A novel ablation strategy of slow/fast atrioventricular nodal reentrant tachycardia targeting the atrial activation focusing point at the inferior Koch's triangle: Identification of entrance site of slow pathway using high-resolution 3D mapping
Graphical Abstract
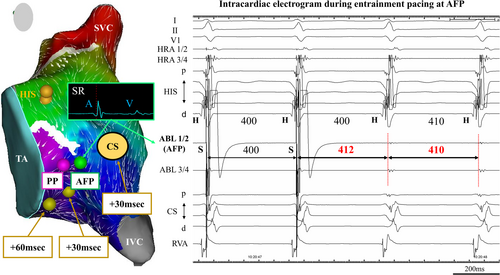
This is the case of a 29-year-old female with sudden palpitations due to tachycardia. The atrial activation focusing point (AFP) was presumed to be the atrial entrance site within the reentry circuit of slow/fast atrioventricular nodal reentrant tachycardia by postpacing interval mapping. AFP is a novel ablation site because it is more distal to the His-bundle recording site and more atrial site compared to the conventional SP ablation site.
Atrioventricular nodal reentrant tachycardia (AVNRT) is one of the most common types of supraventricular tachycardia (SVT), and the success rate of catheter ablation (CA) is high with low arrhythmia recurrence; however, it has been reported that collateral atrioventricular block (AVB) could occur in 0.4%–1% of cases.1, 2
We report a novel ablation strategy of the slow pathway (SP), targeting the atrial activation focusing point (AFP), which was visualized at an inferior site below the coronary sinus ostium (CS–OS) in the Koch's triangle (KT) using a high-resolution three-dimensional (3D) mapping system; AFP was proven to be on the reentry circuit of slow/fast (S/F) AVNRT by postpacing interval (PPI) mapping.
A 29-year-old female who presented with sudden palpitations due to tachycardia was referred to our hospital. The 12-lead electrocardiogram showed normal sinus rhythm (SR) without ventricular preexcitation at baseline; furthermore, it showed a narrow QRS complex without obvious retrograde P wave during tachycardia. She had no significant comorbidities. An electrophysiological study was performed with patient consent.
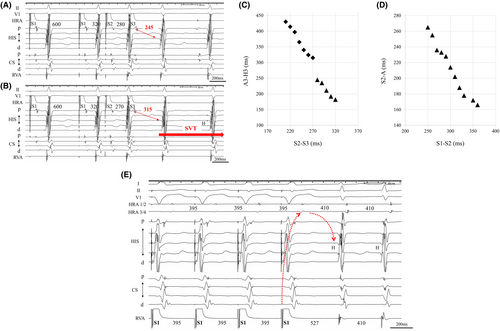
Four multielectrode catheters were positioned in the high right atrium (HRA), right ventricular apex (RVA), bundle of His, and coronary sinus (CS). The A–H and H–V intervals were normal at baseline (A–H interval, 78 ms, H–V interval, 40 ms). During the basic cycle length (BCL) with S1–S1 600 ms and S1–S2 320 ms; when the atrial premature stimulation (S2–S3) was shortened from 280 to 270 ms, the A3–H3 interval was suddenly prolonged by 65 ms, and the supraventricular tachycardia (SVT; tachycardia cycle length [TCL] with 410 ms) was reproducibly induced (Figure 1A,B), and the SVT was continued without ventriculo-atrial (VA) block. Figure 1C shows the atrioventricular (AV) conduction curve. Notably, SVT was reproducibly induced with A–H jump-up from fast to slow pathways. However, the VA conduction curve was smooth, and the retrograde conduction had the earliest atrial activation at His-bundle electrogram recording site (Figure 1D). During SVT, burst pacing (S1–S1 385 ms) from the RVA showed entrainment of the atrial electrogram and a V–A–V response,3 which was unlikely to be diagnosed as atrial tachycardia (Figure 1E). Furthermore, the PPI-TCL was 117 ms (>110 ms) by burst pacing from the RVA during SVT,4 suggesting AVNRT. The orthodromic reciprocating tachycardia via a nodoventricular or nodofascicular pathway was unlikely because a single premature ventricular stimulus delivered during the His refractory period did not reset the subsequent His activation during SVT. Therefore, we diagnosed the SVT as S/F AVNRT.
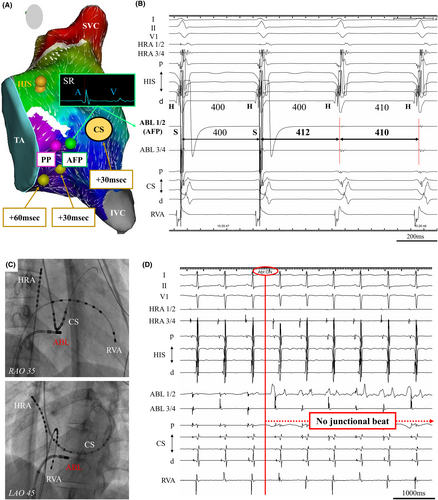
We performed 3D mapping using a high-density multielectrode catheter (PentaRay and CARTO 3; Biosense-Webster Inc., Diamond Bar, CA, USA) during SR to visualize the SP excitation within KT, and we identified the pivot point (PP) and atrial activation focusing point (AFP). The local electrograms were annotated to the latest local activation points. The PP is identified as being below the collision site between the fast pathway and SP.5 However, we also indicated a convergent point where atrial activation was focusing from multiple sites around the KT, inferior vena cava, and tricuspid annulus (TA); this region is defined as the AFP. We created propagation and coherent mapping during SR to identify and depict AFP more clearly. The lower threshold of the early meets late was set at 22 in the present case to show the functional block line (Movie S1). A pacing output of 10 V was required to capture local potentials. Entrainment pacing at AFP showed that PPI was identical to TCL, and its atrial point was presumed to be on the tachycardia circuit, as previously reported by Yamabe et al. in 1999.6 Similar entrainment pacing was performed slightly below the AFP around the 4 o'clock position of the TA, PPI was longer than TCL by 30 ms. Moreover, entrainment pacing was performed at the 6 o'clock position of the TA, PPI was longer than TCL by 60 ms. Additionally, the entrainment pacing at the CS–OS, PPI was also longer than TCL by 30 ms (Figure 2A). At the AFP, the atrial electrogram was considerably larger than the ventricular electrogram, which was located posterior-inferior site to the conventional SP ablation area and approximately 24 mm away from the His-bundle recording site on 3D mapping system. The AFP was presumed to be the atrial entrance site within the reentry circuit of S/F AVNRT because of identical PPI after entrainment pacing to TCL (Figure 2B), and it could be a safer ablation point because it was more distal to the His-bundle recording site compared to the conventional SP ablation area. Therefore, we performed RF ablation targeting the entrance site of the SP at the AFP by using a conventional 3.5-mm irrigated-tip contact force-setting ablation catheter (SMART TOUCH SF; Biosense-Webster Inc., Diamond Bar, CA, USA). RF application was delivered intensively at the AFP during SR, limited to 30 W with the contact force maintained between 5 and 10 g. Fluoroscopic imaging showed the site of successful ablation was located at the septal atrium just beneath the CS–OS (Figure 2C). No junctional rhythm was observed during successful RF application (Figure 2D).
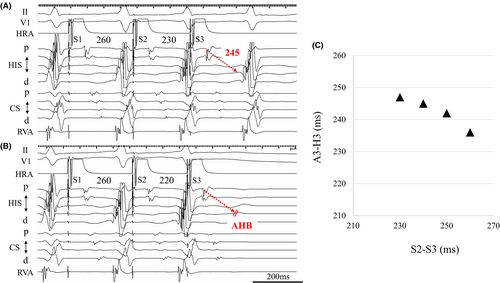
After RF application to the AFP site, programmed atrial stimulation was repeated, and the complete elimination of SP conduction was achieved. When the atrial premature stimulation (S2–S3) was shortened from 230 to 220 ms, A–H block was shown (Figure 3A,B). Figure 3C shows the AV conduction curve after RF application. Moreover, the AVNRT was no longer inducible with or without isoproterenol infusion. The AH and HV intervals were unchanged, and no other complications occurred. The patient has had no tachyarrhythmias for 2 years.
We hypothesized that the AFP was located close to the entrance of the SP, and that RF application to the AFP could eliminate conduction to the SP site at a site further upstream compared to the conventional ablation site. In this case, the AFP was also close to the PP. However, from our experience, even if the AFP is far from the PP, the SP could be eliminated at the AFP site, although multiple RF applications may be necessary in cases with a broader entrance of the SP. Coherent mapping with a vector map using the CARTO 3 system could make the AFP more visible compared to other 3D mapping systems.5, 7 However, ablation to AFP sometimes needs a broader RF application than ablation to SP, probably because a more proximal site of SP is targeted. More investigation is warranted in terms of how much area should be ablated when AFP is targeted.
In this case study, high-resolution 3D mapping during SR might have revealed the entrance of SP, how the atrial activation propagated into the SP through the AFP in KT. By confirming that PPI is equal to TCL with entrainment pacing at the AFP, the AFP was shown to be on the circuit of S/F AVNRT. AFP could be considered a novel ablation target to minimize the risk of AVB for S/F AVNRT.
ACKNOWLEDGMENTS
The authors have nothing to report.
FUNDING INFORMATION
This research did not receive any specific grants from funding agencies in the public, commercial, or not-for-profit sectors.
CONFLICT OF INTEREST STATEMENT
Authors declare no conflict of interests for this article.
ETHICS STATEMENT
Not applicable.
PATIENT CONSENT STATEMENT
Patient consent for publication was obtained.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this research are available from the corresponding author upon reasonable request.