract
Microscopy techniques are often exploited by virologists to investigate molecular details of critical steps in viruses’ life cycles such as host cell recognition and entry, genome replication, intracellular trafficking, and release of mature virions. Fluorescence microscopy is the most attractive tool employed to detect intracellular localizations of various stages of the viral infection and monitor the pathogen-host interactions associated with them. Super-resolution microscopy techniques have overcome the technical limitations of conventional microscopy and offered new exciting insights into the formation and trafficking of human viruses. In addition, the development of state-of-the art electron microscopy techniques has become particularly important in studying virus morphogenesis by revealing ground-braking ultrastructural details of this process. This review provides recent advances in human viruses imaging in both, in vitro cell culture systems and in vivo, in the animal models recently developed. The newly available imaging technologies bring a major contribution to our understanding of virus pathogenesis and will become an important tool in early diagnosis of viral infection and the development of novel therapeutics to combat the disease.
Abbreviations
-
- AAV
-
- adeno associated virus
-
- CFP
-
- cyan fluorescent protein
-
- CLEM
-
- correlative light electron microscopy
-
- DC SIGN
-
- dendritic cell specific intercellular adhesion molecule-3-grabbing non-integrin
-
- DenV
-
- dengue virus
-
- EM
-
- electron microscopy
-
- ET
-
- electron tomography
-
- FIB/SEM
-
- focused ion beam/scanning electron microscopy
-
- FlAsH
-
- fluorescein biarsenical hairpin binder
-
- FLIM
-
- fluorescence life time microscopy
-
- FLIP
-
- fluorescence loss in photobleaching
-
- FM
-
- fluorescence microscopy
-
- FRAP
-
- fluorescence recovery after photobleaching
-
- FRET
-
- fluorescence resonance energy transfer
-
- GFP
-
- green fluorescent protein
-
- GSD
-
- ground state depletion
-
- HA
-
- hemagglutinin
-
- HBsAg
-
- hepatitis B surface antigen
-
- HBV
-
- hepatitis B virus
-
- HCI
-
- high content imaging
-
- HCV
-
- hepatitis C virus
-
- HIV
-
- human immunodeficiency virus
-
- HPV-16
-
- human papillomavirus 16
-
- HSV-1
-
- herpes simplex virus type 1
-
- JFH-1
-
- Japanese fulminant hepatitis 1
-
- LACV
-
- La Crosse virus
-
- MSIM
-
- multifocal structured illumination microscopy
-
- PALM
-
- photoactivation localization microscopy
-
- PRR
-
- pathogen recognition receptor
-
- ReAsHs
-
- red biarsenical hairpin binder
-
- RIG-1
-
- retinoic acid inducible gene I
-
- RSV
-
- respiratory syncytial virus
-
- SEM
-
- scanning electron microscopy
-
- SIM
-
- structured illumination microscopy
-
- SMT
-
- single molecule tracking
-
- STED
-
- stimulated emission depletion
-
- STORM
-
- stochastic optical reconstruction microscopy
-
- TEM
-
- transmission electron microscopy
-
- TIRF
-
- total internal diffraction fluorescence
-
- vMIA
-
- viral mitochondria localized inhibitor of apoptosis
-
- VSV
-
- vesicular stomatitis virus
-
- YFP
-
- yellow fluorescent protein
Introduction
Despite numerous molecular and biochemical techniques which have emerged in the last years to complete the research on human viruses, microscopy techniques are still the leading approaches in the identification of new viruses and characterization of pathogen-host cells interactions after viral infection (Fig. 1).
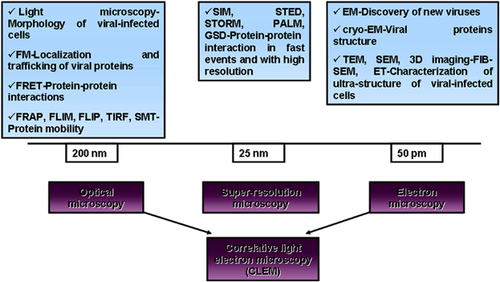
One of the most potent techniques for identification and characterization of ultra-structural features of human viruses and infected cells remains the electron microscopy (EM). Fluorescence microscopy (FM) tools contributed enormously in understanding the architecture of viral protein localizations in infected cells as well as the interactions established between viral and cellular proteins during viral life cycle progression. To date, new super-resolution (SR) imaging tools are being developed to over-come the diffraction limit and consecutively new mathematical applications have been developed in order to perform computational image processing and analyze the newly generated data in a quantitative manner. Correlative light and electron microscopy (CLEM) is a technique of interest for many virological issues. It combines the potency of FM for finding rare events in a population and the resolution of EM for imaging their ultrastructure.
Bright field light microscopy
Bright field microscopy is the simplest optical microscopy illumination technique and it usually requires a highly contrasted sample. If the sample is semi- transparent and contains only a few highly refractive regions, the phase contrast microscopy is used to render a contrast-enhancing effect in the internal cellular components of the specimen to be visualized. Since viruses are too small to be seen in bright field light microscopy, this illumination technique is usually employed to investigate the stage of virus infection in cells in general and to detect hallmarks of necrosis or apoptosis in infected cells in particular. This technique is extensively used in viral pathology to view stained sections of fixed tissue.
For identification of viruses and investigation of intimate structures of the viral particle, more powerful imaging techniques such as FM or EM are required.
Fluorescence microscopy
FM techniques consist of simply labeling the viral protein of interest with specific fluorophores. One should keep in mind that in the case of super-resolution FM (SR-FM) the choice of the dyes should suit the SR method selected by the microscopist. Natural autofluorescent proteins such as green fluorescent protein (GFP) have demonstrated to be invaluable for viral proteins labeling. However, many common fluorescent proteins used to tag viral protein in SR techniques are unsuitable and new fluorophores should be developed soon (Table 1). In addition, this direct labeling may not be compatible with the viral protein native structure and function, therefore, through investigation of the properties of the labeled proteins is strongly recommended.
| Fluorophore location | Method of labeling | Virus and references |
|---|---|---|
| Various viral proteins | Fusion proteins (GFP, mCherry) | Vaccinia virus 105, HIV 106-109, ASLV 110, HBV 4 |
| Envelope | Lipophilic dyes (DiO, DiI, R18) | Influenza A virus 111, HCV 112, Ebola virus 113, HIV 107, HBsAg particles 114, VSV 115 |
| Membrane protein | Enzymes (SNAP, sortase) | HIV 116, Influenza A virus 117 |
| Membrane protein | Quantum dots | Adeno-associated virus 118, VSV-G pseudotyped lentivirus or retrovirus 119 |
| Membrane protein | pH sensitive dye | Poliovirus 120 |
| Membrane or capsid protein | Covalent linkage (hydrazine, maleimide, NHS) | HPV-16 121, VSV 122, 123, HBsAg particles 114 |
| Non-covalent linkage (biarsenical dyes FlAsH fluorescein; ReAsH resurfin) | HIV 53, 55, 124, Influenza A virus 125, HCV 126-128, HBV 129 |
- GFP, green fluorescent protein; DiO, oxacarbocyanines; DiI, indocarbocyanines; R18, octadecylrhodamine B; SNAP, derivative of alkyl-guanyl-transferase; NHS, N-HydroxySuccinimide; FlAsH, fluorescein biarsenical hairpin binder; ReAsH, red biarsenical hairpin binder; HIV, human immunodeficiency virus; ASLV, avian sarcoma and leukosis virus; HBV, hepatitis B virus; HCV, hepatitis C virus; HBsAg, hepatitis B surface antigen; VSV, vesicular stomatitis virus; HPV-16, human papillomavirus-16.
Based on FM, Eyre et al. 1 has investigated hepatitis C virus (HCV) replication and assembly, by employing a pulse-chase imaging approaches. The group was able to show that newly synthesized NS5A-positive foci differ in size and position from aged foci in HCV-infected cells. Using a unique dual fluorescently tagged HCV chimera (tetracystein motif-tagged HCV proteins were labeled with the biarsenical dyes FlAsH and ReAsH) the authors have demonstrated the association of NS5A proteins with the core-capped lipid droplets (LDs). The results have indicated the tight interconnection between the replication complexes and the HCV assembly pathways 1.
FM is a very useful technique to investigate virus internalization in permissive cells. When direct labeling of virus particles is not possible, cellular components of the endocytic and trafficking pathways, and well-characterized internalization markers can be fluorescently labeled instead, followed by quantitative analysis of viral components (either proteins or genomes) in relation with these markers. Using such an approach, our group was able to show that HBV requires functional Caveolin-1- to initiate productive infection in HepaRG cells 2 and that HBV infection strongly depends on the viral particles transport from early to mature endosomes 3. More recently, the group has investigated the possibility to fluorescently label the HBV envelope proteins, in order to facilitate direct monitoring of viral particle assembly. The results have shown that the HBV middle (M) envelope protein can tolerate an N-terminal fusion with the enhanced GFP (EGFP), without losing the native conformation and the dimerisation capacity. However, viral particles secretion was impaired from cells co-expressing the fluorescent fusion protein suggesting that viral assembly was not very efficient 4. Using FM and co-localization experiments, Dorobantu et al. have shown that in contrast to HCV, hepatitis B virus (HBV) morphogenesis occurs independently of LDs 5.
Immuno-fluorescence microscopy (IFM) uses specific antibodies which are tagged with fluorophores in order to identify the presence of viral proteins inside infected cells and their specific locations. As in any antibody recognition-based approach, the data obtained using this technique must be confirmed by alternative molecular and biochemical experiments. As opposed to conventional light microscopes, IFM can be used to label human viruses directly with fluorescent dyes, but has a light diffraction limit dictated by optical properties of light of ∼200 nm. Under these circumstances, fluorescence-labeled particles will appear as merged fluorescent spots with a diameter of ∼200 nm. As a consequence, one cannot differentiate between individual fluorescence-labeled viral particle and clustered virions.
A very useful microscopy approach employed for host cell-virus interactions characterization, especially in genome-wide reverse genetic screens, is the High-Content Imaging (HCI) technique 6. HCI is a multi-stage process which combines high-throughput microscopy analysis with automated, multiparametric imaging detection. Briefly, cells are treated with siRNA to down regulate the expression of a particular cellular target gene, hypothetically involved in viral infection. Infected cells are then subjected to immunostaining and processed for FM to detect a specific viral antigen and thus to quantify the percentage of infection. The researcher can thus determine hundreds of cellular genes involved in the inhibition of the viral processes after only one round of infection. In HCI multi-well imaging plates and robotic liquid handling equipment are used to investigate the effect of libraries of thousands of siRNA on infected cells. High-throughput microscopes record fluorescence images from multiple channels and from multiple fields in a single well and then collect the data from multiple wells of the same plate. Special imaging software analyze and quantify rapidly the data obtained from thousands of aquired images. HCI-based technology proved to be also useful especially for a rapid screening of potential anti-viral compounds, evaluation of their cytotoxicity, and mode of action.
Over traditional highthroughput screening assays, HCI-based analysis is an extremely effective drug discovery tool that offers more informations regarding specific virus-host protein interactions the compounds are acting on. The use of this method has increased the efficiency of the drug discovery development process. Recently, small-molecule libraries containing compounds targeting proteases or cellular signal transduction pathways have been used with HCI for new anti-viral agents discovery. Thus several compounds active against Rift Valley fever virus (RVFV) and other highly pathogenic RNA viruses such as Venezuelan equine encephalitis virus (VEEV), Chikungunya virus (CHIKV), Lassa virus (LASV), Junin virus (JUNV), Marburg virus (MARV), and Ebola virus, were reported by Mudhasani et al. 7, 8.
Using HCI assay with a genome-wide siRNA screens it was thus possible to detect mammalian cells genes involved in replication step of human immunodeficiency virus-1 (HIV-1) 9, 10, HCV 11, 12, influenza A virus 13, 14, and West Nile virus 15.
Advanced microscopy
New image-based techniques exploit the spatial distribution of the fluorophores intensities to measure the accurate position and brightness of the labeled-viral protein, leading to improved resolution. Advanced microscopy techniques also are useful to determine more accurately temporal molecular interactions between viral proteins and viral-host proteins, such as the kinetic of viral assembly process or plasma membrane microdomains formation. Based on the IFM principles, more advanced FM techniques were developed over time.
One powerful method to detect molecular interactions between two viral proteins in infected cells is the fluorescence resonance energy transfer (FRET). This method is based on the energy transfer between two fluorescently-labeled molecules and is able to determine if the two chromophores are situated at a relative significant distance of each other. Emission from the donor is transmitted to the acceptor through long-range dipole–dipole intermolecular coupling and only the second signal is registered if the two fluorophores are associated. By registering increased fluorescence of the acceptor, detecting changes in fluorescence life-time or determine the photobleaching rate of the donor fluorophore when the acceptor fluorophore is present or absent, one can determine the efficiency of this technique. The most common fluorophores used in FRET are the yellow fluorescent protein (YFP) and the cyan fluorescent protein (CFP). However, FRET does not necessarily occur when the two fluorophores involved actually interact with each other because there is a limited angle for the position of the donor and acceptor fluorophores in order for FRET to occur 16. Therefore, backup techniques, such as co-immunoprecipitation are commonly used to confirm the absence of interaction between the two viral proteins.
Other useful techniques in visualizing the dynamics of human viruses in host cells are the fluorescence loss in photobleaching (FLIP) and the fluorescence recovery after photobleaching (FRAP). In these approaches, a confocal microscope is employed for the photobleaching of an individual area within a cell where the fluorescently labeled-protein is localized. Usually green GFP is used to tag the protein-of-interest for a photobleaching experiment. In FRAP, the diffusion coefficient of the fluorescently-labeled-protein or the kinetic coefficient of the reaction of binding/unbinding between two proteins of interest can be detected by the evaluation of the rate at which the fluorescence is restored in that specific photobleached region. When the fluorescence level is not replenish at the initial level, it indicates that a fraction of the fluorescence is immobile and thus cannot be restored by diffusion. For instance, FRAP studies can be employed in investigation of the continuity of organelles at the membranous level. In protein–protein interaction experiments if one of the interactors is static, the fluorescence replenishing is dictated by the association and disassociation rate of the interaction process. The experiments should be carefully designed since employing powerful laser may result in photobleaching or quenching of a fraction of fluorophores in the area studied thus leading to incomplete data acquired by the detector in the recovery of fluorescence after photobleaching process 17.
FLIP is also based on detecting the loss of fluorescence in the area surrounding a multiple-photobleached region. Since this technique is not limited by a distinct photobleached region-of-interest, it can be used to investigate intracellular protein trafficking such as protein shuttling between different intracellular compartments.
In total internal reflection fluorescence (TIRF) microscopy, the fluorophores in the sample are excited by the evanescent wave, resulted after total internal reflection produced at the interface between the coverslip and the sample. A small portion of the fluorophores, which are located mainly at the surface of the sample (cell membrane) are excited, and in the same time only few other fluorophore molecules located outside the evanescent field (cytoplasm) emit signal. Thus, during TIRF illumination signal loss is minimal while the background noise is very low and does not interfere with the signal generated by the protein-of-interest. As a consequence, this technique is useful for studying plasma membrane processes, virus infection, and secretion from the infected cells.
Based on FRAP/Single molecule analysis, Krementsov et al. have shown for the first time the distribution and dynamics features of tetraspanins in HIV-1-infected cells. The authors have demonstrated that viral assembly process, mainly the structural Gag protein, may influence agglomeration and immobilization of tetraspanin protein CD9 at raft lipids, leading to its partitioning in microdomains at the plasma membrane of infected cells 18.
Munro et al. 19 have employed single molecule FRET and TIRF microscopy to investigate the structural rearrangement of HIV envelope during infection. The authors have introduced small peptide tags inside the gp120 envelope glycoprotein that enabled its enzymatic labeling with fluorophores. By monitoring gp120 conformational dynamics the group was able to show the remodeling of the HIV-1 envelope landscape as a consequence of the interaction with its co-receptor, the CD4 molecule 19.
Recently, El Meshri and collaborators used FLIM combined with FRET to demonstrate the key role of nucleocapsid (NC) domain of HIV-1 Gag in the virus assembly and trafficking towards the cell membrane. Using Gag with deletions in the NC domain the authors have observed that after binding to the nucleic acids, the NC domain determines the oligomerization of Gag in the cytoplasm and the progressive transfer of the closely packed population of oligomers to the plasma membrane 20.
Using FRET with FLIM technique Popescu et al. have studied the interactions between HCV proteins NS2 and the viral porin p7 or the E2 viral glycoprotein. Based on complementary techniques it was shown that these interactions are required for HCV assembly 21.
Multicolor super-resolution images of HIV-1 proteins and plasma membrane microdomains were obtained by TIRF microscopy. The Lehmann's group has shown that a low number of clustered tetherin dimmers, which are interferon-induced transmembrane proteins, is sufficient to inhibit the release of newly formed HIV-1 virions. The authors underlined the importance of a new antiviral strategy consisting of association of cellular protein tetherin with HIV budding sites 22. Using TIRF microscopy Scheffer et al. 23 have performed live-cell imaging, to record the dynamics of papillomavirus particles (HPV16) in HaCaT and HeLa cells, in the context of plasma membrane microdomains of CD151 tetraspanin. The authors concluded that HPV16 co-localize with CD151 on the cell surface and remains associated during lateral movement, suggesting that the association with the tetraspanin is a precondition in HPV16 endocytosis and infection 23.
Imaging techniques have been employed to investigate the role of tetraspanins, a family of proteins that forms dynamic clusters with various other proteins in the plasma membrane, in virus entry. FRET microscopy was used by Harris et al. in a series of studies to investigate the behavior of CD81, which is a major receptor for HCV, in viral infections. The authors demonstrated that for an efficient HCV entry into its target cells the direct interaction of the tetraspanin protein CD81 with the tight junction protein claudin-1 is requested 24-26. Moreover, with FRAP/TIRF techniques the authors demonstrated that the dynamics features of CD81 at the plasma membrane, such as lateral diffusion, might limit HCV internalization in polarized hepatoma cells 27. Potel et al. 28 confirmed these data by single-molecule tracking experiments (SMT), which consists of labeling of a very low number of molecule in order to be able to follow individual molecules over time. Using TIRF technique, the authors showed that EWI-2wint, an inhibitor of HCV entry into the cells, could determine a change in the partitioning of CD81 at the plasma membrane as well as influence the co-localization of CD81 with claudin-1, and thus leading to the inhibition of HCV entry into the target cell 28.
FLIM microscopy was used on Huh-7 cells to demonstrate that over-expression of the domain 2 (D2) of the HCV core protein (D2-GFP fusion protein), which disrupts mitochondrial electron transport, increased the level of free reduced nicotinamide adenine dinucleotides (NAD(P)H) and altered NAD(P)H microenvironment. The changes of metabolic co-enzymes correlates with D2 core protein induced-influence on host lipid metabolism 29.
Using FRET, Thaa et al. 30 have shown that clustering between influenza A virus hemagglutinin (HA) and M2 viral protein is reduced upon disruption of HA's lipid rafts association features (acylation, transmembranous valine-isoleucine-leucine motif). However, the association between the two viral proteins remains unchanged when M2 lacks acylation and/or cholesterol binding sites 30. By combining FLIM–FRET and FRAP techniques, the same group has shown a strong association of cytoplasmic tail of the CFP-labeled HA with Myr-Pal-YFP, which is considered a marker for the rafts located at the inner face of the plasma membrane 31. A comprehensive review regarding advanced microscopy techniques useful for viral proteins detection on influenza virus was recently published by Veit et al. 32.
Super-resolution microscopy
The use of conventional wide-field fluorescence microscope in virology is limited by the fact that the size of a viral particle is below the diffraction limit of light. In other words, 200 nm is the highest resolution that can be obtained by conventional microscopes. Different SR microscopy techniques that make use of new physical and chemical concepts have been developed in recent years to allow researchers to break the resolution limit of conventional microscopes and visualize subcellular details of biological events 33, 34. In virology, this breakthrough is particularly important in order to investigate molecular details within the viral particles.
SR microscopy gives the virologists an excellent opportunity to address scientific issues impossible to investigate by conventional FM. Usually, the questions the biologists are trying to answer when using SR microscopy are as follows: a) what is the virus structure and localization; b) how is the viral proteins assembly process regulated; c) which are the molecular details of infection and replication of the viral particle within the host cells.
Various SR microscopy techniques have been used recently to reveal new exciting features in the field of virology such as: structured illumination microscopy (SIM), stimulated emission depletion (STED), photo-activated localization microscopy (PALM), and stochastic optical reconstruction microscopy (STORM). Some of these techniques can even be combined (Table 2).
| Microscopy technique | Resolution (nm) | Fluorescent probe used | Virus study application |
|---|---|---|---|
| SIM/MSIM | 50–150 | RFP-A3 viral core protein, YFP-B5 viral integral membrane protein, YFP-A36 viral envelope protein | Vaccinia virus 38, 39 |
| NS3 and NS4B viral replication proteins and DNAJC14 cellular chaperone protein immunostained with Alexa Fluor 488- or 594-conjugated Abs | Yellow fever virus 40 | ||
| Fluorescein-conjugated flavivirus-derived cell-penetrating peptide (CPP) | Flaviviruses 41 | ||
| Nuclear (N), glycoprotein (G), and matrix (M) viral proteins immunostained with Alexa Fluor 488- or 568- conjugated Abs | Hendra virus 69 | ||
| EGFP-vMIA viral protein | HCMV 71 | ||
| STED/GSTED | 50–125 | ATTO-647N–labeled p24 capsid viral protein, EGFP-Vpr, Env immunostained with ATTO-565-conjugated Abs | HIV-1 45, 46 |
| viral DNA stained with Alexa Fluor 488-azide,capsid viral protein immunostained with AbberiorSTAR-440SX-conjugated Abs | Adenovirus 68 | ||
| EGFP-vMIA viral protein | HCMV 71 | ||
| PALM | 20–50 | Gag-mEosFP, FlAsH-tagged integrase viral protein, Gag-EosFP, AlexaFluor 647-labeled Gag-FLAG, Gag-mEos2 | HIV-1 52, 53, 56, 60, 61 |
| L-EGFP catalytic subunit of viral polymeraseEGFP-P template-binding protein subunit of viral polymerase | VSV 66 | ||
| PAmCherry-vMIA viral protein | HCMV 71 | ||
| STORM/dSTORM | 20–50 | Env immunostained with Alexa Fluor 647-conjugated Abs, FlAsH-tagged integrase viral protein, p24 capsid viral protein immunostained with Cy5-conjugated Abs, Gag-SNAP labeled with Alexa Fluor 647, Nucleoporin Nup153-Alexa568 (green) and histone H3K36me3-Cy5 (red), matrix viral protein immunostained with Cy5-conjugated Abs | HIV-1 52-55, 57 |
| Dengue virus immunostained with ATTO-488-conjugated Abs, DC-SIGN immunostained with AlexaFluor 647-conjugated Abs | Dengue virus 63 | ||
| AlexaFluor 647-labeled CD81 cellular protein and ATTO-488-labeled PB1 viral protein of Udorn strain | Influenza A virus 65 | ||
| core, Jc1- FLAG −E2 and JFH1- FLAG −E2 envelope viral proteins immunostained with AlexaFluor 488- and AlexaFluor 647-conjugated Abs | HCV 64 | ||
| GSD | 25–50 | Nuclear (N) and glycoprotein (G) viral proteins immunostained with AlexaFluor 488- and AlexaFluor 568- conjugated Abs | Hendra virus 69 |
| LACV viral particle and RIG-I cellular RNA helicase immunostained with AlexaFluor 488- and AlexaFluor 647-conjugated Abs | LACV 67 |
- RFP, red fluorescent protein; YFP, yellow fluorescent protein; EGFP, enhanced green fluorescent protein; PAmCherry, photoactivatable variant of mCherry; ATTO-647N, highly photostable red fluorescent dye; EosFP, green-to-red photoswitchable fluorescent protein; FlAsH, fluorescein biarsenical hairpin binder; SNAP, derivative of alkyl-guanyl-transferase; DC-SIGN, dendritic cell-specific intercellular adhesion molecule-3-grabbing non-integrin; Abs, antibodies; HIV, human immunodeficiency virus; DENV, Dengue virus; HCV, hepatitis C virus; JFH1, Japanese fulminant hepatitis-1; Jc1, HCV genotype 2a/2a chimera; LACV, La Crosse virus; VSV, vesicular stomatitis virus; HCMV, human cytomegalovirus; vMIA, viral mitochondria-localized inhibitor of apoptosis; RIG-I, retinoic acid-inducible gene I; SIM, structured illumination microscopy; MSIM, multifocal SIM; STED, stimulated emission depletion; GSTED, gated STED; PALM, photoactivation localization microscopy; STORM, stochastic optical reconstruction microscopy; dSTORM, direct STORM; GSD, ground state depletion.
In SIM, patterns of light such as parallel stripes are projected onto the sample. Several images are then acquired with the orientation of the light pattern slightly changed as it moves along the sample and is being rotated under various angles. Using computer algorithms, these coarser interference patterns will be used further to generate by reconstructions a final image with information extracted from below diffraction limit and with doubled resolution as compared to conventional microscopy 35. One major drawback in this technique which has limited its application in biological samples studies was the rate of fluorophores photobleaching leading to tissue damaging. The problem was solved using reversible photoswitchable fluorescent protein 36. Based on this method cellular structures were visualized even at 50-nm resolution 37.
3D-SIM imaging was employed to investigate cells infected with vaccinia virus 38. Both, the A3 core protein and the B5 integral membrane protein were fluorescently labeled and investigated as markers for the particle lumen and the viral envelope, which cover the core. The authors have demonstrated that the outer shell of the vaccinia virus was different from the viral core protein and succeeded to study the formation of the virus at subviral resolutions. In further studies the authors have investigated the transmembrane protein A36 at these resolutions and produced new data on viral egress from the infected cell by interaction with cytoskeletal structures 39. 3D-SIM super-resolution technique was used by Yi et al. 40 to investigate the replication complexes of Yellow Fever virus which contain dsRNAs, NS3, and NS4B proteins organized as a network of clustered proteins. Applying direct interaction approach Yi et al demonstrated the association of DNAJC14 protein, a member of the HSP40 family, with the viral replication complexes, providing additional information beside co-immunoprecipitation experiments 40.
Chua et al. have reported the utility of SIM technique in the localization of flavivirus-derived cell-penetrating peptides in a discrete area in the membrane of endosomes, which was previously inaccessible using conventional microscopy techniques 41.
As it uses direct wide-field illumination, sample preparation for SIM is not complicated and usually it uses similar dyes with those used for conventional FM. Also, these aspects allow the technique to perform multi-color imaging and to capture fast events such as human viruses’ internalization and trafficking. One major drawback in this technique is that SIM photobleaches fluorophores. Thus, the use of new generation fluorophores which are more resistant to photobleaching, is strongly recommended.
Stimulated emission depletion (STED) is a confocal microscopy technique that employs two overlaid lasers which one laser beam excites the fluorophore in a conventional manner, while the second laser beam depletes the excited state of the fluorophore in such a way that it inactivates the fluorescence of a region around a 10–100 nm size spot. This gives the possibility to examine only the individual signals emitted by the proteins located in this discrete spot 42. By increasing the intensity of the irradiation of the second laser, the size of the focused region of interest will be decreased. STED uses the feature of fluorophores to give a non-linear response upon excitation to obtain a final image with an enhanced resolution. The final imaging data obtained by STED are not computationally-generated and do not need complicated image processing analysis, which makes this technique user-friendly and more similar to conventional FM in terms of data processing. However, STED still requires complex microscopes with high power lasers, synthetic, photostable dyes, and well-trained microscopists. For STED imaging one can use non-fluorescent, genetically encoded tags such as the SNAP-tag, a 20-kDa derivative of alkyl-guanyl-transferase which covalently self-labels with different fluorescent substrate derivatives 43. When performing live cells experiments a variety of available cell membrane permeable fluorescent substrates or even some particular photostable fluorescent proteins can be employed 44.
By employing STED imaging of HIV-1 virions in dendritic cells, it was shown that viral particles co-localize with intracellular actin at immunological synapses. The dendritic cells were co-cultured with CD4+ T cells and the model was used to establish the functional relevance of the immunological synapses before employing EM techniques 45. STED microscopy was also used by Chojnaki et al. to investigate the distribution of HIV-1 Env glycoprotein. The authors have demonstrated that the clustering of Env on the surface of the viral particle is dependent of the maturation of viral structural Gag polyprotein by proteolysis and of the interaction of Env-tail domain with Gag. These rearrangements in virus architecture were essential for a productive entry of HIV-1 in target cells 46.
Other SR microscopy techniques which also break the limit of resolution obtained by light were developed to yield higher quality images and with resolutions in the nanometer-range.
Single-molecule localization microscopy (SMLM) is based on two techniques: photo-activated localization microscopy (PALM) and stochastic optical reconstruction microscopy (STORM). These are two stochastic functional SR microscopy techniques 47 which use photo-switchable fluorophores that can be converted between two states (on/off) in a manner controlled by light. The experimental set-up proposes that only a small number of distant fluorophores emits a fluorescence signal upon excitation for a defined short period of time. This means that the signal obtained from the fluorophores that are in the fluorescent state will be recorded as signals from each individual molecule. The fluorophores in this subset are next deactivated and another small fraction of fluorophores will be excited to reach the fluorescent state and the process is repeated. Using mathematical algorithms, the precise localization of the molecule can be detected and the final super-resolution image of the structure of interest is reconstructed from the data collected. These wide-field microscopy techniques can be performed using commercially available microscopes that exploit specific characteristics of the conventional fluorophores in order to increase the resolution. Usually, an experiment involves acquisition of tens of thousands images and each frame is recording only a small fraction of individual fluorophores.
In the original STORM method, the fluorophore reaches the metastable “off” state when oxygen scavenging enzymes and/or reducing agents are added in the buffer and after that the fluorescence is stochastically recovered when the first dye is coupled to a secondary dye 48. On the contrary, in direct STORM (dSTORM) the recovery of the fluorescence is based on the permanent switch of fluorophore between the two states 49.
Another SMLM-based technique is the ground state depletion microscopy followed by individual molecule return (GSDIM or even shorter, GSD). In this technique, high power lasers are applied to the sample in order to move the fluorophore molecules from ground to a long-lived dark state (a non-fluorescent state) which allows for individual fluorophore molecules to return stochastically, emit fluorescence and be registered separated from each other. The SR image with the recorded position of each individual fluorophore molecule is obtained using a software algorithm 50, 51. The microscope used for GSD imaging resembles the one employed in STED experiments, however this technique requires special fluorophores.
Muranyi et al. have revealed novel insights in HIV-1 biology by capturing the fundamental assembly and budding processes of HIV-1 in great details using PALM/STORM technique combined with TIRF microscopy. Remarkably, the authors have succeeded to image individual assembly site as a single 130 nm diameter punctum very close to the plasma membrane 52. Only recently molecular imaging on HIV-1 intimate structure has been made available by using super resolution techniques like PALM /dSTORM. The conical morphology of the capsid, the architecture of envelope glycoproteins, and the localization of the FlAsH tagged integrase viral protein inside the capsid were revealed by Lelek et colab. Using nanoscopic measurements of distinct viral particles with PALM 53. In a very recent article, the same group has demonstrated that chromatin organization at the nuclear pore favors HIV replication based on high resolution dual-color STORM analysis of histone H3K36me3 and nuclear pore complex 54.
An increase of the cluster size (∼230%) of the HIV capsid, compared to the matrix protein, during infection was reported based on the SR microscopy technique dSTORM 55.
The structure of HIV structural protein Gag was studied with sptPALM, a microscopy technique combining localization microscopy and SMT. Dynamic analysis experiments revealed that a fraction of the population of membrane-associated viral Gag protein was selected and localized into distinct assembly clusters at the plasma membrane, previously inaccessible using diffraction-limited FM. Using sptPALM the authors showed that the clusters contained dramatically different numbers of molecules as compared to the data obtained after TIRF microscopy 56.
PALM/STORM techniques applied in experiments on fixed cells have shown that the assembly sites of HIV-1 were roughly spherical and of ∼130 nm in diameter, which is in agreement with the already known size of the mature viral particle obtained by the EM 57, 58. Using two-color localization microscopy it was shown that the recruitment of ring-shaped HIV Env proteins takes place around the assembly sites and that the association of Env viral protein with the assembly complex was dependent on the long C-terminal tail of Env and on the matrix region of Gag structural polyprotein 58, 59. Very recently, van Engelenburg et al. have used three-dimensional (3D) localization microscopy with particle averaging to demonstrate that the host ESCRT (endosomal sorting complex required for transport) machinery is recruited by Gag protein to the assembly site of the newly formed virion in order to help the release of HIV viral particles from the cell by membrane fission 60. Based on super-resolution techniques able to quantitate the molecular analysis of the fluorescent HIV-1 protein clusters, it was possible to investigate hundred different labeled Gag clusters during virions assembly at the plasma membrane of the host cells 61.
DC-SIGN (dendritic cell-specific intercellular adhesion molecule-3-grabbing non-integrin), a receptor on the surface of dendritic cells for viruses such as HIV and Dengue virus, was studied using STED microscopy on various receptor mutants. The results have revealed that the viral receptor expressed by the antigen presenting cells was organized in clusters at the viral entry point and that the extracellular neck domain was important for the DC-SIGN clustering and virus internalization 62. The deletion of the cytoplasmic tail of viral receptor DC-SIGN lead to the absence of clustering at the plasma membrane. The microdomains were nano-sized and the smallest clusters contained only 4–8 molecules of DC-SIGN, as shown by dSTORM 63.
Using dSTORM, the relative localizations of core protein and E2 envelope protein of HCV inside the infected cells were determined. The two viral proteins were found on ∼0.2 μm diameter lipid droplets distributed very close (∼1 µm) from the cell membrane and, surprisingly, very little subcellular colocalization of the two viral protein was observed in the individual regions studied using this super-resolution technique. Thus the improved resolution of dSTORM technique versus confocal allowed for the demonstration of very little colocalization of the viral proteins 64.
By using STORM and EM techniques Zhaung and coworkers have recently evaluated whether the tetraspanin CD81 plays a role in the replication process of Influenza A virus, in addition to the well-established function in viral entry. The authors have used fluorescently labeled influenza virus particles to reveal that the virus enters the cells by CD81- positive endosomes, replicate and then assemble the at the cell surface. Moreover, it was observed that the fusion of viral particle with cell membrane was impaired in the absence of CD81. STORM SR analysis of the 3D distribution of CD81 with a ∼20 nm lateral (xy) and ∼50 nm axially (z) resolution revealed that the tetraspanin protein is arranged in a 150 nm distance pattern along filamentous influenza A viral particles 65.
Recently, the sub-virion localization of the asymmetrically packed polymerase complex, in the vesicular stomatitis virus (VSV), a model for Ebola virus, was assessed using high resolution fluorescence imaging such as fPALM and STORM. The study revealed the localization of the recombinant viral protein subunits, L and P linked with EGFP, toward one end of the bullet-shaped virion. Moreover, the polymerase complex was shown to occupy ∼50 nm of a 150 nm central cavity inside the recombinant viral particle 66.
Weber et al. 67 have employed GSD microscopy to image the interaction of retinoic acid-inducible gene I (RIG-1), a cytoplasmic RNA helicase and intracellular pathogen recognition receptor (PRR), with a triphosphorylated 5′ terminus double stranded RNA of encapsidated RNA virus genomes at a 20 nm resolution. The interaction of RIG-1 with dsRNA was found to be crucial for triggering the interferon-based rapid innate immune response and was independent of viral RNA synthesis of RNA viruses such as: influenza A virus, VSV, and La Crosse virus (LACV) 67.
Super-resolution gated STED (gSTED) with dual color was very useful to track the localizations of adenovirus DNA (vDNA) and viral capsid protein after 30 min of viral infection. The viral capsid size was found to be ∼120 nm and the vDNA was ∼80 nm. The images processed at single-molecule resolution suggested that the virus-associated DNA is more compact after encapsidation as compared to free cytoplasmic vDNA. The results confirmed data obtained using X-ray diffraction measurements 68.
Using both GSD and SIM techniques, the localization and dimension of Hendra viral proteins was determined 69. Thus, the diameter of the viral particle was found ∼300 nm, in agreement with EM experiments. The M matrix protein was reported to be rather associated with the complex containing the viral RNA genome than with the viral envelope.
Multifocal structured illumination microscopy (MSIM) is based on illuminating the sample at precise spots and in sequential steps and thus minimizes scattered light. A series of images are then recorded and processed by defining the exact location of the focal spots to obtain a super-resolution image. This powerful technique maintains the optical sectioning of confocal microscopy and provides a twofold resolution improvement beyond the diffraction limit. Recently, a 3D super-resolution image of microtubules in live transgenic zebra fish embryos was obtained using MSIM 70.
Combining superresolution approaches such as gated STED, MSIM, and PALM it was possible to reveal the localization of human cytomegalovirus viral mitochondria-localized inhibitor of apoptosis (vMIA). Thus, the ∼100 nm diameter clustered distribution of vMIA was detected in the outer mitochondria membrane (OMM) and not in mitochondrial matrix as established previously by deconvolution of confocal microscopy imaging 71.
Electron microscopy
The most powerful imaging techniques to visualize and investigate virus internal ultra-structures are EM methods which can reach a resolution of up to 50 pm. The EM investigations enable the elucidation of nanoscopic structures in the biological specimen that would otherwise be impossible to decipher using conventional light microscopy techniques.
Transmission electron microscopy (TEM) is based on the assemble of electromagnetic and electrostatic lenses which are able to transmit through an ultra-thin sample a high voltage electron beam in vacuum. The image of the sample is composed of the unscattered electrons which are captured on a fluorescent screen. If the sample is very dense, the number of electrons passing through is less, and the image is darker.
Scanning electron microscope (SEM) directs an electron beam towards the specimen in a similar way to TEM. The principle of SEM is based on an electron beam which strikes the sample surface, leading to some of the electrons to be backscattered. X-rays will be emitted from underneath the sample surface and at the same time a consistent number of secondary electrons will be emitted from atoms within the sample depending on the angles of collision between the incident electron beam and the sample surface. All these emissions are then measured by special detectors leading to construction of an image of the surface topography of the sample.
Common sample preparation methods include chemical fixation using glutaraldehyde, formaldehyde, and osmium tetroxide. For embedding resin during sample preparation araldite is commonly used or LR White for immuno-gold labeling to preserve the antigens. Cryo-techniques can also be employed in samples preparation for TEM investigations in order to provide better preservation of structures of the samples.
In order to obtain 3D image of a sample, investigators use the electron tomography (ET), which is derived from the conventional TEM technique, or the focused ion beam (FIB). The ET functioning principle is based on an electron beam which is able to penetrate the sample at incremental degrees of rotation about the centre of the sample to be investigated. The 3D image is constructed based on the collection of images collected with a resolution of 2.4-angstrom 72. ET technique has been used widely to study the lifecycles of different viruses such as HIV, herpesvirus, vaccinia virus, and influenza virus 73-75.
The FIB technique relies on the use of a focused beam of ions (for example, gallium) to image samples. In a similar way to SEM, the image construction is based on the collection of the signals from the secondary electrons and sputtered ions. Since FIB is destructive for the sample, scientists can combine both FIB and SEM technique. The “DualBeam” technique (FIB–SEM) employs the non-destructive electron beam and in the same time mills with the ion beam to remove materials at specific sites. FIB–SEM has the advantages that a more precise cross-sectioning of the sample can be performed, while the ion beam preserve the fluorescence signal from the samples, thus allowing the correlation between FM imaging and EM imaging.
A major drawback in the identification of the localization of proteins-of-interest in infected cells at the ultra-structural level is that the experimentalist has to prepare two different samples to obtain IFM and EM images. Moreover, enormous amount of time is spent on viewing random EM sections in the hope to identify a section showing the desired biological phenomenon. Correlative light-electron microscopy (CLEM) was developed in order to bridge the gap between IFM and EM. In CLEM, fluorescently-labeled proteins-of-interest can be imaged first using IFM and then using EM to visualize ultra-structures of the viral proteins. The main advantage when employing this technique is that first one can locate the region-of-interest at the cellular level using conventional FM before reaching the EM level to investigate the selected region-of-interest 76, 77.
A recent breakthrough in EM techniques was direct imaging of a sample in situ in solution. In this method, molecular processes are captured live at high resolution since neither chemical fixation nor cryo-techniques are required. An affinity capture device or a silicon nitride microchip coated with functionalized lipids disposed in monolayers are used with a microfluidic-based sample holder 78.
Gilmore et al. imaged with direct imaging of a sample in situ in solution using TEM the assembly process of rotavirus double-layered particles. Affinity microchips previously coated with antibody against VP6 viral protein were used in this approach 79.
In their study, Jun et al. 80 have offered new structural details of the interaction of HIV-1 with HeLa cells, by first using confocal live-cell microscopy then cryo-EM tomography analysis. The study provided evidence that a hyperstable capsid mutant, E45A, revealed delayed capsid disassembly compared to wild-type viral capsid 80.
By using the CLEM approach, Romero-Brey et al. were able to reveal the precise localization of HCV NS5A viral protein as very close to the ER, in double membrane vesicles. After ET analysis and 3D reconstructions it turned out that these vesicles were protrusions from the ER 81. CLEM experiments were also performed on Huh7 cells infected with HCV to reveal the ultrastructure details of viral host cells (Fig. 2) (unpublished data).
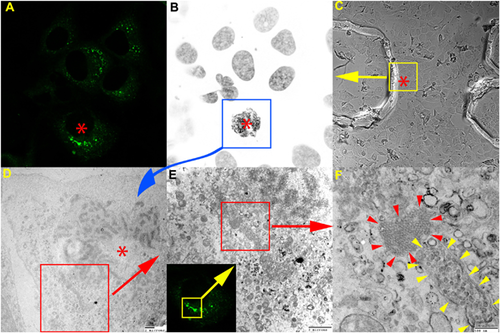
Based on FIB-SEM 3D tomography, investigations on vaccinia virus entry steps have revealed that the pathogen induced large membrane blebs to facilitate its internalization via macropinocytosis. The viral particle-membrane bleb and filopodia interactions were visualized at ultrastructural level using 3D imaging 82, 83.
The Cryo-EM techniques have also contributed to the bullet-shaped model design of the vesicular stomatitis virus, based on the symmetry mismatches between various forms of RNA helix in the viral cavity 84.
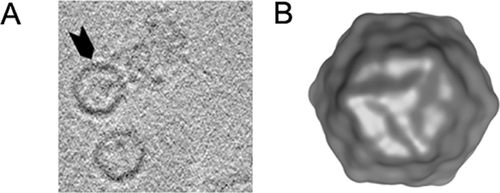
The atomic model at 3.6 A resolution of human adenoviruses was observed by cryo-EM, revealing interactions within three protein–protein viral network. The authors suggested that future cancer and gene therapy may be oriented towards these networks 85. Very recently atomic models of single-particle polioviruses at 30 A resolution were descipherated using cryo-EM 86-88 (Fig. 3).
Animal models for human viruses imaging
Despite the advantages of investigating viral infections in susceptible cell lines in vitro, clearly, these are not able to reproduce entirely the physiological microenvironment that viruses meet in the host. Therefore, when possible, small animal models are desirable to study the viral infection in vivo and test new therapeutic approaches.
The non-invasive bioluminescence system for detection of viral infection promises many benefits to traditional dissection of euthanized animals such as the possibility to study viral infection and reinfection in the same animal and to investigate the correlation between disease progression and viral replication.
Development of a humanized mice supporting HCV infection, which expressed human genes encoding for essential factors for HCV entry 89, made possible for the first time the imaging of cellular reporters and visualization of HCV infection in vivo.
The Cre-loxp reporter system consists of a recombinant HCV expressing Cre recombinase and mice expressing reporter genes after releasing of transcription at loxP site. After intravenously injection of HCV and viral polyprotein translation, Cre recombinase is able to re-localize from the cytosol to the nucleus and excises a transcriptional loxP-flanked stop cassette that blocks the expression of a reporter gene such as luciferase or fluorescent protein in the mice hepatocytes 90, 91.
Although the Cre reporter system is not suited for HCV replication study, detection of the HCV infected cells in situ remains very important, as it enables the study of HCV entry in the three dimensional microenvironment of the hepatic tissue 92.
Other cellular encoded reporter is MAVS, an innate immune signaling protein located in peroxisomes and mitochondria, which after cleavage by HCV encoded NS3/4A protease allows the viral infection to persist in hepatocytes. MAVS was fused with a fluorescent protein which contains a nuclear localization signal (TagBFPnlsMAVS) and after HCV infection and protein cleavage the fluorescent protein is translocated to the nucleus 93. Multiple HCV genotypes can thus be detected in mice expressing transgenically the TagBFPnlsMAVS fusion protein by FM or flow cytometry on single cell suspension of mice hepatocytes 94.
Nogales et al. 95 have developed a replication-competent influenza A virus which expresses the mCherry red fluorescent protein fused with viral non-structural 1 protein. The recombinant virus was able to lethally infect mice allowing for influenza A virus replication to be measured in excised lungs using imaging systems 95.
Fukuyama et al. 96 have generated multi-spectral fluorescent reporter influenza viruses to analyze the progression of influenza infection in lungs in in vivo animal models. However, the monitorization of the viral infection cannot be performed in live animals non-invasively since none of the fluorescent reporter proteins is inside the “biological optical window” (650–900 nm) to be detected 96.
Several other attempts to monitor influenza virus replication in vivo have proven to be more difficult since the luciferase reporter system used in these studies has the disadvantage of systemic inoculation of the substrate in the animal each time the detection of the influenza virus is desired. It is worth noting, that Fukuyama et al. also succeeded in monitoring the cellular immune mechanisms involved in influenza infection, while the groups using the luciferase reporter system lack the resolution with their bioluminescence system to perform such investigations 97-99.
Using in vivo imaging systems (IVIS) and bioluminescent enzyme, Poussard et al. was able to visualize encephalitic virus replication in a murine model in vivo, 3 days before the appearance of clinical symptoms at the level of the central nervous system, and thus could identify more quickly therapeutic possibilities for the disease 100.
Rameix-Welti et al. 101 proposed a recombinant human respiratory syncytial virus (RSV) in which firefly luciferase gene was inserted into the virus genome as a tool to visualize the replication of the virus in living mice. The bioluminescence of the recombinant luciferase-expressing RSV was detected in the snout and lungs of virus-infected mice. The authors concluded that this recombinant virus could be used for both screening of anti-viral drugs and detection with high sensitivity of the effect of anti-viral compounds in vivo in mice 101.
Burke et al. have used a luciferase-encoding Sendai virus (the murine form of the human parainfluenza virus) to detect non-invasively the dynamics of viral infection, systemic immunological response, and protection from reinfection in living mice using bioluminescence imaging 102.
Liang et al. have described a new bioluminescence assay for in vivo imaging of the kinetics of HBV clearance in the liver, after hydrodynamic injection of the luciferase gene and over-length, linear viral DNA into hepatocytes. The authors have developed a nontransgenic HBV animal model where the reporter luciferase gene was placed under the control of HBV core promoter allowing for direct in vivo bioluminescence detection of hepatitis B core antigen (HBcAg)-specific immune responses in infected mice 103.
Yun et al. used reverse genetics to monitor viral spread in interferon α/β receptor knockout mice infected with luciferase-expressing hepanipaviruses Nipah and Hendra virus. The bioluminescence imaging system was also used to investigate the differences in budding and fusogenic properties between the two henipaviruses 104.
Conclusion
The virology field has greatly benefited from the innovative advances of the microscopy technology in the last decade, many details of the life-cycle of important human viruses being deciphered using these techniques. The major challenge for the virologists is now to translate this technological progress into modern tools for early diagnosis of viral infection and monitoring of the disease pathogenesis and progression. At the same time, a better understanding of the viral structure and interaction with the host cells will certainly contribute to the development of novel therapeutics to combat the disease.
Acknowledgments
This paper is supported by the Sectoral Operational Programme Human Resources Development (SOP HRD), financed from the European Social Fund and by the Romanian Government under the contract number POSDRU/159/1.5/S/137390 and by Romanian Academy Project 3 of the Institute of Biochemistry. The image displayed in Fig. 2 was kindly provided by Dr. Nicolas Barois from Institut Pasteur de Lille, France and the image displayed in Fig. 3 was kindly provided by Dr. Mihnea Bostina from University of Otago, New Zealand.
The authors have declare no conflict of interest.




