Mirror neurons and their relationship with neurodegenerative disorders
Abstract
The finding of mirror neurons (MNs) has provided a biological substrate to a new concept of cognition, relating data on actions and perceptions not only to integrate perception in action planning and execution but also as a neural mechanism supporting a wide range of cognitive functions. Here we first summarize data on MN localization and role in primates, then we report findings in normal human subjects: functional magnetic resonance imaging and neurophysiological studies sustain that MNs have a role in motor learning and recognizing actions and intentions of others, and they also support an embodied view of language, empathy, and memory. Then, we detail the results of literature searching on MNs and embodied cognition in Parkinson's disease (PD), frontotemporal dementia (FTD)/amyotrophic lateral sclerosis (ALS), and in mild cognitive impairment (MCI)/Alzheimer's disease (AD). In PD the network of MN could be altered, but its hyperactivation might support motor and cognitive performances at least in early stages. In the ALS/FTD continuum, preliminary evidence points out to an involvement of the MN network, which could explain language and inter-subjectivity deficits shown in patients affected by these clinical entities. In the MCI/AD spectrum, a few recent studies suggest a possible progressive involvement from posterior to anterior areas of the MN network, with the brain putting in place compensatory mechanisms in early stages. Reinterpreting neurodegenerative diseases at the light of the new views about brain organization stemming from the discovery of MN could help to better comprehend clinical manifestations and open new pathways to rehabilitation.
Abbreviations
-
- ACE
-
- action compatibility effect
-
- AD
-
- Alzheimer's disease
-
- AOT
-
- action observation training
-
- BDNF
-
- brain-derived neurotrophic factor
-
- bvFTD
-
- behavioral variant of frontotemporal dementia
-
- CBD
-
- corticobasal degeneration
-
- DBS
-
- deep brain stimulation
-
- DLB
-
- dementia with lewy body
-
- EEG
-
- electroencephalography
-
- fMRI
-
- functional magnetic resonance imaging
-
- FoG
-
- freezing of gait
-
- FTD
-
- frontotemporal dementia
-
- IFG
-
- inferior frontal gyrus
-
- IPL
-
- inferior parietal lobule
-
- MCI
-
- mild cognitive impairment
-
- MEP
-
- motor evoked potentials
-
- MI
-
- motor imagery
-
- MNs
-
- mirror neurons
-
- MotND
-
- motoneuron disease
-
- PD
-
- Parkinson's disease
-
- PDD
-
- Parkinson's disease dementia
-
- PET
-
- position emission tomography
-
- REM
-
- rapid eye movements
-
- RME
-
- reading the mind in the eyes test
-
- TMS
-
- transcranial magnetic stimulation
Significance
This work deals with a groundbreaking pathway of research: the relevance of the mirror neuron (MN) network to the interpretation and possible prevention and treatment of neurodegenerative diseases. We revise findings about the role of MNs in monkeys, normal human subjects, and people affected by different neurodegenerative diseases. These findings may have interesting applications in aging neuroscience as the MN exemplify a neuronal network interconnecting perception, action, and cognition; characterizing their functioning in neurodegenerative diseases could be stimulating from a speculative point of view, shed an innovative light on the clinical picture, and open interesting possibilities for rehabilitative treatment.
1 INTRODUCTION
The concept of “embodied cognition” considers that the classical perception-cognition-action architecture proposing a sequential flow of processing with clean cuts between all modules is not appropriate to understand the behavioral effect of neurodegenerative disorders and to find innovative therapeutic solutions. In the last decades, the discovery of mirror neurons (MNs) has provided a biological frame to this theoretical perspective; MNs are now thought to associate information about actions and perceptions to both integrate perception in action planning and execution and as a neural mechanism supporting a wide range of cognitive functions, for example empathy and language. At the same time, it is now clear that in each neurodegenerative disease both cognitive and motor symptoms are represented along a continuum. The main purpose of this review is to investigate the integrity of the MN network in neurodegenerative diseases such as Parkinson's disease (PD)/dementia with lewy bodies (DLB), frontotemporal dementia (FTD)/amyotrophic lateral sclerosis (ALS), and the mild cognitive impairment (MCI)/Alzheimer's disease (AD) spectrum. Describing the functional state of the MN network in neurodegenerative diseases would provide us with a better comprehension of pathophysiological mechanisms and symptoms of these diseases. It would also enable us to capitalize on these kinds of neurons in the rehabilitation of motor and cognitive manifestations.
2 MIRROR NEURONS
MNs represent a groundbreaking discovery in the field of neuroscience. They are a special population of neurons that discharge both when a subject accomplishes an action and when she/he watches another subject performing the same or a similar action.
2.1 Mirror neurons: Research from primates
MNs were originally discovered in monkey's brain through single neurons recording in the ventral premotor area F5 (and above all its F5c cytoarchitectural area) and in the inferior parietal lobule (IPL) (di Pellegrino, Fadiga, Fogassi, Gallese, & Rizzolatti, 1992; Rizzolatti & Fogassi, 2014). In area F5, besides purely motor neurons, researchers found two categories of visuomotor neurons, namely “canonical neurons” (which respond to the vision of three-dimensional items; Fadiga, Fogassi, Pavesi & Rizzolatti, 1995) and “mirror neurons.” MNs discharge when the subject views motor acts performed by other individuals (Gallese, Fadiga, Fogassi, & Rizzolatti, 1996; Rizzolatti, Fadiga, Gallese, & Fogassi, 1996). In particular, monkey's MNs have showed to discharge to the observation and execution of hand actions (e.g., grasping, locating, manipulating with the fingers, and clutching) and mouth actions (e.g., eating-related gestures such as breaking food items, chewing, and sucking, and communicative gestures such as lip-smacking or protrusion and tongue-protrusion; di Pellegrino et al., 1992; Ferrari, Gallese, Rizzolatti, & Fogassi, 2003; Gallese et al., 1996). Even if initially F5 MN did not appear to react if the same action (e.g., grasping) was performed with a tool, more recent data indicate that after a protracted visual exposure of the monkey to motor acts performed with a tool, a subset of MN in the ventral part of area F5c responds also to this type of visual stimuli (Ferrari, Rozzi, & Fogassi, 2005). Additional generalization properties of some F5 MNs have been subsequently reported, for example some of them reacted in a similar way to the same action executed with different body parts (e.g., grasping with the hand and grasping with the mouth; Ferrari et al., 2003; Rizzolatti, Fogassi, & Gallese, 2001), to the sound associated with familiar actions (Kohler et al., 2002), and even to partially covered actions that can be deduced only from their initial motion track (Umiltà et al., 2001). Other authors have shown that the response a subset of IPL MN (i.e., in the cytoarchitectonic area PF and PFG: in the macaque monkey, the rostral part of the inferior parietal lobule is divided into area PF and area PFG; Fogassi et al., 2005), during both the observation and execution of a complex grasping act, is dependent on the final goal of the motor act (placing or eating) for which the grasping was performed (Bonini et al., 2010; Fogassi et al., 2005); MNs have a smaller response if the piece of food is clutched to place it into a box. Another significant characteristic of these cells is that their activity appears not to be strictly related to the precise timing of the observed action; indeed, a certain part of parietal MN is activated before the achievement of the end-goal (Fogassi et al., 2005). Fogassi and colleagues considered this kind of response a demonstration of the possible explicit cognitive role of IPL MN of encoding the intentions of other people (Fogassi et al., 2005). More recent investigations have displayed similar reaction properties in F5 MN (Bonini et al., 2010), even if to a lesser degree (Rizzolatti & Fogassi, 2014). Other studies suggest that MNs assimilate into their responses additional pieces of information that are not directly related to the action itself. MN activity can change accordingly to the distance at which the observed actions were executed with respect to the monkey, that is in the peri-personal space or in the extra-personal space of the monkey (Caggiano, Fogassi, Rizzolatti, Thier, & Casile, 2009). The distance between viewer and performer can be important in choosing possible subsequent behaviors (e.g., interactive or approaching behaviors). Thus, the MN system could be part, together with other brain areas, of a system whose aim was not only to understand what other people are doing, but also in deciding the most appropriate behavioral response (Caggiano et al., 2011). According to another study, the majority of F5 MNs appear to visually encode motor acts in a view-dependent way (Caggiano et al., 2011). In this study, MN’s firing to action observation was studied by displaying the same actions from different points of view, comprising the performer's own point of view. The responses of MNs were modulated not only by the kind of action being observed but also by the point of view under which it was observed (e.g., a frontal or a side view).
2.2 Mirror neurons: Studies in humans
In humans, the presence of MNs has been suggested through neuroimaging (functional magnetic resonance imaging, fMRI) and neurophysiological techniques (electroencephalography—EEG, evoked potentials, transcranial magnetic stimulation—TMS; Cochin, Barthelemy, Roux, & Martineau, 1999; Fadiga et al., 1995). MN’s activity has been found in the premotor cortex (posterior regions of the inferior frontal gyrus—IFG—which is thought to be the human homologue of the monkey F5; Ferri et al., 2015; Kilner, Neal, Weiskopf, Friston, & Frith, 2009) and in the IPL (Arnstein, Cui, Keysers, Maurits, & Gazzola, 2011; Chong, Cunnington, Williams, Kanwisher, & Mattingley, 2008; Molenberghs, Cunnington, & Mattingley, 2012; Rizzolatti, 2005; Rizzolatti & Craighero, 2004; Rizzolatti et al., 2001). The presence of MN activity was additionally signaled in the primary motor cortex (Fadiga, Craighero, & Olivier, 2005) and even in the hippocampus (Mukamel, Ekstrom, Kaplan, Iacoboni, & Fried, 2010). Moreover, the functional properties of Superior Temporal Sulcus neurons suggest that these neurons supply the essential cortical visual input to MNs (Nelissen et al., 2011). STS could also be fundamental in social communication (Allison, Puce, & McCarthy, 2000). In fact, Montgomery, Isenberg, and Haxby (2007) discovered significant response in the STS and the MN system for the observation, imitation, and execution of both object-directed hand movements and communicative hand gestures.
Studies utilizing fMRI have shown that a parietofrontal network, analogous in monkeys and humans, is activated during the observation and execution of hand grasping actions (Grèzes, Armony, Rowe, & Passingham, 2003), as well as during the observation of grasping acts performed with tools ( Peeters et al., 2009), accordingly with previous single neuron studies in monkeys (Ferrari et al., 2005). fMRI has identified regions of premotor cortex (BA6 and BA44) and inferior parietal areas that are functioning during both action observation and execution (Aziz-Zadeh, Wilson, Rizzolatti, & Iacoboni, 2006; Buccino, Binkofski, & Riggio, 2004; Carr, Iacoboni, Dubeau, Mazziotta, & Lenzi, 2003; Grèzes et al., 2003; Iacoboni et al., 1999; Leslie, Johnson-Frey, & Grafton, 2004; Tanaka & Inui, 2002; Vogt et al., 2007).
In human subjects, both goal-directed actions with the object present and goal-directed pantomimed actions (Buccino et al., 2001; Montgomery et al., 2007) seem to implicate the MN system, in the same way as communicative actions do. In a fMRI experiment in which subjects observed, imitated, and produced communicative hand gestures and object-directed hand movements, the observation and execution of both types of hand gestures activated the MN system to a similar degree (Montgomery et al., 2007). A fMRI study in humans (Buccino et al., 2001) also showed the activation of different subdivisions of Broca's area and premotor cortex during the observation of hand, mouth, and foot actions. This activation is associated with the effector of the observed action, following a somatotopic pattern which looks like the classical motor cortex homunculus. Therefore, some authors (Buccino, Solodkin, & Small, 2006; Rizzolatti & Arbib, 1998; Tettamanti et al., 2005) have claimed that there is a homology between the motor-related part of Broca's region, contained in the opercular portion of the inferior frontal premotor cortex (mainly in area 44 of Brodmann) and the area F5 in monkey.
As far as more posterior areas of MN human system are considered, a precise part of human IPL (left anterior supramarginal gyrus, aSMG) would be a region exclusive to hominid evolution as it is activated in humans, but not in monkeys, during observation of actions made with tools (Peeters et al., 2009). Figure 1 shows the cerebral location of human MN regions.

An extensive quantitative meta-analysis (Molenberghs et al., 2012) of fMRI data from 125 studies revealed that human MN areas are localized in the inferior frontal gyrus, in the ventral premotor cortex, and in the IPL (which are considered “classical” MN areas and are expected to be part of the MN system based on studies in monkeys), but also in the primary visual cortex, in the cerebellum, and in the limbic system.
Several studies based on neurophysiological recordings showed a reduction in magnitude of the μ rhythm—an EEG oscillation occurring within the standard “alpha” band (i.e., ~8–13 Hz in adults; ~6–9 Hz in children) in the rolandic areas (e.g., Fox et al., 2016)—during action observation and execution. These studies led to the conclusion that this rhythm is an indicator of a brain system that is functionally comparable to the monkey's MN network (Lepage & Théoret, 2006; Muthukumaraswamy, Johnson, & McNair, 2004; Pineda, 2005). μ suppression studies in humans revealed that a stronger suppression occurred when viewing another's hand in a precision grasp (i.e., a grasp to be used to get an object) rather than in a neutral, non-grasping position, and that interaction with an object determined greater μ suppression than situations without object interaction (Muthukumaraswamy & Johnson, 2004; Muthukumaraswamy et al., 2004). These groundbreaking studies, indicating that μ suppression reflects human MN system, have led to many action observation experiments. Fox et al. (2016), who recently reviewed this branch of investigation in a meta-analysis, concluded that μ suppression can be used as a surrogate for human MN activity. Indeed, most results indicate consistent EEG μ desynchronization (suppression) during both action execution and observation, regardless of the type of stimulus and action (e.g., object-directed and non-object-directed) observed. Otherwise, it has been supposed that MN responses to non-object-directed actions a human peculiarity denoting a departure from our common ancestors which represent a milestone in the evolution of language (Rizzolatti & Craighero, 2004).
However, other researchers have questioned that μ suppression match MN activity. In point of fact, μ suppression studies have been unsuccessful in providing robust evidence of the role of the MN system due to limitations in methodology, for instance, a few studies describe changes in power at locations other than the central electrodes, one can therefore wonder whether observed effects are not due to changes in power in other areas (Hobson & Bishop, 2016). Coll, Bird, Catmur, and Press (2015) described that μ suppression was associated with sensory mirroring but not motor mirroring, a finding that weakens the essential connection between action and perception that MNs are thought to represent. For these reasons the legitimacy of μ suppression as a measure of the human MN system still represents a matter of discussion. Some authors have also emphasized that the rolandic μ rhythm consists of two spectral peaks and that its arch-like form depends on the contribution of both the alpha and the beta range activities (Niedermeyer & da Silva, 2005). The ß frequency band is usually defined as a rhythm between 13 and 35 Hz, with a typical peak frequency of about 20 Hz (Niedermeyer & da Silva, 2005). Like μ, ß activity is suppressed by voluntary movements, motor imagery (MI) and action observation (Babiloni et al., 2002), so changes in ß activity have also been considered to be an index of MN activity (Hobson & Bishop, 2016; Pozzo et al., 2017).
Neurophysiological and fMRI data have also been correlated during action observation tasks (e.g., see Braadbaart, Williams, & Waiter, 2013). These authors found that μ power modulation was negatively associated with BOLD response in supposed MN areas: right inferior parietal lobe, premotor cortex, and inferior frontal gyrus. Thanks to μ suppression they have also identified a variety of structures that control motor preparation and respond to visual input including, but not limited to, the human analogue of the MN system cluster (bilateral cerebellum, left medial frontal gyrus, right temporal lobe, and thalamus) (Braadbaart et al., 2013).
Most functional studies on MNs have explored hand actions and very few have studied how MNs respond to mouth actions or communicative gestures. A recent study by Ferrari and colleagues has shown the presence of MNs in different cytoarchitectonic areas and that their specific properties are related not only to the type of effector involved (hand or mouth) but also to the different anatomical pathways (Ferrari, Gerbella, Coudé, & Rozzi, 2017). In particular, this study showed that the mouth and hand MN sectors have distinct and specific connections. Unlike hand MNs, mouth MNs do not receive their visual input from parietal regions. This information concerning face/communicative behaviors could come from the ventrolateral prefrontal cortex. Other strong connections derive from the limbic structures involved in coding emotional facial expressions and motivational/reward processing (e.g., anterior cingulate cortex, anterior and mid-dorsal insula, orbitofrontal cortex, and the basolateral amygdala). The mirror mechanism is therefore composed and supported by at least two different anatomical pathways: the first concerns sensorimotor mirroring in relation to reaching and hand grasping within the traditional parietal-premotor circuits; the second is related to mouth/face motor control, is connected with limbic structures, and potentially explains the function of MN system in empathy and social interaction.
2.2.1 MN functions in humans
Recognizing actions of others
The first function attributed to MNs in humans has been recognizing actions performed by other people. In a TMS study (Fadiga et al., 1995) subjects were submitted to stimulation (with an increase of left motor cortex excitability) during observation of grasping acts, purposeless movements, and static objects. Evoked potentials of hand muscles were more marked during grasping observation, even if they were also present during observation of aimless movements. The most important point is the fact that evoked potentials were present in muscles specifically involved in the execution of the observed act.
Another emblematic study was conducted with fMRI on patients with upper limbs aplasia (Gazzola et al., 2007). The observation of manual grasping by subjects that never used hands activated MNs at the same extent than acts having the same aim and performed by the subjects with the mouth or the foot.
Interpreting action intention
Other studies on MN function investigated whether they were also involved in interpreting the intention embedded in the action. Iacoboni et al. (2005) performed a fMRI study where participants observed three types of stimuli: grasping hand actions without a context, context only (scenes containing objects), and grasping hand actions performed in two different contexts. In the latter condition, the context suggested the intention linked to the grabbing action (either drinking or cleaning). Actions embedded in contexts, differently than actions in the other two conditions, produced a significant signal intensification in the posterior portion of the inferior frontal gyrus and the adjacent subdivision of the ventral premotor cortex, where hand actions are represented. The authors concluded that premotor MN areas play a role in understanding the intentions of others.
Motor imitation and motor learning
The MN system is thought to be concerned in imitational learning of familiar elementary movements. Iacoboni et al. (1999) demonstrated the activation of the inferior frontal cortex (pars opercularis) and of the superior parietal lobule during the observation, spontaneous execution, and imitation of a motor task in a fMRI study. The involvement of the pars opercularis of the frontal lobe in imitation has been also confirmed by a TMS study in which the cortical area was excited using a stimulous frequency of 5 Hz (Heiser, Iacoboni, Maeda, Marcus, & Mazziotta, 2003). Another study suggesting the role of the MN system in imitation is the already cited research by Molenberghs, Brander, Mattingley, and Cunnington (2010). Buccino et al. (2004) have also suggested that the MN system is involved in the acquisition of new motor skills. In a fMRI study, researchers recruited subjects who had never played a guitar. The subjects watched a video showing a music teacher who played some chords. After a pause, they had to reproduce the chords. Control conditions were represented by: (a) observing the video without reproducing chords, (b) playing accords spontaneously, (c) observing a video with a guitar alone, before imitating, and (d) executing movements not related to the music, after chords observation. Watching the video and then imitating was the condition which activated the MN system the most, while observation not followed by imitation activated the MN circuit (IPL and the posterior part of the inferior frontal gyrus plus the adjacent motor cortex) at a lesser extent. In imitation-based motor learning, the creation of permanent motor memories was considered in the past to be done through the physical practice of movements. According to the new view, in this kind of learning the MN system enables motor learning simplifying the physical performance of the proper training movements. Mattar and Gribble (2005) utilized kinematic analyses to demonstrate that the achievement of complex motor behaviors is eased by a previous observation of subjects mastering the novel task.
The role of MN in higher cognitive functions
MN discovery received a lot of attention from specialists (not only neuroscientists but also psychologists and philosophers) both in scientific and public media. The MN network, besides playing a role in action understanding and imitation, is now considered to be involved in many other sophisticated human behaviors such as empathy, language, and learning (Cook, Bird, Catmur, Press, & Heyes, 2014; Corradini & Antonietti, 2013; Oztop, Kawato, & Arbib, 2013; Rizzolatti & Fogassi, 2014). For instance, the finding of MNs has given a biological complement to the simulation theory, suggesting that actions involve both an overt and a covert stage (Jeannerod, 2001; Jeannerod & Frak, 1999). The covert stage is a kind of implicit cognitive representation of the future and comprises the goal of the action, the means to get it, and its consequences on the organism and the external world. Covert and overt stages would represent a continuum; every overtly executed action indicates the existence of a covert stage, although the cognitive covert stage of action does not necessarily turn out into overt action (Jeannerod, 2001). In a further development of this research line, the existence of the MN system supports the theoretical perspective of the “embodied” or “motor” cognition. Among the multitude of ways to explain human and animal behaviors, there is a growing body of experimental evidence indicating that cognition and action production are mutually dependent and that motor representations are present at each level of cognitive (language, memory, spatial and temporal representations, and social cognition) processes (Barsalou, 1999, 2008; Gallagher, 2005; Gavazzi, Bisio, & Pozzo, 2013; Noe, 2004; Wheeler, 2005; Wilson, 2002). More generally, the evolution of the brain and its volume increase is associated with more complex behavior, meaning more sensorimotor sophisticated response to environmental constraints, an idea highly consensual in evolutionists (Krubitzer, 2007; Northcutt, 2002). The discovery of a category of neurons that links knowledge about actions and perceptions has thus given a possible neural motor substrate to an extensive array of high cognitive functions, including attention, meaning, concepts, goals, and intentions understanding, in addition to communication for social interaction (Pulvermüller, Moseley, Egorova, Shebani, & Boulenger, 2014).
2.2.2 Inter-subjectivity, empathy, and social cognition
Experimental evidence strongly suggests that the MN system (along with other cerebral circuits, such as the social cognition network), contributes to understand not only other people's actions but also their emotions (Agnew, Bhakoo, & Puri, 2007; Gallese, 2003; Gallese, Keysers, & Rizzolatti, 2004; Pineda & Hecht, 2009; Wicker et al., 2003). In this sense, a crucial role of MN would be to foster empathy. Empathy is a subjective experience. It can be viewed as the process by which a person resonates and tunes to others’ affective and cognitive states (Levy & Feldman, 2019). It plays a key role in social relations and allows humans to connect and understand each other and to create social and cultural groups. Studies on emotional states demonstrate that a large-scale neural network—including the ventral premotor cortex and the inferior frontal gyrus (two reputed MN areas), in addition to classical cerebral regions involved in feeling emotions, such as the anterior insula and the amygdala—is active for instance during facial expression observation and imitation (Budell, Jackson, & Rainville, 2010; Carr et al., 2003; Grosbras & Paus, 2006; Iacoboni, 2009; Rizzolatti & Craighero, 2004). In children, activity in the frontal part of the MN system, produced by observation and imitation of emotional expressions, correlates with measures of empathic behavior and interpersonal skills (Pfeifer, Iacoboni, Mazziotta, & Dapretto, 2008). Likewise, it is now proposed that the core deficits of autism, which are motor, language, and social impairments, are indicative of dysfunction of the MN system (Dapretto et al., 2006; Fishman, Keown, Lincoln, Pineda, & Müller, 2014), and various works suggest a role of MNs in schizophrenia (Kato et al., 2011; Saito et al., 2017; Schilbach et al., 2016). In contrast, as suggested by Linkovski, Katzin, and Salti (2017), caution in theoretical generalization of experimental procedures is needed. As an example, researches on MNs and schizophrenia do not report a correlation between social dysfunction severity and the abnormality of the MN network (Enticott et al., 2008; McCormick et al., 2012).
2.2.3 Language
Some studies have shown that MNs have a main role in different aspects of language acquisition (Theoret & Pascual-Leone, 2002), speech perception (Glenberg et al., 2008) and production (Kühn & Brass, 2008), and evolutionary language development (Arbib, 2008). Tettamanti et al. (2005) demonstrated that listening to sentences describing actions performed with the mouth, the hand or the leg activated a left fronto-parietal-temporal network that included the pars opercularis of the inferior frontal gyrus (Broca's area), the intraparietal sulcus, and the posterior middle temporal gyrus, in a somatotopic manner. Based on these studies, it has been suggested that cerebral areas establishing a correspondence among performed and observed actions in monkeys (F5Area) match to those that are appointed to language production in humans (Broca's Area; Molnar-Szakacs, Iacoboni, Koski, & Mazziotta, 2005, Tettamanti et al., 2005). However, a recent study raised doubts about this presumed matching (Cerri et al., 2015). Other neuroimaging studies revealed zones in the human premotor cortex that activate both when participants see actions being performed and when they read sentences concerning those actions (Aziz-Zadeh et al., 2006). Furthermore, Hauk, Johnsrude, and Pulvermüller (2004) found a somatotopically organized activation in the motor and premotor cortex when subjects passively read action-associated words (i.e., leg-associated action words lead to activations in medially situated areas while arm or face-associated action words lead to lateral areas activation).
More generally, the role of the MN system in evolutionary language development is recurrently proposed (Chwilla, Virgillito, & Vissers, 2011; Fischer & Zwaan, 2008; Meteyard, Zokaei, Bahrami, & Vigliocco, 2008; Rizzolatti & Arbib, 1998), even if it is still under discussion (Caramazza, Anzellotti, Strnad, & Lingnau, 2014).
2.2.4 Memory
Several behavioral investigations support the idea of the embodied nature of memory processes, that is the idea that memory is the recall of a sensation stored after an action production generating that sensation. According to this view, memory is considered a dynamic process instead of a static storage such as a library or a video archive and learning is influenced by sensorimotor coupling. For instance, when normal subjects are asked to retain lists of objects in memory while performing a congruent or incongruent action with these objects, performing an incongruent action impaired memory performance, in comparison to congruent action. Interestingly, the quantity of manual experience with the object regulates the quantity of interference (Yee, Chrysikou, Hoffman, & Thompson-Schill, 2013). Accompanying words or sentences in a foreign language with the corresponding gestures conducts to better learning (Macedonia, 2014); gestures activate motor neurons and could activate MN, which are a subset of motor neurons. Neurophysiological studies have indicated a direct involvement of the MN network in the imitational learning of familiar elementary movements (Iacoboni et al., 1999) and in the acquisition of new motor skills (Buccino et al., 2004; Gatti et al., 2013; Mattar & Gribble, 2005). Stefan et al. (2005) demonstrated that a specific motor memory like the one induced by practicing movements is formed by observation. Viewed together, these studies suggest that MNs could affect memory by improving encoding. In contrast, the MN system seems to influence not only encoding, but also retrieving; autobiographical memories in body-congruent and body-incongruent positions relative to that of the original experience also influences performance (Dijkstra, Kaschak, & Zwaan, 2007). Gelbard-Sagiv, Mukamel, Harel, Malach, and Fried (2008) showed, in patients with pharmacologically intractable epilepsy implanted with depth electrodes, that neurons in the medial temporal lobe are reactivated during spontaneous recall of previously observed audiovisual sequences (episodic memory). According to the authors, these neurons could match the vision of actions executed by other people with the memory of those same actions completed by the observer. This concept is in agreement with the idea that during action accomplishment, a memory of the performed action is formed, and this memory trace is reactivated during action observation. The same group (Mukamel et al., 2010), by recording extracellular activity, described neurons with mirror properties in the hippocampus (that is neurons which responded to both observation and execution of actions). This important observation suggests that in humans, multiple neuronal systems might be endowed with mechanisms of mirroring others’ actions; the functional significance of the mirror mechanism might vary according to the position of the MN, thus supporting different functions, memory included. Even if these data need to be confirmed by further studies, it opens exciting perspectives for a novel interpretation of memory mechanisms.
In conclusion, MNs represent the neuronal population which link perception and action, cognition and motility. For a summary of the literature about MN functions in humans, see Supporting Information Appendix A.
3 MIRROR NEURONS AND NEURODEGENERATIVE DISEASES
The possible role of MNs in cognition suggests a hypothetic involvement of this neuronal network in neurodegenerative diseases. In the last decade it has become increasingly evident that the classic distinction between cognitive and motor neurodegenerative disorders is blurred and that in each neurodegenerative disease both cognitive and motor symptoms are represented in a sort of continuum; in PD, initially classified as a movement disorder, it is recognized that non-motor symptoms (cognitive, autonomic, affective, and behavioral disorders) are relevant in the course of the disease (Moustafa & Poletti, 2013). This disease has a strong relationship with the second most common cause of neurodegenerative dementia, DLB (McKeith et al., 2017). In contrast, AD, the most common cause of dementia, and MCI, which is now seen as a pre-dementia stage of the same disease, particularly in its amnesic form, have always been seen as “pure” cognitive disorders (Rossor, 1992). However, in the last decade, there has been a growing recognition of a connection between deficiencies in motor function and these two clinical entities. (Bisio et al., 2012; Ghilardi et al., 1999; Yan, Rountree, Massman, Doody, & Li, 2008). Nevertheless, the most striking example of combining cognitive and motor symptoms in neurodegenerative disease is the continuum of ALS-FTD.
To investigate the possible role of MNs in neurodegenerative diseases we performed a quasi-systematic search of sources, to appraise the existing studies considering the MN system, the embodied cognition theory, and these three main categories of neurodegenerative diseases.
3.1 Empathy deficit in neurodegenerative conditions
As previously cited, the MN network is thought to greatly contribute to social relationships. Early loss of empathy (accompanied by reduced response to other people's needs and feelings, and diminished social interest) is one of the six core symptoms in the revised diagnostic criteria of the behavioral variant of FTD (Rascovsky et al., 2011) and is frequently encountered in this disease (Baez et al., 2014; Laforce, 2013). Impaired performance at a test which is considered a measure of empathic abilities showed to better discriminate behavioral variant of frontotemporal dementia (bvFTD) patients from normal controls or AD patients than altered performance in executive tests. This result highlights the importance of social cognition abnormalities in bvFTD diagnosis (Schroeter et al., 2018). Interestingly, loss of empathy (Girardi, MacPherson, & Abrahams, 2011) and altered emotional empathy attribution (Cerami et al., 2014) have also been described in ALS patients without dementia.
However, empathy loss has also been described in neurodegenerative diseases other than the FTD/ALS continuum; several studies (Ariatti, Benuzzi, & Nichelli, 2008; Herrera, Cuetos, & Rodríguez-Ferreiro, 2011; see also Péron, Dondaine, Jeune, Grandjean, & Vérin, 2012 for a review) have shown emotion recognition deficits in PD patients when compared to matched healthy controls. Deficits in recognizing others' emotions are reported in AD (Fernandez-Duque, Hodges, Baird, & Black, 2009; Martinez et al., 2018) and even in MCI (Teng, Lu, & Cummings, 2007).
These data are important for caregivers of persons with neurodegenerative diseases; in point of fact, this emotional impairment can affect both sides, that is the sender and the receiver of the emotional communication. A recent study has shown not only that subjects with AD and PD are significantly impaired in recognizing emotions, but also that their caregivers do not recognize these deficits, and this is linked with increased caregiver burden and depression (Martinez et al., 2018). We found several studies linking the MN network to neurodegenerative diseases in our literature revision. Their results will be detailed in the following chapters.
3.2 Mirror neurons and Parkinson's disease
Most available studies considering both cognition and action systems are focused on PD, the most common extrapyramidal disorder, with controversial results. The functioning of the MN network in PD has been studied, as in normal subjects, through neurophysiological techniques (EEG and TMS), neuroimaging (fMRI), and kinematic studies. Several studies suggested rehabilitative training based on stimulating the MN network and the embodied cognition framework in PD. An in-depth presentation of the studies on MN and PD is shown in the Supporting Information Appendix B.
3.2.1 Neurophysiological studies
Many studies on MNs in subjects with PD have used the “Action Observation” (AO) approach. This approach includes both observation and execution of an action (Ertelt & Binkofski, 2012) and it is based on the core property of MN. The subthalamic nucleus is directly linked with the frontal (motor and premotor) cortex through the so called “direct” pathway of the motor circuit (Nambu, 2004) and by the cortico-strio-pallido-subthalamic projection (the “indirect” circuit). The direct pathway conveys a monosynaptic excitatory input from the frontal areas to the subthalamic nucleus. Cortical activity triggered in the MN network by AO could therefore easily propagate to the basal ganglia and particularly to the subthalamic nucleus through these pathways. This entry might modulate basal ganglia output in order to facilitate/inhibit competing motor programs according with the expected results of action (Alegre et al., 2010).
According to these views, Alegre et al. (2010) have shown that the subthalamic nucleus displays variations in activity during both movement observation and movement execution. The authors recorded EEG and local field potentials in 18 patients with PD through surgically implanted electrodes for deep brain stimulation (DBS). Oscillations in electrical activities were recorded during AO and two control conditions. During AO (observing wrist extension movements of an examiner seated in front), a significant bilateral decrease of beta range activity in the subthalamic nucleus was recorded in both on and off medication states (even if higher in the first one). This result suggests a substantial conservation of the MN network in PD, with a possible influence of the medication state. In line with this evidence, another study regarding DBS-implanted patients (Marceglia et al., 2009) found a change in subthalamic nucleus oscillatory activity during AO.
Other studies however showed opposite results; in a study, µ-rhythm desynchronization usually recorded during movement observation was reduced in patients with early PD (Heida, Poppe, Vos, Putten, & Vugt, 2014). Tremblay, Léonard, and Tremblay (2007) tested 11 PD patients in the on condition and the same number of healthy elderly controls, by recording motor evoked potentials (MEP) amplitudes in four conditions: rest, AO (a video depicting a hand cutting a piece of paper with scissors), MI, and active action imitation. The MEP amplitude (recorded at the first dorsal interosseous and abductor digiti minimi) increased in PD patients during active imitation but neither during AO nor during action imagery. This would indicate a failure to involve the motor system more at the covert than at the overt phase of action execution. This would be due, according to the authors, to a deficit in motor activation affecting critical nodes in the motor cortical circuit physiologically involved in action preparation. Tremblay et al. hypothesize that these nodes could be the supplementary motor area and the inferior parietal cortex. However, these remarks may also be explained by the involvement of the frontal-subcortical circuits that link the basal ganglia to the premotor cortex (Alegre, Guridi, & Artieda, 2011; Alegre et al., 2010; Alexander, Crutcher, & DeLong, 1990).
Contrasting results in PD studies could depend on several factors, namely, most importantly, the stage of the disease (not always precised in the different studies), then the recruited population (e.g., a bias depending on the recruitment of very small samples cannot be discarded), the dopaminergic state and maybe the clinical form of the disease (tremor vs. akinetic form), an information which is seldom reported.
3.2.2 fMRI studies
In an interesting neuroimaging study, Anders et al. (2012) recruited eight pre-symptomatic carriers of a single mutant Parkin gene, who exhibited a slight but significant decrease of dopamine metabolism in the basal ganglia. Indeed, it is well known that PD has a long pre-symptomatic stage, during which the brain compensates for dopaminergic degeneration by augmenting motor-related cortical activity (Morrish, Sawle, & Brooks, 1996). This work aimed to study whether comparable compensatory mechanisms were operative in non-motor basal ganglia-cortical gating circuits. As execution and perception of facial gestures are thought to be related to MNs in the ventrolateral premotor cortex (Hennenlotter et al., 2005; Leslie et al., 2004), Parkin mutation carriers first underwent fMRI while observing neutral and affective dynamic facial expressions and then performed a facial emotion recognition task. As expected, recruited participants showed a significantly higher activity in the right ventrolateral premotor cortex during execution and perception of affective facial gestures than healthy controls. Furthermore, Parkin mutation carriers showed a slightly reduced ability to recognize facial emotions that were inversely proportional to the increase of ventrolateral premotor activity. According to the authors, these findings are consistent with the hypothesis that compensatory activity in a MN area during processing of affective facial gestures can lessen impairment in facial emotion appreciation in subclinical Parkin mutation carriers. A failure of this compensatory mechanism could conduct to the impairment of facial expressivity and facial emotion recognition observed in clinically evident PD. In contrast, it is possible that the stimulation of MNs could favor these compensation mechanisms at least in the first phases of the disease, thus allowing PD patients to have better social interaction.
Additional support to the putative link between MN area impairment and the well-known emotional deficit of PD patients (Péron et al., 2012) comes from a similar recent study by the same group (Pohl et al., 2017). In this investigation, 13 PD patients and controls performed an emotion recognition task during fMRI measurement. Subjects watched video clips displaying emotional, non-emotional, and neutral facial expressions or were requested to make these facial expressions. Patients completed the emotion recognition task a little worse than controls, but only for the most difficult expressions to be interpreted. Inferior frontal and anterior inferior parietal MN areas activated during observation and execution of the emotional expressions in both groups, but at a lesser extent in PD patients; furthermore, activation of the right anterior IPL positively correlated with patients’ emotion recognition ability.
Péran et al. (2009) compared fMRI cortical activation during the production of action verbs with activation during object naming, in 14 PD patients using a common set of objects pictures. Data revealed the participation of a large cortical circuit during action verb generation, with differences from the object name production situated above all in the premotor and prefrontal cortices. These results suggest an important role of the frontal cortex in action verb generation. They also suggest that a motor striatal-frontal loops impairment leads to the compensative enrollment of a cortical network. Abrevaya et al. (2016) found similar results when subjects listened to action verbs and nouns. The verb lexical category elicited connectivity between primary motor areas and anterior areas (implied in action observation and imitation), in normal controls, while activated posterior areas in PD patients, thus suggesting that patients might afford alternative pathways to process words when motor substrates are altered.
3.2.3 Behavioral studies
Poliakoff, Galpin, Dick, Moore, and Tipper (2007) evaluated the effect of movement-relevant visual stimuli on reaction times in mild-to-moderate PD patients. In the first experiment, participants had to classify visual stimuli (graspable door handles and the control bar condition) according to shape and orientation. No difference was detected in the overall reaction times for patients and controls. However, the spatial compatibility effect (that is faster reaction times when the response hand and the stimulus direction were compatible) was larger in the handle than in the bar condition for controls, but not for PD patients. In the second experiment, the two groups observed video clips of finger or object movements and had to answer as quickly as possible if an X appeared at the end. Both patients and normal participants reduced significantly their reaction times after observing finger compared to objects movements, indicating partial preservation of MN network in PD. However, patients with PD showed a spatial compatibility effect only for non-living object movements leading the authors to propose that in PD the MN system is not completely preserved and that external cues would act through low-level visual processes.
However, other findings suggest a normal coupling between perception and action systems in PD. In an interference task, healthy controls and PD patients, (tested in off period) completed horizontal and vertical arm movements, while watching a person, or a moving dot, performing similar movements in the same-congruent or orthogonal-incongruent plane (Albert, Peiris, Cohen, Miall, & Praamstra, 2010, see also Alegre et al., 2011). No difference was found between patients and controls.
Castiello, Ansuini, Bulgheroni, Scaravilli, and Nicoletti (2009), examining PD patients’ motor imitation ability, showed kinematic facilitation effects only when the model was a patient with PD—who performed slower and less fluid movements—in contrast to a healthy model. Therefore, differently than normal controls, patients could re-enact their motor representations only when the visual model belonged to the patient's motor repertoire. In line with this, the authors have proposed that basal ganglia play a role in setting frontal and parietal cortices; this role is important not only for the execution of actions but also for the internal simulation of observed behaviors, given that the internal state of the simulated action is available.
Reaserchers have also studied the ability of PD patients to perceive and recognize human action. Precisely, when displayed with limited visual attributes (a point-light walker) persons with PD showed reduced visual sensitivity to biological motion (Jaywant, Shiffrar, Roy, & Cronin-Golomb, 2016). Likewise, when observing point-light human figures that carried communicative and non-communicative gestures, patients were impaired, relatively to normal controls, in describing the meaning of non-communicative gestures, while they normally perceived communicative gestures. However, men were more compromised than women and their ability to recognize both types of gestures was reduced.
3.2.4 Neuropsychological studies
Action naming is an ability that depends on the inferior frontal gyrus, a “classical” MN area (see Kemmerer, Rudrauf, Manzel, & Tranel, 2012). Action verb impairment (with relative preservation of noun processing) has been repetitively described in PD. In the same vein, it has been reported that motor-language coupling (i.e., the influence between verbal processes and voluntary body movements, see García & Ibáñez, 2014) is altered in PD. For a detailed list of publications on these topics one can refer to the reviews by Cardona et al. (2013), Silva, Machado, Cravo, Parente, and Carthery-Goulart (2014), and Birba et al. (2017).
Neuropsychological studies (Jacobs, Shuren, Bowers, & Heilman, 1995; Livingstone, Vezer, McGarry, Lang, & Russo, 2016; Marneweck, Palermo, & Hammond, 2014; Ricciardi et al., 2017), similar to the already cited study of Pohl et al. (2017), have also reported the existence of a significant relationship between voluntary control of facial muscles and emotion recognition deficits in PD patients, an interesting finding at the light of the embodied cognition framework. If the MN system is implicated in both production and perception of facial emotional expression, one can really expect an alteration of MN in PD, even if the MN network is not the only neuronal system involved in emotion perception (Wang, Larson, Bowen, & van Belle, 2006). A possible involvement of the MN network in PD empathy deficits is also suggested by a study of Nobis et al. (2017). This research shows a relationship between the performance on the reading the mind in the eyes test (RME, Baron-Cohen, Wheelwright, Hill, Raste, & Plumb, 2001), a test measuring empathic abilities, and the severity of motor symptoms, along with disease duration, in PD patients. It is also interesting to mention that similar data to those reported in PD have been obtained in Huntington disease (Clark, Neargarder, & Cronin-Golomb, 2008; Sprengelmeyer et al., 1996; Trinkler et al., 2017).
Overall, fMRI data (Anders et al., 2012; Pohl et al., 2017) and neuropsychological studies listed above considerably support the contention that empathy deficits in PD are linked to an impairment of the MN network.
3.2.5 Action observation training for PD rehabilitation
Several works found that action observation training (AOT), which includes observation and execution of an action (Ertelt & Binkofski, 2012) and is evidently based on the stimulation of the MN system (Shih et al., 2017), has a positive effect on PD rehabilitation. During a typical rehabilitation session, patients must observe a specific daily action presented through a video clip on the computer screen and then perform what they have observed. Typically, 20 daily actions are performed and chosen based on their ecological value (e.g., drinking coffee, reading the newspaper) during a rehabilitation treatment that lasts 4 weeks (5 days a week; see Buccino, 2014). This supplementary top-down therapeutic tool was first developed for patients with stroke, with the aim to improve brain plasticity and functional outcomes (Carvalho et al., 2013) but subsequently it has also been used in motor deficits of children with cerebral palsy (Bassolino, Sandini, & Pozzo, 2015; Buccino et al., 2012; Sgandurra et al., 2011) and in PD (Buccino, 2014). Most of the studies in PD have focused on walking disturbances.
Efficacy of AOT in motor rehabilitation
A pioneering study by Buccino et al. (2011), in a randomized controlled trial, explored the efficacy of AOT as an added therapy to standard pharmacological and rehabilitative treatments. After treatment, the experimental group reported better scores in the Unified Parkinson Disease Rating Scale and the Functional Independence Measure, while there was no objective variance in gait performance. Jaywant, Ellis, et al. (2016) found similar results—no difference in gait performance was observed; however, the AOT group obtained a reported significantly improved mobility measured through a subscale of self-perceived mobility. Santamato et al. (2015) performed a study on the efficacy of AOT in balance and walking of PD patients. They obtained negative results, but a limitation of this study was the absence of a control group.
In contrast, a supplementary effect of AOT on regaining of walking ability in PD patients with freezing of gait (FoG) was observed by Pelosin et al. (2010). The patients, while performing a standard physical therapy treatment, were randomly attributed to an “action group” or to a control group. Patients in the action group watched video clips showing specific movements and strategies to avoid freezing events, while patients in control group watched video clips of static pictures showing different landscapes. Another study (Agosta et al., 2016) confirmed the positive effect of AOT on FoG, showing that this improvement was associated with brain functional changes. The protocol reproduced the one developed by Pelosin et al. (2010). After 4 weeks, both AOT and standard therapy groups showed lessening of FoG and enhanced walking speed and quality of life. Only in the AOT group motor disability was additionally reduced and balance ameliorated. After 8 weeks, only the AOT group still exhibited a positive effect on motor disability, walking speed, balance, and quality of life. Patients were then scanned while executing foot movements, a MI task related to FoG, and during AOT. The AOT group displayed amplified recruitment of frontoparietal mirror areas during fMRI tasks. In the same group, functional brain changes were mirrored by clinical progresses. It was concluded that AOT may enhance motor learning and facilitate the construction of a motor memory in PD to circumvent FoG through MN network activation.
Pelosin, Bove, Ruggeri, Avanzino, and Abbruzzese (2013) have also shown that watching video clips showing repetitive finger movements paced at 3 Hz increased the spontaneous rate of finger movements. AOT significantly affected movement rate in both the on and off conditions, but 45 min later the effect was still evident only in the on condition. The authors conclude that AOT could be a possible method for the rehabilitation of bradykinesia, even if the dopaminergic state participates in the effects of AOT. In another work focusing on arm bradykinesia, Bieńkiewicz, Rodger, Young, and Craig (2013) demonstrated that it was possible to improve motor performances in PD patients by a LED display simulating biological motion. Among rehabilitative approaches thought to be based on MN stimulation and aimed to reduce hand bradykinesia in PD (Bonassi et al., 2016) we must cite the use of “mirror box.” In this approach a motor training is performed with the non-affected limb, while the patient receives visual feedback from the affected limb. This procedure has been used above all for stroke patients (Ramachandran & Altschuler, 2009). Among its possible neural substrates, it has been proposed the involvement of the MN system or at least some areas strictly linked to it (such as the superior temporal gyrus; Deconinck et al., 2014).
A recent review about the efficacy of AOT and MI in PD (Caligiore, Mustile, Spalletta, & Baldassarre, 2017) concluded that AOT facilitates motor behavior at least in the earlier stages while there is less agreement about MI efficiency. Another recent review (Patel, 2017) has taken into account AOT studies in PD rehabilitation as a mean to modify postural way and gait. As in the case of Caligiore et al., the general conclusion of the author was that AOT could be effective if used in conjunction with traditional treatment in rehabilitation, by exploiting the MN network and its ability to understand the motor planning of others, which could favor anticipatory postural responses and reactive reflexes.
The mechanism through which AOT improves motor performances in PD needs, however, to be further elucidated. AOT does not directly stimulate basal ganglia but might activate MN prefrontal areas that rely on the basal ganglia through frontal-subcortical circuits. Such a kind of connections has been proposed by several authors (Alegre et al., 2011; Bonini, 2016) and claimed to explain results of several studies (see previous paragraphs). Recent anatomical data (Gerbella, Borra, Mangiaracina, Rozzi, & Luppino, 2015) have in fact revealed that most of the regions forming the cortical MN system for hand actions such as the inferior parietal areas, the ventral premotor cortex, and the ventrolateral prefrontal cortex (which, according to some authors -Borra, Gerbella, Rozzi, & Luppino, 2011; Gerbella, Borra, Tonelli, Rozzi, & Luppino, 2012; Nelissen et al., 2011 could also have MN like properties) send convergent projections to specific sectors of the putamen. In birds, imitation learning would develop, assuming a Hebbian model, using pathways between basal ganglia and MN areas (Giret, Kornfeld, Ganguli, & Hahnloser, 2014).
Music in PD rehabilitation
We now know that MN are multimodal cells, which can be stimulated not only by action observation and execution but also by the acoustic perception of action-related sounds (e.g., breaking a peanut, paper ripping) (Aziz-Zadeh, Iacobon, Zaidel, Wilson, & Mazziotta, 2004; Fadiga et al., 1995; Gazzola, Aziz-Zadeh, & Keysers, 2006; Kohler et al., 2002). Using fMRI, Gazzola and colleagues searched for brain areas that respond both during motor execution and when individuals listened to the sound of an action performed by the same effector. In the study, the authors tested the auditory and motor properties in the same subjects on two separate days. For auditory activity, the subjects listened to the sounds of five categories: mouth action sounds (for example kissing, gurgling), hand action sounds (e.g., opening of a hinge, crushing a soft- drink can), ambient sounds (e.g., train passing by), scrambled mouth actions, and scrambled hand actions. During the motor execution task, subjects had to perform actions similar to those used in auditory stimuli. The authors showed that in both cases a left-sided temporo-parieto-premotor hemispheric circuit is activated, providing evidence for a human auditory mirror system (Gazzola et al., 2006). Moreover, the cortical facilitation due to AO is at its maximum for both acoustic and visual stimuli linked to the action (Alaerts, Swinnen, & Wenderoth, 2009). This result agrees with the idea that daily life activities are typically sustained by multiple sensorial experiences and that action production is always multimodal. These MN properties suggest the fascinating possibility to combine the visual information with the action sound to maximize the positive effect of AOT in subjects with PD. Schiavio and Altenmüller (2015) have suggested the use of music in the rehabilitation of PD. It is well known that periodicity, such as the fact of matching walking to a musical beat or to a metronome, improve velocity, cadence, and stride length of parkinsonian gait (del Olmo & Cudeiro, 2005). Indeed, as Schiavio and Altenmüller underline, “timing and periodicity are fundamental aspects of human gait and because basal ganglia-cortical circuitry is typically involved in time-related processes,” musical stimulation could offer a way to obtain a better sensorimotor coupling (Schiavio & Altenmüller, 2015). Bacigalupe and Pujol (2014) propose that the external auditory and visual cues (often used in PD rehabilitation) represent the main stimulus for the paradoxical kinesia, the phenomenon according to which PD patients improve their motor performance thanks to external stimuli relevant for movement (e.g., bradykinetic PD patients begin, suddenly and for a while, to run). Provoking this phenomenon would be possible by exploiting the two main streams of the perceptual-action coupling (but above all the dorsal stream, more linked to action) and the MN system. Exploiting paradoxical kinesia could be useful in rehabilitation and it could be done by using motor affordances in recreational and artistic activities.
Efficacy of AOT on cognitive functions
Two recent studies have further examined the role of AOT in PD rehabilitation, showing significant results in terms of improvement not only from the motor but also from the cognitive point of view. In a study by Di Iorio et al. (2018) in cognitively conserved patients with PD, the improvement in cognitive and motor performance (including FoG) thanks to AOT was correlated with a reduction in P300 latency duration (finding which points to changes in cortical activity). Another study (Caligiore et al., 2019) showed that long-term AOT could also lead to cognitive improvement in PD patients if utilized within a dual task framework. In fact, participants significantly progressed in both short-term and long-term verbal memory tasks, in long-term visuospatial memory, and in some attentional/focusing aspects (Stroop Test) after the intervention. Interestingly, the improvement in both short and long-term memory tests persisted at follow-up, one month later.
3.2.6 Motor imagery
Motor imagery is a cognitive process in which subjects imagine performing a movement without actually doing it (Jeannerod, 2001; Jeannerod & Frak, 1999). MI would mirror the result of conscious access to the intention to move (Jeannerod & Decety, 1995). Since the last decade of the 20th century, many studies have shown that this process involves many areas also recruited during action execution (e.g., Abbruzzese, Trompetto, & Schieppati, 1996; Decety, 1996). Among these areas, there are regions thought to be part of the MN network in humans, such as the premotor cortex and the IPL. Differently than AOT, which is an implicit and automatic online process, MI can only be performed offline that is when one is disconnected from other potential interactions with other subjects or objects. It is also clear that there are obvious behavioral differences among AO, MI, and action execution; they are linked, for example to motivation, attentional processes, the recollection of a sensitive initial state to be able to recall kinesthetic sensation for MI—which is not present in AO—,and the absence of a risk linked to a real execution in AO and MI. Recently, a review has recapitulated data from neuroimaging experiments examining MI, AO, and related control tasks implicating movement execution (Hardwick, Caspers, Eickhoff, & Swinnen, 2018). These behavioral and neural differences should be taken into account when planning a rehabilitative training in different neurodegenerative diseases.
Several studies investigated MI in PD with different approaches (electrophysiological, neuroimaging, and neuropsychological ones)—results are once again controversial. A TMS research (with an increase of cortical excitability; Tremblay et al., 2007) and a neurophysiological study (Cunnington et al., 2001) recording movement-related potentials (usually associated with voluntary movements preparation and execution) reported an impaired facilitation by MI in PD. Conson et al. (2014) tested whether the most affected body side influenced PD patients' ability to mentally manipulate images. PD patients were specifically compromised in judging laterality of the hand corresponding to their own affected side when presented with back-facing human figures, in comparison with normal subjects. However, no difference was found for front-facing figures. Authors hypothesized that two kinds of whole-body transformation could exist, namely an “embodied” one, for back-facing figures, and a “perspective” one, activated for front-facing bodies. However, their interpretation is questionable and their results could be also interpreted in another way—a defective “embodied” mechanism could succeed in judging front-facing figures, but not back-facing ones, because this kind of judgment is less frequently requested (and therefore exerted) in everyday life. It must be also kept in mind that aging affects MI abilities, particularly for the non-dominant side of the body (Saimpont, Mourey, Manckoundia, Pfitzenmeyer, & Pozzo, 2010; Saimpont, Pozzo, & Papaxanthis, 2009); this could exert a confounding effect in studies focusing on MI, PD, and laterality.
Recent investigations testing MI through ad hoc assessment batteries did not found differences between PD patients and normal controls (Heremans et al., 2011; Maillet et al., 2015) both during the on and off phases (Peterson, Pickett, & Earhart, 2012, see Abbruzzese, Avanzino, Marchese, & Pelosin, 2015 for a complete review). A position emission tomography investigation showed that MI in PD was associated with a normal activation in the supplementary motor area and a significant activation of the ipsilateral inferior parietal cortex (both in the “off” and the “on” state) and the ipsilateral premotor cortex (when “off” only). Inferior parietal cortex hyperactivation could compensate the reduced activation of other areas in comparison with controls, including the right dorsolateral prefrontal area, which deals with the working memory component of the imagery task (Cunnington et al., 2001). Other studies (Helmich, Lange, Bloem, & Toni, 2007; Maillet et al., 2015) hypothesized that neural compensation mechanisms can help patients to maintain a good performance in MI tasks.
3.3 Mirror neurons and amyotrophic lateral sclerosis-frontotemporal degeneration continuum
3.3.1 MRI studies
Most of the research into MN and ALS (a form of motoneuron disease [MotND] in which both central and spinal motor neurons degenerate) or FTD (a common presenile dementia which can display a spectrum of clinical syndromes, ranging from behavioral impairment to language or motor deficit, tightly linked to ALS both from the clinical and genetic point of view) were conducted using magnetic resonance imaging (MRI). For a summary of the literature searching about MN and FTD/ALS, see Supporting Information Appendix C.
Li et al. (2015) conducted a study on 30 patients with ALS and 30 matched healthy controls. The participants performed fMRI while observing a video of repetitive flexion-extension of the fingers at three frequencies or difficulties. AO activated brain areas belonging to the MN system in both ALS and healthy subjects. In ALS patients, however, the dorsal lateral premotor cortex, inferior parietal gyrus, and supplementary motor area were more activated in comparison with controls. Augmented activation within the primary motor cortex, the dorsal lateral premotor cortex, the inferior frontal gyrus, and superior parietal gyrus was linked with hand movement frequency/complexity in patients. This finding indicates a constant compensatory process happening within the motor-processing network of ALS patients, in order to compensate the loss of function. However, a limitation of this study is the fact that it was restricted to action observation.
Another study suggesting a compensatory response of the MN system during AO processing in ALS was performed by Jelsone-Swain, Persad, Burkard, and Welsh (2015). Using fMRI, while subjects observed an actor's hand rhythmically squeezing a ball or squeezed themselves a ball, the authors recorded greater activity in ALS patients than in controls in MN regions, particularly the right frontal inferior operculum and the right frontal and left parietal lobes. In the second part of this same study, Jelsone-Swain et al. (2015) investigated ALS patients’ ability to recognize actions of other people. Participants watched a short video of an actor pantomiming an action with his hands; they had either to passively observe it or to “actively recognize” the action by choosing the correct action from two phrases displayed on the screen. The contrast analysis of the cerebral activity during active recognition versus passive observation displayed greater activity in various anterior and posterior regions in the normal group; on the contrary, only in the ALS group the right occipital activity increased. Patients were then separated into two groups according to their performance at the recognition task—only the best performers showed activation in bilateral frontal superior gyrus. Their performance was also proportional to performance at the RME test. Thus, social cognition would be affected in some ALS patients, an impairment which may be related to a MN system dysfunction (Jelsone-Swain et al., 2015).
Jastorff et al. (2016) investigated brain areas which process emotional stimuli in patients diagnosed with bvFTD. They used both behavioral testing and structural and resting state imaging. bvFTD patients performed worse than controls in emotion detection and in emotion categorization tasks. Their performance in emotion categorization inversely correlated with atrophy of the left inferior frontal gyrus (IFG), a MN area. Functional connectivity analysis also found lessened connectivity of this area. An explanatory hypothesis could be that, due to the atrophy of the IFG, the absence of motor mirroring could prevent the recognition of emotions. Similarly, Brioschi-Guevara et al. (2015) discovered that the atrophy of prefrontal and premotor regions at structural MRI inversely correlated with the ability of bvFTD patients to infer intention and emotional beliefs of others. Marshall et al. (2016) found that grey matter correlates between impaired emotion recognition and automatic imitation depended on the different clinical form of FTD. The brain regions involved delineated a distributed neural network previously associated with embodied cognition. We can conclude that data from bvFTD patients highly support the role of the MN system in emotion recognition.
Finally, another study in ALS deserves to be reported. Verstraete, Veldink, van den Berg, and van den Heuvel (2013) investigated the longitudinal effects of the disease on brain networks using diffusion tensor imaging. Their analyses revealed an expanding sub-network of impaired brain connections over time, with a central role of the primary motor cortex and loss of structural connectivity mainly propagating to frontal and parietal brain areas (regions also belonging to the MN network). In fact, some authors suggest a possible role of the MN network alteration in the pathogenesis of ALS and FTD. According to Eisen, Turner, and Lemon (2013), the ALS/FTD complex might be associated with a disruption of MN. In particular, the damage would involve MN projecting to the pyramidal tract, along with their sophisticated braking mechanism, which allows a subject to observe an actor and use his own motor repertoire to recognize and categorize the observed movement, without activating his own movements (Kraskov, Dancause, Quallo, Shepherd, & Lemon, 2009). In ALS/FTD the MN damage would lead to an impairment of different evolutionarily interlinked functions, associated with the different clinical forms of the disease, in particular: (a) hand function specialization and the associated development of bipedalism (associated with the “classical” form beginning from the upper limbs or to the pseudo-polineuritic form, beginning from the lower limbs), (b) sound production, swallowing, and breathing (which the authors designate as “the brainstem functional complex”; this impairment would be associated with the “bulbar” ALS variant), and (c) cognitive functions linked to social behavior and communication ability, through gestures and language (associated with FTD). Previously, Bak and Chandran (2012) had already proposed that dementia associated with MotND could be interpreted as the fifth major clinical form of the disease, together with bulbar, thoracic and upper and lower limb presentation. Accordingly, the authors underline that in ALS the most impaired cognitive functions are those with the tightest functional relations to the motor system, such as verb and action processing (see also Bak, 2013).
3.3.2 Neuropsychological studies
A series of neuropsychological researches showed an impairment of action rather than object naming in ALS (Bak & Hodges, 1997; Bak, O’Donovan, Xuereb, Boniface, & Hodges, 2001; Grossman et al., 2008; York et al., 2014) and FTD (Cappa et al., 1998; Hillis, Oh, & Ken, 2004). Combining neuropsychological assessment with morphological MRI, York et al. (2014) showed that performance in action verb judgment was related to gray matter atrophy in bilateral frontal regions, including motor association cortex and pre-frontal regions. Similarly, Grossman et al. (2008) had shown that cortical atrophy in premotor areas associated with the representation of the face, arm, and leg correlated with performance on measures requiring action knowledge. This supports the hypothesis that the difficulty shown by ALS patients in naming actions is partly related to the degradation of action-related conceptual knowledge represented in the motor-associated cortex.
Fiori et al. (2013) investigated controls and ALS patients’ responses during a task testing the effects of biomechanical constraints on MI. Effects present in normal subjects (slower responses and lower accuracy when participants judged the laterality of a hand displayed in a position difficult to reach with a real movement awkward positions) were compromised in ALS, at least for the proximal muscles.
Vannuscorps, Dricot, and Pillon (2016), longitudinally examined a patient with basal cortical degeneration, a clinical picture related to FTD, and found contra-indicative data compared to previously cited studies. Throughout 4 years, the patient exhibited worsening action production disorders with associated growing bilateral atrophy in cortical and subcortical regions linked to sensorimotor control (tsuperior parietal cortex, primary motor and premotor cortices, inferior frontal gyrus, mostly on the right side, and basal ganglia). Differently, the patient's performance in processing action-related concepts (e.g., action naming and action comprehension) was spared during the same period. These data would defy the idea that action concept processing is based on the same cognitive and neural networks underlying the sensorimotor control of actions (Meteyard, Cuadrado, Bahrami, & Vigliocco, 2012). However, the moderate involvement of MN areas on the left side could have allowed the maintenance of behavioral performances.
3.4 Mirror neurons and mild cognitive impairment/Alzheimer's disease continuum
Over the years, many studies have investigated the neurophysiological and neuroimaging correlates of AD, the most frequent cause of dementia, and MCI—a category characterized by cognitive decline greater than expected for an individual's age and education level without loss of independence in everyday life, at high risk of developing dementia (Busse, Bischkopf, Riedel-Heller, & Angermeyer, 2003; Petersen et al., 1999, 2001). Among these works, only a few studies have focused on the link between MCI/ AD and MN. For a summary of the literature investigating MN and AD/MCI, see Supporting Information Appendix D.
3.4.1 EEG spectral analysis
Recently, 74 adult subjects with MCI undertook EEG recording and high-resolution MRI by Moretti (2016). Alpha3/alpha2 frequency power ratio and cortical thickness were computed for each subject. This ratio represents the increase in high alpha frequencies relatively to low alpha power; it has been demonstrated as a reliable EEG marker of hippocampal atrophy and its increase has been found in MCI subjects who will convert in AD (Moretti et al., 2011). High EEG alpha3/alpha2 frequency power ratio was correlated with atrophy of cortical regions pertaining to the posterior MN network (IPL) areas in MCI subjects. Thus, a possible pathological uncoupling of the MN system would justify the cognitive deficits in prodromal AD. The location of the IPL at the junction of the parietal, temporal, and occipital lobes makes it ideally situated to perform cross-modal integration (hearing/vision/proprioceptive); its impairment would induce both praxis, language, calculation, and topographical deficits characterizing AD, a hypothesis which agrees with traditional neurological views (Greene, Killiany, & Alzheimer's Disease Neuroimaging Initiative, 2010).
3.4.2 TMS studies
In a study by Cotelli et al. (2006), TMS of both left and right dorsolateral prefrontal areas—with a frequency of 20 Hz leading to an increase of cortical excitability—ameliorated action naming but not object naming in 15 AD patients. Similar results were obtained for a mild AD group (Cotelli, Manenti, Cappa, Zanetti, & Miniussi, 2008), while an improved naming accuracy for both action and objects was found in the moderate to severe group. These results raise the interesting possibility of improving language performance in AD via magnetic stimulations of the motor system, but are difficult to interpret. The role of the dorsolateral prefrontal cortex in action naming must be further elucidated. A possibility might be the fact that this cerebral area can intervene by selecting and combining motor representations in the MN system (Vogt et al., 2007); therefore, stimulation of dorsolateral prefrontal cortex might facilitate MN activation and consequently naming of action (if we accept the embodied nature of language). In contrast, according to a recent study (Lanzilotto, Gerbella, Perciavalle, & Lucchetti, 2017) in the dorsolateral prefrontal cortex there are also neurons with mirror-like properties, which react to both self and other people head rotation.
3.4.3 fMRI studies
Rattanachayoto, Tritanon, Laothamatas, and Sungkarat (2012) examined the MN system abnormalities in MCI and AD through a fMRI study. Ninety-two subjects (five MCI, seven mild AD, and 80 cognitively normal) were studied. Participants had to observe a video showing the movement of a hand (tearing a piece of paper) or a control condition (observing a fixation point). Observing the hand movement elicited significant activations of the inferior bilateral frontal lobule and the IPL, but brain activations of controls were significantly higher than those in the MCI and mild AD groups (with no significant difference between these last two groups). Similar to Moretti's study (2016), this study suggests that a dysfunction of the MN system might play a role in cognitive impairment due to MCI/AD, even if we must be cautious in this conclusion because the number of MCI and AD subjects examined was low.
Recently, another study investigated the MN network functioning in people with MCI and AD, alongside with its relationship to cognitive performance (Farina et al., 2017). In this study, three matched groups of 16 subjects each (normal elderly, amnesic MCI with hippocampal atrophy, and AD) were assessed with a fMRI task specifically created to test MN and a focused neuropsychological battery. The MN network appeared largely conserved in aging, while it seemed involved according to an anterior–posterior gradient in neurodegenerative cognitive deterioration. In AD the MN network appeared clearly deficient. The authors speculate that the conservation of the anterior part of the MN network, found in MCI, could possibly compensate the initial decay of the posterior part, preserving cognitive performance, particularly in task (such as RME) related to abilities thought to be linked to MN (see Figure 2 for a graphical representation of the results of study). It is interesting to note that an fMRI study focusing on mindreading (or theory of mind; Baglio et al., 2012) similarly found a preserved performance at RME in people with MCI, despite a reduced activation of some posterior regions of the mind reading network. In the study of Farina et al. (2017) an increased activation of the left Broca area (B44), thought to be a node of the MN system, was observed. This result suggests that the preservation and maybe hyperactivation of MN prefrontal areas could play a compensatory role.
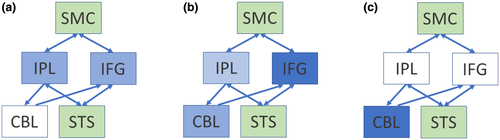
Another study, conducted by Peelle, Powers, Cook, Smith, and Grossman (2014), tested a semantic judgment task, involving shape and color of natural kinds or manufactured items. AD patients were impaired in this task, exhibiting significantly reduced activation in the left temporal-occipital cortex than healthy elderly people and diminished activity in the left inferior frontal cortex. According to the authors, these results agree with a sensory-motor approach of semantic memory; knowledge associated with object concepts would be stored in brain regions that are responsible for perceptual and motor functions of a certain material (Barsalou, Simmons, Barbey, & Wilson, 2003).
Finally, an fMRI study by Lee, Sun, Leung, Chu, and Keysers (2013) aimed at evaluating neural activities during the processing of facial expressions in AD patients. The patients showed weaker activations in left cerebral regions associated with MN or empathic simulation (ventral premotor cortex, anterior insula, and frontal operculum) compared to matched controls. Notably, levels of brain activation in those regions forecasted the level of affect in the AD group. Thus, neural changes in areas associated with motor and emotional simulation (comprised MN regions) could be relevant in the progress of AD symptoms and explain affective impairment associated with this disease.
3.4.4 Behavioral studies
In a first study Bisio et al. (2012) tested if the implicit imitation is preserved in mild to moderate AD patients. AD patients and healthy elderly controls had to observe a dot moving on a screen and to point to its final position when it stopped. The dot speed similarly influenced the actions of AD patients and of healthy elderly participants, suggesting that perception-action matching is not precluded by the disease. In contrast, only patients had an anticipatory motor response, that is they began to move before the end of the stimulus motion, in contrast to the request by the examiner. The imitation of the stimulus speed suggests a conserved ability to match the internal motor representations with that of the visual model; however, the uncontrolled motion initiation would indicate AD patients’ inability to voluntarily inhibit response production. The authors hypothesize that the first part of their results would point to the relative preservation of the MN network (responsible of perception-action matching). However, MN parietal areas such as IPL would be more affected than frontal ones, leading to impaired movement inhibition.
In the second experiment (Bisio et al., 2016), the authors tested voluntary imitation in AD and how this ability was influenced by the nature of the observed stimulus. To this end they compared the capability to reproduce the kinematic features of a human model with that of a dot moving on a screen. The dot's motion (at three different velocities) reproduced the characteristics of a human vertical movement. Participants had to observe the movement of the dot until it reached its final position. When the dot stopped, the subjects were asked to replicate its movement. The human model was a young person previously trained to make vertical simple arm movements at three different velocities. Results showed that, when asked to imitate the velocity of the stimulus, AD patients presented an intact capacity to reproduce it. This capacity improved when the stimulus was a human agent. These data suggest that high-level cognitive processes linked to voluntary imitation could be conserved in mild-moderate stages AD and that voluntary imitation capacities could profit from the implicit interpersonal communication existing between the patient and the human model. These findings make available new clinical views and innovative methods in training programs for people with early dementia. The conservation of the motor resonance mechanisms, not reliant on conscious awareness, finds an intact basis upon which clinicians could model both physical and cognitive interventions for healthy elderly, MCI, or early AD patients. Furthermore, by stimulating the voluntary imitation of everyday activities, the caregivers might help the patients to maintain intact, as long as possible, the ability to easily move in their familiar environment and to feel that they are still part of their family community.
3.4.5 Neuropsychological studies
Some studies pointed out a more severe loss in action than in object naming in AD patients (Druks et al., 2006; Kim & Thompson, 2004; Robinson, Grossman, White-Devine, & D'Esposito, 1996), even if at a minor extent than in FTD (Cappa et al., 1998). In contrast, other researchers reported that nouns were more impaired than verbs in AD patients (Williamson, Adair, Raymer, & Heilman, 1998) or did not report any difference between nouns and verbs (Masterson et al., 2007; Rodríguez-Ferreiro, Davies, González-Nosti, Barbón, & Cuetos, 2009).
Other studies have shown deficits among individuals with AD in recognizing facial emotions (Bediou et al., 2009; Guaita et al., 2009; McLellan, Johnston, Dalrymple-Alford, & Porter, 2008), mainly in the recognition of happy, sad, and fearful expressions (Kohler et al., 2005). These data, alongside with the fMRI study by Lee et al. (2013), appear coherent with a putative impairment of the MN network in AD, since this network (and particularly the inferior frontal gyrus and the ventral premotor cortex) is involved in recognizing other people emotions. However, we must recognize that data about the relationship between emotion recognition and the MN system in AD are scarce in comparison with PD and FTD.
De Scalzi, Rusted, and Oakhill (2015) found a conservation of the action compatibility effect in people with AD; when patients are requested to make judgments on sentences that designate a transfer of an object toward or away from their body, they are faster to answer when the response needs a movement in the same direction as the transfer defined in the sentence. This raises the possibility to exploit the motor systems to improve language comprehension of AD (e.g., by requiring performing the action while listening to a sentence).
3.4.6 Rehabilitative studies
AOT, which is a mean to stimulate the MN network, has been proposed as a rehabilitative motor tool (see Bassolino et al., 2015 for a review). However, AOT could also play a role in maintaining cognitive functions, if one accepts the indissociable nature of cognition from the motor system. So far, the only published research about AOT with AD is from Eggermont, Swaab, Hol, and Scherder (2009). Nursing home residents with dementia observed either videos showing hand movements (19 subjects) or a documentary (25 subjects) for 30 min, 5 days a week, for 6 weeks. At the end of treatment, patients showed improvements in attention and facial recognition (which depends on the superior temporal sulcus, a region tightly linked to the MN system). A preliminary research in progress, performed on small groups (nine each) of patients diagnosed with mild-to-moderate AD, compared AOT with multidimensional stimulation (Spada, 2012). The data analysis showed a significant improvement between test and retest in a naming action task. However, recently an intervention focused on MN in AD patients showed negative results (Caffarra et al., 2016, oral communication at XI Sindem National Congress, Florence, Italy). If the MN network is already disturbed in the pre-dementia phase, as suggested by Moretti’s (2016) and Farina et al.'s (2017) studies, AOT would result ineffective as a rehabilitation technique in moderate AD, and it should be rather performed in the MCI or at least in the early phase to achieve positive results.
4 CONCLUSION AND PERSPECTIVES: FUTURE DIRECTIONS FOR CLINICAL APPLICATION RELYING ON THE RELATIONSHIP BETWEEN THE MN SYSTEM AND NEURODEGENERATIVE DISEASES
The traditional model, according to which almost all studies on neurodegenerative diseases have been conducted, considers cognitive abilities as high-level supervisor functions, which utilize perception and action as separated low-level systems. Reinterpreting neurodegenerative diseases at the light of the new views about brain organization stemming from the discovery of MN and the embodied cognition hypothesis could help a better comprehension of clinical manifestations and open new pathways to rehabilitation. According to Vallet (2015), exploring embodiment (supported by the MN system) in normal and abnormal aging would be of interest from a theoretical point of view, as becoming older is accompanied by changes in all cerebral, sensory, and bodily functions. It could also be useful in clinical practice, because it would possibly allow to develop interventions to help patients.
Data about the status and role of the MN network in neurodegenerative diseases are poor with the partial exception of PD. A hypothetic conclusion of existing studies in PD could be that the MN network is in some way altered in this disease (the rationale in this case could be the degeneration of cortical-subcortical loops linking the basal ganglia to the frontal cortex); an hyperactivation of this system might support motor and cognitive performances, at least in early stages, while in later stages the more severe impairment of the MN network could prevent compensation at least in a part of patients (see Figure 3). The dopaminergic state and DBS could have a role in compensating the functional deficits of circuits linked to motor cognition, too. The compensation mechanism could explain why studies on AOT as a rehabilitative method for people with PD report positive results. Interestingly, a very recent study on mild-to-moderate PD patients showed that they can be highly influenced by motor contagion induced by AOT also in a negative sense (Pelosin et al., 2018). Most studies in the field of PD rehabilitation have recruited very small samples. In contrast, it must not be forgotten that, in rehabilitative studies, studying large samples of people does not allow to individualize the treatment according to the clinical picture that, as we have seen, can be very various along the course of the disease in each patient. Therefore, in the field of rehabilitation, the ideal situation would be often to characterize each patient to follow him/her as a single case experiment.
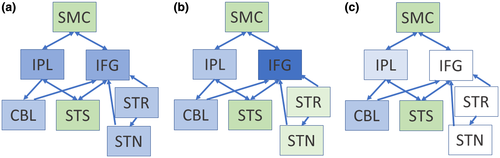
In the ALS/FTD continuum, preliminary evidence points out an involvement of the MN network, a possibility which is not unexpected, as MN are primarily motor neurons. The MN system decay could explain language and inter-subjectivity deficits shown in these patients. In the MCI/AD spectrum, data are very scarce. Most recent studies suggest a possible progressive involvement from posterior to anterior areas of the MN network, with the brain putting in place compensatory mechanisms for this decay (Farina et al., 2017; Moretti, 2016; Moretti et al., 2011). However, more researches are needed to confirm this hypothesis, also because previous behavioral researches partly pointed in the opposite direction. In general, it could be very stimulating to study MNs with neurophysiological techniques (maybe easier to perform with cognitively compromised people) or fMRI and correlating data with neuropsychological tests exploring functions traditionally linked to MN, behavioral measures, and motor scales.
AOT, which stimulates the MN network, might be a powerful tool to improve motor, language, and/or social cognition deficits in people with PD or FTD. Based on the supposed role of the motor system in memory building-up, it may also be hypothesized that MN stimulation through AOT could maintain and speed up the execution of complex motor sequences needed to perform activities of daily living. It could also facilitate the formation of memory traces of actions executed during a normal day in people with AD or at least with MCI. The possibility to exploit the motor resonance to stimulate cognitive function in the frame of rehabilitation of neurodegenerative pathologies appears fascinating because it could represent a therapeutic solution that does not exist yet. Specific drugs have still not been approved for the MCI phase and pharmacotherapy effects are extremely limited in AD (Petersen et al., 2017; Raina et al., 2008); cognitive stimulation and serious game trainings have positive but limited effects (Hill et al., 2017; Manera et al., 2017; Woods, Aguirre, Spector, & Orrell, 2012) probably because of training performed in artificial environment and developing specific skills not transferable to daily normal life activities. No substantial pharmacological therapy is available for ALS/FTD. Even in PD, where various drugs are available, they have only symptomatic effects and do not avoid progression of the motor and cognitive impairment. Other advantages of a rehabilitative treatment based on motor cognition are the fact that it promotes an active involvement of the patient in her/his recovery process instead of rendering him/her a passive medicine consumer, and the large possibility to easily personalize the training.
Finally, protocols based on motor cognition, which is easy and cheap to implement and less risky than pharmacotherapy, might have a strong impact on prevention of neurodegenerative diseases in the growing elderly population, a very interesting perspective from the public health point of view. As usual in neurodegeneration, a preventive intervention could in fact be far more effective than an intervention applied in the overt phase of the disease. This view is supported both by data coming from PD studies (Anders et al., 2012) and by recent data on the MCI/AD spectrum (Farina et. al., 2017; Moretti, 2016; Vallet et al., 2017).
ACKNOWLEDGMENTS
The authors are very grateful to professor Leonardo Fogazzi for his precious suggestions and revisions. They are also grateful to Angelo G. Gaillet and Shaun Stratford for English revision.
CONFLICT OF INTEREST
The authors have no competing interest to declare.
AUTHOR CONTRIBUTIONS
Conceptualization, E.F. and T.P.; Data Curation, E.F. and F.B.; Formal Analysis, E.F.; Funding Acquisition, E.F.; Investigation, E.F.; Methodology, E.F.; Writing – Original Draft, E.F. and T.P.; Writing – Review & Editing, E.F., F.B., and T.P.; Project Administration, T.P.; Supervision, T.P.; Validation, T.P.