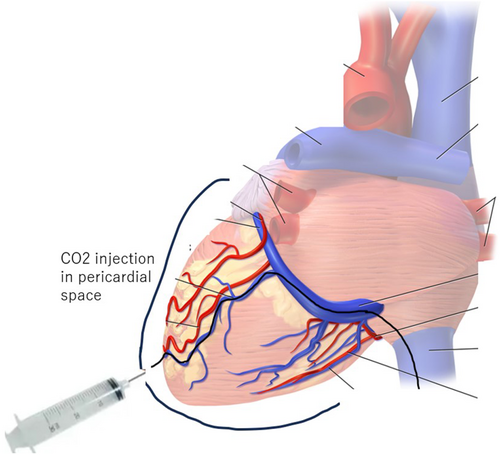
Carbon dioxide insufflation to facilitate epicardial access in ECMO-supported ventricular tachycardia ablation
Graphical Abstract
Successful catheter ablation of ventricular tachycardia (VT) occasionally requires epicardial access. However, puncture of pericardial space with minimal fluid is technically challenging and several complications such as inadvertent right ventricle puncture and injury to right coronary artery or the liver are not uncommon.
Here, we reported a case of a patient who has failed an initial endocardial catheter ablation attempt for VT. We performed epicardial access with insufflation of pericardial space using carbon dioxide (CO2) based on the technique which has been previously reported.1-3 To the best of our knowledge, this is the first reported case of such procedure ever done in Japan.
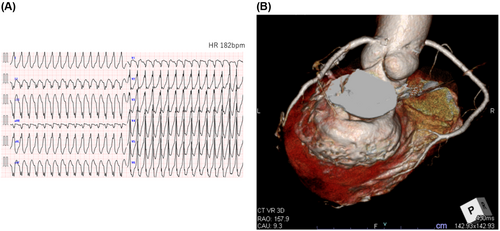
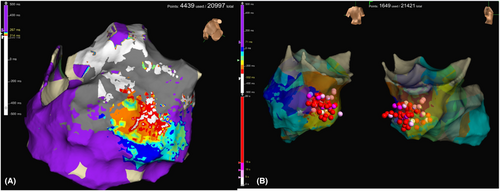
The patient was a 67-year-old female who had myocardial disease from scleroderma. She presented to our emergency department with repeated episodes of VTs (Figure 1A) Endocardial ablation was done and VT circuit was localized to the basal part of the inferior wall of left ventricle (LV) which correlates well to scar area on CT scan (Figure 1B). However, the precise circuit of VT was unmappable due to hemodynamic instability. An implantable cardioverter-defibrillator (ICD) (Cobalt™ XT DR, Medtronic, Minneapolis, MN) was implanted after the initial ablation. Two weeks later, she had multiple ICD shocks per day after several cycles of ineffective intrinsic anti-tachycardia pacing (iATP). Amiodarone was avoided because of interstitial pneumonitis from scleroderma.
In order to increase success rate, we thought that second ablation would require epicardial access, with extracorporeal membrane oxygenation (ECMO) support. However, her hepatic left lobe was on the trajectory of subxiphoid puncture and she had been on steroid therapy for several years, leading to fragile connective tissue, both of which increase the risk of complications during epicardial puncture. We though that this CO2 method would drastically reduce the risk. Written informed consent was obtained from the patient, and the procedure was approved by the board of medical ethics of our hospital.
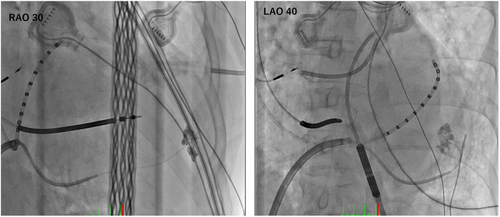
The procedure was done while the patient was awake under moderate analgesia by 0.15 mg of fentanyl. Tactiflex SE catheter (St. Jude Medical, St. Paul, Minnesota) was used to cannulate coronary sinus (CS) via transfemoral vein, and an Agilis sheath (St. Jude Medical) was then advanced in to CS along the catheter. Venography was performed through Arrow Wedge Pressure catheter (Teleflex Medical Japan, Tokyo, Japan) to visualize the branches of coronary sinus. The first posterior branch was selected with a hydrophilic 0.018″ wire and then exchanged to 0.014” Cruise wire (Asahi Intecc, Seto, Japan) through the Arrow Wedge Pressure catheter. We then advanced a microcatheter Finecross GT (Terumo, Tokyo, Japan) to the distal part of the branch and performed tip injection with contrast. First tip injection showed muscular stain, indicating cannulation of a LV perforating branch (Figure 2). After reselecting another straight branch, we exchanged the wire to CTO tapered wire Conquest Pro 0.009/0.014 (Asahi Intecc) and were able to easily penetrate the vein. We confirmed that the wire was in the pericardial space by advancing Cruise wire to make a large loop inside the pericardial space and injecting contrast through the microcatheter.
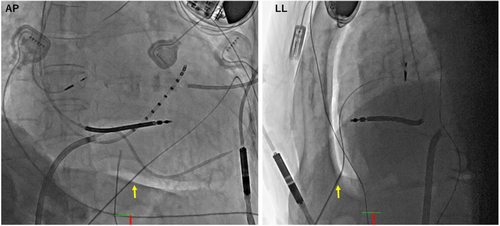
In total, 140 mL of CO2 was then gradually injected through the microcatheter creating fluoroscopically visible space on anteroposterior and lateral view (Figure 3). Subxiphoid punctured was performed by aiming at the space on fluoroscopy with AMC/4 Arterial Needle (18ga × 7 cm) (Argon Medical devices, Texas). Another Agilis sheath was inserted into the pericardial space, and CO2 aspiration was subsequently undertaken to avoid interference with the impedance during epicardial mapping. 3D electroanatomical mapping was performed by using HD Grid catheter on EnSite NavX (St. Jude Medical) mapping system.
Endocardial and epicardial maps during sinus rhythm, pacing from and posterolateral coronary venous branch (Figure 4A), and VT were created. We shifted to general anesthesia and ECMO support before induction of VT. Three different VTs were induced. Combination of ablation from endocardial right ventricle, epicardial on the LV posterior wall, transeptal and transaortic approach to endocardial LV were needed to successfully ablated all types of VT (Figure 4B). The patient was discharged 5 days after the procedure and remained event free on remote patient monitoring of ICD. In summary, we used CO2 injection technique to safely access epicardial space and successfully ablate the VT circuit by combination of endocardial and epicardial ablation.
1 DISCUSSION
Since the introduction of epicardial access for ablation in 1996, the technique of subxiphoid puncture with Tuohy or micropuncture needle has not changed substantially with complications up to 10%. CO2 insufflation enlarges the pericardial space and makes it visible on fluoroscopy, leading to a straightforward puncture route with a straight needle. This innovation of has rendered epicardial access safe and can be readily adopted in any center after relatively short learning curve.
Distal part of any branches (mid cardiac vein, posterior, lateral, or anterior interventricular vein3, 4) can be equally selected for intentional perforation. The important key while advancing CTO wire is to avoid the perforating branches of coronary veins that run toward LV muscle. This can be easily differentiated by fluoroscopic view that is perpendicular to the perforating branches. Premature ventricular contractions (PVCs) after advancing the wire into those branches and stain in cardiac muscles after contrast injection (Figure 2) are signs of perforating branch cannulation. If this goes undetected, CO2 could be injected in to the ventricles, misleading the operator to puncture of the chamber.5
This patient's hepatic left lobe was on the course of the subxiphoid puncture trajectory. Generally, we would need her to inhale deeply and hold her breath to avoid injury to the liver. However, with this CO2 insufflation technique, holding breath was deemed unnecessary since the diaphragm was pushed downward with CO2 inflation, creating adequate space to puncture.
We did not observe any drop in BP during tamponading the heart with CO2. BP remained stable at 120–130/80–90 throughout the puncture procedure. We also regularly checked the color of pericardial fluid to confirm that there was no bleeding from venous exit site with 0.014″ wire, although previous studies have stated that bleeding is not an issue in this situation1-5.
Considering the safety of this procedure, we think that this method should be considered as a preferred option for epicardial access in patients with high risks.
FUNDING INFORMATION
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
CONFLICT OF INTEREST STATEMENT
All authors do not have any potential sources of conflict of interest regarding the content of this study.
ETHICS STATEMENT
The study was approved by the board of medical ethics of our hospital.
PATIENT CONSENT STATEMENT
Written informed consent for the study and publication was obtained from the patient.
PERMISSION TO REPRODUCE MATERIAL FROM OTHER SOURCES
This study is not citing any material from other sources that need permission.
CLINICAL TRIAL REGISTRATION
This study is not part of any clinical trial registration.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author, [T.T], upon reasonable request.