Effectiveness of upgrade left bundle branch area pacing for right ventricular pacing-induced cardiomyopathy: Extra QRS shortening matters
Hao Huang and Xiaofeng Li were authors contributed equally to this work.
Abstract
Background and Objectives
Left bundle branch area pacing (LBBAP) has developed as a strategy for patients with pacing-induced cardiomyopathy (PICM). We aimed to compare the upgrade effectiveness between LBBAP and traditional biventricular pacing (BVP) in PICM patients.
Methods
Consecutive PICM patients with successful device upgrades were enrolled. The primary outcome was the echocardiographic response, defined as absolute left ventricular ejection fraction (LVEF) improvement ≥5% at 6-month follow up.
Results
A total of 92 patients were included. 61 underwent BVP and 31 underwent LBBAP. The median RVP burden was 96.8% (IQR: 93.0–99.0%). LBBAP achieved a shorter paced QRS duration (QRSd) compared with BVP (145.9 ± 22.4 ms vs. 157.5 ± 26.5 ms; p =.031). At 6 months, LBBAP had a higher echocardiographic response rate than BVP (67.7% vs. 39.3%, p =.019). LVEF increased from 37.8% ± 9.2% to 44.8% ± 10.2% (p <.001) in LBBAP compared with an improvement from 35.7% ± 8.9% to 38.2% ± 12.1% (p <.01) in BVP, with significantly greater change from baseline in LBBAP (7.0% ± 7.0% vs. 2.5% ± 8.7%; p =.024). Narrower pacing QRS after upgrade was associated with better echocardiographic response only in LBBAP but not in BVP. (P for interaction <.05). Both groups had similar rates of composite clinical outcome.
Conclusion
LBBAP improved echocardiographic response compared with BVP in PICM patients. The superior efficacy of LBBAP in reverse remodeling was dependent on improved electrical synchrony.
1 INTRODUCTION
Conventional right ventricular pacing (RVP) modality has been established for decades, as it is technically straightforward. However, around 12% of patients with pacemakers or implantable cardioverter defibrillator (ICD) devices experience left ventricular (LV) systolic dysfunction due to intraventricular dyssynchrony induced by RVP.1, 2 In this context, upgrading to cardiac resynchronization therapy (CRT) is recognized as a standard device treatment in patients with high burden of pacing and impaired LV function.3, 4 The indications for de novo CRT are well-defined,5, 6 however, the efficacy of upgrade to CRT is still inferior, probably due to the worse health condition and less optimal timing of intervention.7, 8
Left bundle branch area pacing (LBBAP), as an alternative strategy for CRT, has exhibited promising application prospects. Recent observational study and small randomized clinical trial concluded that it was associated with greater narrowing of QRS duration (QRSd), higher echocardiographic response rate, and significant reduction in composite clinical outcomes compared with biventricular pacing (BVP).9, 10 However, no sub-analyses were presented among RVP patients who require an upgrade to resynchronization therapy, and current guidelines did not recommend a specific pacing modality. Besides, though it seems clear that the acute improvement of electrical dyssynchrony reflected by QRS narrowing results in better CRT response following BVP,11 this conclusion was largely driven by de novo CRT recipients. Whether the favorable clinical effect induced by changing pacing modality depends on the restoration of electrical synchrony remains elusive.
Therefore, this study aimed to assess the electrical synchrony, reverse remodeling effect and clinical outcomes of upgrade to LBBAP, as well as the impact of acute correction of QRSd on reverse remodeling in patients with pacing-induced cardiomyopathy (PICM). Patients upgraded to BVP during the same period were used as controls.
2 METHODS
2.1 Study population
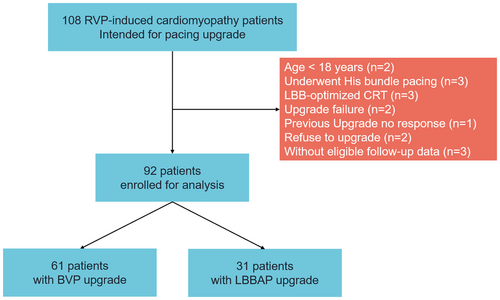
One hundred and eight PICM patients intended for pacing upgrade between January 2018 to December 2023 were retrospectively screened in the study. PICM were defined as: (1) implanted with single/dual-chamber of PM or ICD; (2) had preserved LV ejection fraction (LVEF) without evident structural abnormalities at initial implantation, and newly developed symptomatic heart failure with reduced LVEF or structural heart disease, despite optimal medical treatment; (3) had significant RV pacing burden greater than 20%.3, 4 The following exclusion criteria were applied: younger than 18 years of age (n = 2), underwent His bundle pacing upgrade (n = 3), LBB-optimized CRT (LOT-CRT, n = 3), upgrade failure (n = 2), or previous upgrade with non-response (n = 1). In addition, patients who refused to upgrade (n = 2), or without eligible follow-up data (n = 3) were also excluded (Figure 1). Baseline characteristics, electrocardiographic (ECG), echocardiographic, and pacing parameters, comorbidities, and medications were collected. The institutional review board of Fuwai Hospital approved the study, with adherence to the Declaration of Helsinki. All patients provided informed consent.
2.2 Implantation procedures
The implantation procedures have been previously described.12, 13 All procedures were performed in electrophysiology laboratories, with real-time 12-lead ECG monitoring. The original leads were retained in situ unless infection of the pacemaker system. The upgrade strategy was determined by the experience of the operator. For patients undergoing BVP, a lateral coronary sinus (CS) vein was targeted, while the anterior interventricular (V-V) and middle cardiac veins were not considered suitable. For patients undergoing LBBAP, a fixed-curve sheath (C315HIS, Medtronic) was introduced to the right ventricle under the fluoroscopic right anterior oblique 30°, advancing a pacing lead with fixed helix (Selectsecure model 3830, 69 cm, Medtronic) into the ventricular septum from the right side of V-V septum towards the left bundle branch area. LBBAP was considered to be successful when the unipolar paced QRS morphology demonstrated a Qr or qR pattern in V1 along with any of the following: (1) recording of LBB potential; (2) demonstration of transition from nonselective to selective LBB or LV septal capture during threshold testing; (3) R-wave peak time in leads V6 <90 ms; or 4) V6-V1 interpeak interval >40 ms.14, 15 26/31 (83.9%) patients in LBBAP group were upgraded with CRT devices. For these patients, the RV ports were connected to the original RV pacing leads or defibrillator leads, and the LV ports were connected to the LBBAP leads. The other 5 patients were implanted with dual chamber pacemakers, with LBBAP leads connected to the ventricular port, and the original RV pacing leads were left unused in situ with the connectors encapsulated. Meanwhile, 60/61 (98.4%) patients in BVP group were upgraded with CRT devices, with LV ports connected to the newly implanted CS leads. The only one patient who had persistent atrial fibrillation (AF) were upgraded with a dual chamber pacemaker, with RV lead connected to the RA port, and CS lead connected to the RV port.
2.3 Device programming
Device programming was performed on the next day of operation to obtain the narrowest paced QRSd by modifying the optimal interventricular (V-V) and atrioventricular (A-V) intervals. The QRSd was measured from the onset of the QRS complex to the final deflection in the simultaneous 12-lead ECG, both before or after the upgrade. Patients with CS leads were searched for the most suitable electrode without phrenic nerve stimulation and a threshold <2.5 V. In patients undergoing LBBAP, unipolar and bipolar pacing were tested, and the one that achieved the shortest QRSd was selected.
2.4 Follow-up
All patients were followed up at 6 months to evaluate upgrade response. Postimplantation assessment included ECGs, echocardiography, device interrogation, clinical status and complications. The echocardiographic response was defined as ≥5% absolute improvement in LVEF compared to baseline echocardiographic measurement. The assessment of LVEF was used by Simpson's biplane method. Clinical response was defined as at least 1 New York Heart Association (NYHA) functional class improvement without HF hospitalization. Follow-up visits were continued at 6–12 month intervals thereafter on a phone interview or outpatient service.
The primary endpoint was echocardiographic response at 6 months. Prespecified secondary endpoints included the absolute change in LV end-diastolic diameter (LVEDD) and NYHA class. The clinical outcome included a composite outcome of all-cause mortality, heart transplantation, and HF hospitalization, defined as any hospital admission or emergency care visit requiring intensive treatment for HF with intravenous diuretics or intravenous inotropic medications.
All baseline and follow-up ECGs, echocardiograms, and device interrogation data were independently reviewed by medical staff who were blinded to the patients' group assignments and clinical outcomes.
2.5 Statistical analysis
Continuous variables were expressed as mean ± standard deviation (SD) for normally distributed continuous variables and median [interquartile range (IQR)] for non-normally distributed variables. Categorical variables are expressed as frequencies with percentages. Student's t test and Mann–Whitney U test was used for comparing the differences of continuous variables as appropriate. A paired t-test was conducted to validate the change of key indicators before and after upgrade in each group, including QRSd, NYHA class, and LVEF. χ2 test or Fisher's test was used for comparing the difference of categorical variables. Logistic regression and linear regression analyses were conducted to evaluate the effect of upgrade strategy on echocardiographic response and LVEF improvement, respectively. Cox proportional hazards regression was applied to compare the difference in the clinical outcome between groups. Multivariable regression was also performed with relevant clinical characteristics as covariates, including age, sex, NYHA class, estimated glomerular filtration rate (eGFR), RV pacing QRSd, ischemic cardiomyopathy, AF, and baseline LVEF. A generalized additive model was built to allow curve-fitting to assess potential nonlinear associations between QRSd and echocardiographic response/LVEF improvement in each group. The interaction effect between QRSd reduction/QRSd after upgrade and upgrade strategy was identified by introducing cross-product term (QRSd reduction/QRSd after upgrade * LBBAP) in the regression model with echocardiographic response or LVEF improvement as a dependent variable. The statistical analyses were performed using R version 4.0.3. Two-sided p-value≤0.05 was considered statistically significant.
3 RESULTS
3.1 Baseline characteristics
A total of 92 patients were included in the final analysis. Thirty-one patients underwent LBBAP. The remaining 61 patients underwent BVP. The baseline characteristics are presented in Table 1. Overall, the median age was 57.6 ± 13.1 years. 71.7% were male and 83.7% had non-ischemic cardiomyopathy. The median RV pacing percentage in the last interrogation before upgrade was 99.0%, with no difference between two groups (99.0% vs. 99.8%, p =.253). Similarly, no other differences were observed between patients with BVP and LBBAP, including NYHA class, RV paced QRSd, duration, comorbidities, medications and echocardiographic parameters (all p > 0.05).
| All (n = 92) | BVP (n = 61) | LBBAP (n = 31) | p value | |
|---|---|---|---|---|
| Age (year) | 57.6 ± 13.1 | 56.8 ± 13.3 | 59.3 ± 12.8 | .390 |
| Male | 66 (71.7) | 41 (67.2) | 27 (80.6) | .305 |
| NYHA functional class | 2.6 ± 0.7 | 2.7 ± 0.6 | 2.5 ± 0.8 | .161 |
| NICM | 77 (83.7) | 51 (83.6) | 26 (83.9) | 1.000 |
| Comorbidities | ||||
| Hypertension | 30 (32.6) | 16 (26.2) | 14 (45.2) | .111 |
| Coronary atrial disease | 17 (18.5) | 11 (18.0) | 6 (19.4) | 1.000 |
| Diabetes | 27 (29.3) | 18 (29.5) | 9 (29.0) | 1.000 |
| Hyperlipidemia | 34 (37.0) | 21 (34.4) | 15 (41.9) | .633 |
| Atrial fibrillation | 16 (17.4) | 14 (23.0) | 2 (6.5) | .092 |
| Chronic kidney disease | 15 (16.3) | 12 (19.7) | 3 (9.7) | .353 |
| Medication | ||||
| ACEI/ARB/ARNI | 62 (89.9) | 40 (87.0) | 22 (957) | .481 |
| Beta-blocker | 84 (92.3) | 55 (91.7) | 29 (93.5) | 1.000 |
| Aldosterone antagonist | 83 (91.2) | 55 (91.7) | 28 (90.3) | 1.000 |
| Diuretic | 70 (76.9) | 47 (78.3) | 23 (74.2) | .856 |
| Digoxin | 22 (24.2) | 17 (28.3) | 5 (16.1) | .303 |
| Amiodarone | 24 (26.4) | 19 (31.7) | 5 (16.1) | .179 |
| Echocardiography | ||||
| LVEF (%) | 36.4 ± 9.0 | 35.7 ± 8.9 | 37.8 ± 9.2 | .295 |
| LVEDD (mm) | 61.8 ± 7.5 | 62.5 ± 7.5 | 60.6 ± 7.4 | .268 |
| LAD (mm) | 45.5 ± 7.2 | 45.9 ± 7.0 | 44.7 ± 7.6 | .482 |
| RV pacing QRS duration (ms) | 185.7 ± 25.3 | 187.1 ± 25.4 | 182.9 ± 25.3 | .452 |
| RV pacing duration (day) | 1482 (781, 2441) | 1496 (671, 2252) | 1474 (782, 1820) | .720 |
| RV pacing burden (%) | 99.0 (97.0, 99.9) | 99.0 (96.4, 99.9) | 99.8 (98.1, 100.0) | .253 |
- Abbreviations: ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; ARNI, angiotensin receptor-neprilysin inhibitor; BVP, biventricular pacing; LAD, left atrial diameter; LBBAP, left bundle branch area pacing; LVEDD, left ventricular end-diastolic diameter; LVEF, left ventricular ejection fraction; NICM, non-ischemic cardiomyopathy; NYHA, New York Heart Association; RV, right ventricular.
3.2 Procedural parameters
A total of 77 (83.7%) patients received pacemakers and 15 (16.3%) underwent ICD implantation before upgrade. The proportion of patients receiving ICD increased to 41.3%, with no difference between two groups (42.6% vs. 38.7%, p =.842). 5 (16.1%) patients received dual-chamber devices in the LBBAP group, while almost all patients undergoing BVP upgrade used CRT devices, except for one (1.6%) patient with persistent AF used two ventricular pacing leads and a dual-chamber device. All patients maintained a high ventricular pacing proportion after the upgrade (median 99.0%, IQR: 97.0%–9.9%). The average s-LVAT time in the LBBAP group was 84.5 ± 17.9 ms. The pacing threshold of upgrade LBBAP leads remained lower at baseline and follow-up (Table 2). One patient in the LBBAP group and one patient in the BVP group had device removal for infection in 9- and 11-month follow-ups, respectively. No pneumothorax, pericardial tamponade, lead dislodgement or perforation was observed.
| All (n = 92) | BIVP (n = 61) | LBBAP (n = 31) | p value | |
|---|---|---|---|---|
| Type of previous device | 1.000 | |||
| Pacemaker | 77 (83.7) | 51 (83.6) | 26 (83.9) | |
| ICD | 15 (16.3) | 10 (16.4) | 5 (16.1) | |
| Type of upgrade device | ||||
| Pacemaker | 54 (58.7) | 35 (57.4) | 19 (61.3) | .892 |
| ICD | 38 (41.3) | 26 (42.6) | 12 (38.7) | |
| Dual chamber | 6 (6.5) | 1 (1.6) | 5 (16.1) | .027 |
| CRT | 86 (93.5) | 60 (98.4) | 26 (83.9) | |
| Pacing parameters (upgrade lead) | ||||
| Baseline pacing threshold | 1.1 ± 0.7 | 1.3 ± 0.7 | 0.6 ± 0.2 | <.001 |
| 6-month follow-up pacing threshold | 1.1 ± 0.6 | 1.2 ± 0.7 | 0.8 ± 0.3 | .002 |
| Baseline impedance | 624.5 ± 246.9 | 667.3 ± 281.9 | 545.8 ± 136.4 | .027 |
| 6-month Follow-up impedance | 512.1 ± 182.7 | 528.6 ± 208.6 | 481.6 ± 118.5 | .284 |
| Pacing percentage after upgrade (%) | 99.0 (97.0, 99.9) | 99.0 (96.4, 99.9) | 99.8 (98.1, 99.9) | .368 |
| QRS duration | ||||
| QRSd reduction | −32.1 ± 26.7 | −29.7 ± 29.2 | −37.0 ± 20.7 | .162 |
| Paced QRSd after upgrade | 153.6 ± 25.6 | 157.5 ± 26.5 | 145.9 ± 22.4 | .039 |
- Abbreviations: CRT, cardiac resynchronization therapy; ICD, implantable cardioverter-defibrillator; QRSd, QRS duration; Other abbreviations as in Table 1.
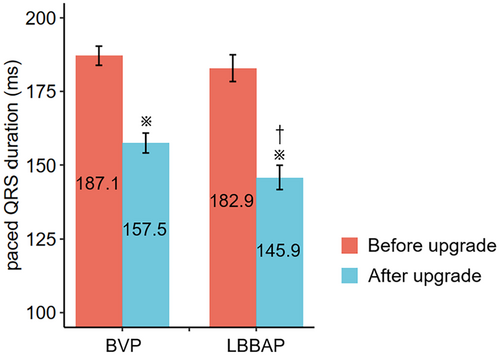
Paced QRSd narrowing was achieved in 82 (89.1%) patients. The average QRSd narrowing was 32.1 ± 26.7 ms. Two pacing modalities exhibited different QRSd and morphology after the upgrade (Figure S1). The paced QRSd was significantly shortened by both LBBAP (−37.0 ms, 95% CI: −44.3 to −29.7 ms, p < 0.001) and BVP (−29.7 ms, 95% CI: −37.0 to −22.3 ms, p < 0.01). LBBAP resulted in shorter paced QRSd compared with BVP (145.9 ± 22.4 ms vs. 157.5 ± 26.5 ms, p =.031) (Figure 2).
3.3 Response to upgrade resynchronization
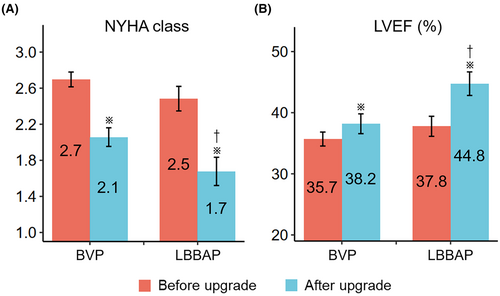
After a six-month follow-up, the NYHA functional class improved from 2.7 ± 0.6 to 2.1 ± 0.8 in the BVP group (p <.001), and from 2.5 ± 0.7 to 1.7 ± 0.9 in the LBBAP group (p <.001). 67 (73.6%) patients had a clinical response, which was similar between both groups (70.5% vs. 80.0%, p =.475) (Figure 3(A)). 45 (48.9%) had an echocardiographic response, and LVEF improved from 36.4 ± 9.0% to 40.2 ± 11.9% (p <.001) in the total population. LBBAP yielded a higher echocardiographic response than BVP (67.7% vs. 39.3%, p =.019). Compared with patients with BVP, those who received LBBAP had a higher LVEF improvement (7.0 ± 7.0% vs. 2.5 ± 8.7%, p =.024). The LVEF after 6 months was also significantly higher in the LBBAP group (44.8 ± 10.2% vs. 38.2 ± 12.1%, p =.016) (Figure 3(B)). When adjusted for multiple clinical variables, LBBAP was independently associated with higher odds of echocardiographic response, and higher absolute LVEF improvement as well (Table 3). The overall data on changes of QRSd, NYHA class and LVEF before and after upgrade was presented in Table S1.
| Models | Echocardiographic response (BVP as reference) | LVEF improvement (BVP as reference) | ||
|---|---|---|---|---|
| OR (95% CI) | p value | β (SE) | p value | |
| Model 1 | 4.26 (1.64–9.44) | .004 | 4.57 (1.90) | .018 |
| Model 2 | 3.86 (1.44–11.18) | .009 | 5.21 (1.94) | .009 |
| Model 3 | 3.68 (1.29–10.14) | .018 | 4.61 (2.05) | .028 |
- Note: Model 1: Univariate model. Model 2: adjusted for age, sex, and NYHA class. Note: Model 3: adjusted for age, sex, NYHA class, ischemic cardiomyopathy, baseline LVEF, and RV pacing QRS duration.
- Abbreviations: OR, odds ratio; Other abbreviations as in Table 1.
3.4 Relationship of corrected QRSd and LVEF improvement
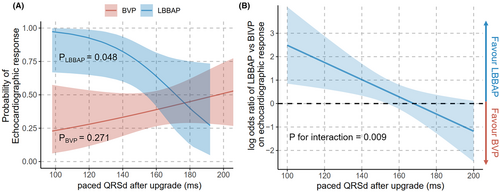
The effect of QRSd correction on echocardiographic response was further investigated. There was a linear correlation between paced QRSd after upgrade and the possibility of echocardiographic response in the LBBAP group, as narrower paced QRS was associated with better echocardiographic response (p =.048). On the other hand, the relationship between corrected paced QRSd and echocardiographic response was not evident after BVP (p =.271, Figure 4(A)). A significant interaction of upgrade strategy with QRSd correction on echocardiographic response was observed after multiple adjustments (p =.009, Table 4). As the paced QRSd increased, this beneficial effect of LBBAP on echocardiographic response gradually diminished until both upgrade methods had equivalent odds ratio of response when it was estimated at 168 ms (Figure 4(B)). Similar trends were found that higher paced QRSd after upgrade was associated with less LVEF improvement in the LBBAP group (p =.013), while this relationship was not significant after BVP (p = 0.184, Figure S2 & Table S2).
| LBBAP * paced QRSd after upgrade | Echocardiographic response | LVEF improvement | ||||
|---|---|---|---|---|---|---|
| β | SE | p value | β | SE | p value | |
| Model 1 | −0.061 | 0.027 | .013 | −0.161 | 0.081 | .050 |
| Model 2 | −0.060 | 0.028 | .031 | −0.178 | 0.082 | .034 |
| Model 3 | −0.084 | 0.032 | .009 | −0.187 | 0.084 | .029 |
3.5 Clinical outcomes
During a median follow-up of 701 days (IQR: 216–1166 days), 8 patients died, 3 received heart transplantation, and 22 were admitted for HF deterioration. In the LBBAP group, two patients underwent heart transplantation after 80 and 404 days, respectively, and six patients had HF rehospitalization. The incidence rates of the composite outcome were 16.1 (6.5–33.2) per 100 person-years in the LBBAP group, and 14.8 (8.6–23.6) per 100 person-years in the BVP group, respectively. LBBAP had a similar risk of composite outcome (HR: 1.16; 95% CI: 0.48–2.82; p = 0.742; Figure S3) compared with BVP. Multivariate analysis indicated that both groups had comparable risk of endpoints after adjusting for clinical variables (Table S3).
4 DISCUSSION
In this retrospectively single-center study, we enrolled patients with significant RV pacing burden and HF manifestation undergoing device upgrade, and compared the efficacy between different pacing strategies. It is concluded that (i) 89.1%, 73.6%, and 48.9% of patients undergoing resynchronization therapy had electrophysiologic, clinical, and echocardiographic response in this cohort; (ii) Both LBBAP and BVP complemented each other as an alternative pacing strategy without significant device- or procedure-related events; (iii) Upgrading to LBBAP was associated with lower pacing threshold, narrower QRS complex, and higher LVEF improvement compared with BVP, and did not show a significant difference regarding clinical outcomes; (iv) The better echocardiographic response for LBBAP was dependent on acute correction of QRSd.
Due to the absence of evidence from large randomized clinical trials, guidelines established recommendations for CRT upgrade by relying on observational or small randomized studies, and the class/level of recommendation for CRT upgrade underwent multiple modifications over the past decade.3, 4, 16, 17 Notably, the BUDAPEST-CRT trial has recently ascertained the advantages of CRT-D upgrade rather than ICD on hard clinical endpoints. This holds true particularly for symptomatic HF patients with indications of defibrillator use who already had an implanted device, a reduced LVEF, and a higher burden of RV pacing.18 The procedural strategy was not specifically described, though. Pooled analysis of randomized controlled trials of BVP upgrade showed an average LVEF improvement of 8.4% from 35.5%, which further necessitates upgrade strategy in clinical settings.19
Despite the benefit of CRT upgrade, its treatment effect compared with de novo CRT implantation was controversial. A meta-analysis in 2017 concluded that CRT upgrade is associated with both similar clinical response and risk for all-cause mortality compared to de novo CRT.20 However, more recent studies with longer duration of follow-up and detailed adjustment for cofounders, have widely reported it as inferior to de novo CRT implantation.7, 8, 21 In a propensity-score-matched cohort consisting of 488 pairs of de novo or upgrade CRT implantation, upgrade to CRT was associated with a higher rate of the composite adverse outcome for both upgrades from pacemaker (HR = 1.33) and ICD (HR = 1.40).8 This phenomenon not only implies an innate unbalanced difference between two different populations, but also brings up the need for better upgrade timing and modality.
In this context, LBBAP has emerged as an alternative cardiac physiologic pacing, with most clinical evidence appearing in de novo implantation. Some small-sample studies showed the effectiveness and safety of LBBAP upgrade when treating RVP-induced cardiomyopathy.22-25 Upgrading to LBBAP could also serve as a rescue for CRT non-responders, achieving significant heart function improvement and better clinical outcomes.26 The meta-analysis of observational studies suggested a similarly significant improvement in LV function, QRSd, and clinical status compared with BVP upgrade.19 In this study, we made a head-to-head comparison between BVP and LBBAP in patients with RVP-induced cardiomyopathy. As a complement to previous findings, it is indicated that LBBAP was a feasible upgrade pacing modality, with a comprehensive superior efficacy in electrical synchrony, pacing threshold, symptom relief, and reverse remodeling over BVP. This finding supported the priority to attempt LBBAP when upgrading RVP to resynchronization therapy.
In theory, recruitment of an intact left conduction system might be more accessible in PICM for pure reasons of long-term pacing rather than those with pre-existing and progressing myocardial lesions. That's why recently LBBAP was mainly taken as a primary strategy for upgrade candidates with prolonged QRSd and left bundle branch block morphology in our center. We proved that acute correction of electrical dyssynchrony is more crucial when performing LBBAP, as narrower paced QRSd and paced QRSd shortening lead to better reverse remodeling. On the other hand, the prognosis of BVP upgrade was not associated with QRS shortening as demonstrated in the previous study.27 What we found interesting is that, once LBBAP could correct QRSd to a certain level, which was estimated at 168 ms of paced QRSd, the superiority over BVP would be evident. This finding may help operators to determine whether the deep screw of LBBAP sites was fair enough, and whether an additional CS lead was necessary to achieve better resynchronization. In another words, in patients with longer paced QRSd after upgrade to LBBAP, we suggest another CS-LV lead may be added to achieve LOT-CRT.
5 LIMITATIONS
The main limitation of this single-center, retrospective study is a relatively small sample size and short follow-up time in the LBBAP group, which may underestimate the true efficacy in terms of long-term clinical outcomes. Although we observed comparable baseline characteristics between the two groups, and finely verified the main results by multivariate adjustments, it remains conceivable that unknown or unmeasured confounders may have an influence. Large-scale, multicenter, prospective randomized controlled trials with substantial power concerning clinical endpoints are further needed to validate our findings.
6 CONCLUSIONS
For PICM patients, an upgrade to LBBAP provided better electrical and pacing parameters, clinical remission, and echocardiographic response compared with BVP. Moreover, the superior efficacy of LBBAP in reverse remodeling was dependent on improved electrical synchrony.
ACKNOWLEDGMENTS
We acknowledged Ms. Jiawei Liu for her help in image visualization.
FUNDING INFORMATION
This work was supported by the National Natural Science Foundation of China (grant number 82070349) and Fundamental Research Funds for Central Universities (grant number 3332019047).
CONFLICT OF INTEREST STATEMENT
The authors have nothing to report.
ETHICS STATEMENT
This study was approved by the Ethics Committee of Fuwai Hospital (Approval No. 2017–856). All procedures followed were in accordance with the ethical standards of the Declaration of Helsinki.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




