Roles of GluN2C in cerebral ischemia: GluN2C expressed in different cell types plays different role in ischemic damage
Abstract
Over the past decade, many studies have focused on clarifying the roles of different N-methyl-d-aspartate (NMDA) receptor subunits in cerebral ischemia, hoping to develop subunit-selective drugs. Recently, more attention was given to studying the role of GluN2C in ischemia damage, which may lead to the development of new NMDA receptor antagonists for cerebral ischemia. Results showed that GluN2C inhibition or knockout can effectively alleviate the ischemic injury caused by middle cerebral artery occlusion and, contrarily, can aggravate the damage to hippocampal CA1 circuit caused by transient global cerebral ischemia. These results indicate the complicated roles of GluN2C in cerebral ischemia. In this minireview, we focus on these findings, describe the roles of GluN2C from different cell origins in ischemic damage, and explain the above inconsistent experimental results.
Significance
Besides cerebellum, thalamus, and olfactory bulb, GluN2C is widely expressed in other brain regions, though in low abundance. Moreover, cerebral ischemia can induce the up-regulation of GluN2C in ischemic regions. Therefore, GluN2C may play a greater role in ischemic damage than we thought. Several recent studies observed opposite effect of GluN2C in cerebral ischemia. In this minireview, we focus on these findings and, further, explain the underlying mechanisms. The opinion described in this article may help to determine the roles of GluN2C under different conditions and also contribute to delineate the roles of different kinds of N-methyl-d-aspartate receptors in cerebral ischemia.
1 INTRODUCTION
N-methyl-d-aspartate (NMDA) receptor is an important therapeutic target for cerebral ischemia. Although a variety of NMDA receptor antagonists have been developed, due to poor outcomes or serious adverse effects, all related drugs failed in clinical trials (Lai, Zhang, & Wang, 2014). So far, many studies have focused on studying the roles of different NMDA receptor subunits in cerebral ischemia, hoping to develop subunit-selective drugs. NMDA receptor is a type of glutamate-gated ion channel. It consists of seven subunits such as GluN1, GluN2 (A-D), and GluN3 (A-B). A typical NMDA receptor constitutes two GluN1 and two GluN2 subunits. Recently, several studies observed opposite results on the roles of GluN2C in cerebral ischemia (Chen & Roche, 2009; Chung et al., 2016; Doyle et al., 2018; Holmes, Zhou, Donahue, Balsara, & Castellino, 2018; Kadotani, Namura, Katsuura, Terashima, & Kikuchi, 1998). The purpose of this minireview is to explain the above contradictory results.
2 FUNCTIONAL PROPERTIES OF GluN2C
Although NMDA receptors have the characteristics of voltage-dependent Mg2+ block (Traynelis et al., 2010; Wyllie, Livesey, & Hardingham, 2013), this voltage-dependent blocking phenomenon mediated by extracellular Mg2+ was stronger in HEK 293 cells expressing GluN2A as compared with cells expressing GluN2C subunits (Burnashev, Zhou, Neher, & Sakmann, 1995). The IC50 values of Mg2+ at −60 mV for blocking GluN2A- and GluN2C-containing NMDA receptors (GluN2A and GluN2C receptors) were 26 and 200 μM, respectively (Bhattacharya et al., 2018). Therefore, one important property of GluN2C receptors is the lower sensitivity to Mg2+ block compared to GluN2A and GluN2B receptors (Burnashev et al., 1995; Kuner & Schoepfer, 1996; Monyer, Burnashev, Laurie, Sakmann, & Seeburg, 1994; Monyer et al., 1992). This property allows GluN2C receptors to be activated by ambient glutamate at resting membrane potential and contributes to higher NMDAR activity at resting potential in some brain regions, such as in layer 4 of barrel cortex (Binshtok, Fleidervish, Sprengel, & Gutnick, 2006) and reticular thalamic nuclei (Fernandez et al., 2017; Zhang, Llinas, & Lisman, 2009).
GluN2C receptors are also low-conductance ion channels. An early study in Xenopus oocytes showed that, contrary to the higher conductance of GluN2A and GluN2B receptors (50pS/40pS), GluN2C receptors have the characteristics of smaller conductance level (36pS/19pS) (Stern, Behe, Schoepfer, & Colquhoun, 1992). Subsequent in vivo results indicated that the appearance of NMDA receptors with low unitary conductance in cerebellum during development is dependent on the developmental expression of GluN2C (Farrant, Feldmeyer, Takahashi, & Cull-Candy, 1994). Moreover, no low-conductance channels were observed in GluN2C knockout mice (Ebralidze, Rossi, Tonegawa, & Slater, 1996).
The third feature of GluN2C receptors is the lower Ca2+ permeability compared to other NMDA receptors (Wyllie et al., 2013). Via measuring fractional Ca2+ currents (the proportion of whole-cell current carried by Ca2+, representing the contribution of Ca2+ to the total current mediated by NMDA receptors), Burnashev et al. found that the fractional Ca2+ currents measured in GluN2A-expressing cells were 30% larger than those of cells expressing GluN2C subunits (Burnashev et al., 1995).
Finally, GluN2C receptors have an unusually very low open probability, which is approximately 44-fold and 10-fold less than the peak open probabilities determined for GluN2A and GluN2B receptors, respectively, in steady-state recording from outside-out patches (Dravid, Prakash, & Traynelis, 2008).
3 EXPRESSION REGIONS AND PATHOPHYSIOLOGICAL FUNCTIONS OF GluN2C
A large number of studies have reported the expression and localization of GluN2C, mainly in the cerebellum, thalamus, and olfactory bulb (Buller et al., 1994; Wenzel et al., 1995) (Table 1). The highest expression region for GluN2C is cerebellum. By using oligonucleotide probe and GluN2C-specific antibody, researchers observed the highest expression of GluN2C mRNA and protein in adult cerebellum (Laurie, Bartke, Schoepfer, Naujoks, & Seeburg, 1997; Wenzel, Fritschy, Mohler, & Benke, 1997). Quantitative analysis results showed that the expression level of endogenous GluN2C mRNA in cerebellum accounted for up to 43.8% of the total expression in the brain (Goebel & Poosch, 1999). Functional studies showed that C-terminal truncation of GluN2C or combined gene disruption of GluN2A and GluN2C could induce deficits in motor coordination (Kadotani et al., 1996; Sprengel et al., 1998). Therefore, cerebellar GluN2C may play important role in the motor coordination. Homocysteine is a full agonist of GluN2C and GluN2D receptors in the cerebellar neurons, and a large population of cerebellar NMDA receptors are highly sensitive to homocysteine, which suggest potential vulnerability of this brain region to pathological hyperhomocysteinemia (Sibarov, Giniatullin, & Antonov, 2018). Another higher expression region for GluN2C is thalamus (Laurie et al., 1997; Wenzel et al., 1997). GluN2C is enriched in parvalbumin-positive interneurons in the reticular nucleus of thalamus (Ravikrishnan et al., 2018). Early research indicated that the mRNA level of GluN2C in the thalamus of patients with schizophrenia is lower than that of comparison subjects (Ibrahim et al., 2000). NMDA receptor antagonist- or GluN2C knockout-induced hypofunction of GluN2C receptors in the reticular nucleus of thalamus may induce delta frequency bursting in the brain which is a well-established phenomenon in schizophrenia (Zhang, Buonanno, Vertes, Hoover, & Lisman, 2012; Zhang et al., 2009). On the contrary, up-regulation of GluN2C function via systemic administration of CIQ, a positive allosteric modulator of GluN2C/D-containing NMDA receptors, attenuated schizophrenia-like phenotypes elicited by MK-801 and methamphetamine (Suryavanshi, Ugale, Yilmazer-Hanke, Stairs, & Dravid, 2014). Consistently, administration of d-cycloserine, a super agonist of GluN2C receptors, produced persistent benefits for negative symptoms and memory deficits (Goff, 2017). In addition, recent study found that at a psychotogenic concentration in humans, ketamine blocks a substantial fraction of GluN2C receptors but has less effect on other GluN2-containing NMDA receptors (Khlestova, Johnson, Krystal, & Lisman, 2016). The above evidence shows that GluN2C may serve as important therapeutic targets for schizophrenia. Olfactory bulb is also a higher expression region for GluN2C (Laurie et al., 1997; Wenzel et al., 1997). The expression cell types may be neuron and non-neuron cells (Alsaad et al., 2019; Karavanova, Vasudevan, Cheng, & Buonanno, 2007). The pathophysiological function of GluN2C in olfactory bulb is not clear.
| Brain regions | Cells expressing GluN2C | Functions | Relevant diseases | References |
|---|---|---|---|---|
| Cerebellum | Cerebellar granule cells | Motor coordination | Hyperhomocysteinemia | Laurie et al. (1997), Wenzel et al. (1997), Kadotani et al. (1996), Sprengel et al. (1998), Sibarov et al. (2018) |
| Thalamus | Neurons (++) and non-neuron cells (+) | ND | Schizophrenia | Laurie et al. (1997), Wenzel et al. (1997), Ibrahim et al. (2000), Zhang et al. (2009, 2012), Ravikrishnan et al. (2018), Goff (2017), Suryavanshi et al. (2014) |
| Olfactory bulb | Neuron and non-neuron cells | ND | ND | Laurie et al. (1997), Wenzel et al. (1997), Karavanova et al. (2007), Alsaad et al. (2019) |
| Cerebral cortex | Neurons in retrosplenial cortex (+) and non-neuron cells (++) | ND | Epilepsy, ischemia | Goebel and Poosch (1999), Sun et al. (2000), Karavanova et al. (2007), Ravikrishnan et al. (2018), Alsaad et al. (2019), Lozovaya et al. (2014), Kadotani et al. (1998), Holmes et al. (2018) |
| Hippocampus | Neurons (+), astrocytes (++) and non-neuron/non-astrocyte cells (+) | ND | Ischemia | Karavanova et al. (2007), Ravikrishnan et al. (2018), Alsaad et al. (2019), Berg et al. (2013), Chung et al. (2016) |
| Suprachiasmatic nuclei | Neurons | Circadian | ND | O'Hara et al. (1995), Clark and Kofuji (2010), Brancaccio et al. (2017) |
| Locus coeruleus | ND | ND | Depression | Karolewicz et al. (2005) |
| Amygdala | Non-neuron cells | Fear memory | ND | Palygin et al. (2011); Ravikrishnan et al. (2018); Alsaad et al. (2019), Hillman et al. (2011), Ogden et al. (2014) |
| White matter | Oligodendrocytes | ND | Ischemia | Karadottir et al. (2005), Salter and Fern (2005), Burzomato et al. (2010), Doyle et al. (2018) |
| Hypothalamus | ND | ND | ND | Goebel and Poosch (1999) |
| Striatum | Neurons (+) and non-neuron/non-astrocyte cells (++) | ND | ND | Goebel and Poosch (1999), Alsaad et al. (2019) |
| Globus pallidus | Neurons (++) and non-neuron/non-astrocyte cells (+) | ND | ND | Alsaad et al. (2019) |
- Abbreviations: ND, not determined; ++, relatively higher expression level; +, relatively lower expression level.
GluN2C also expresses in other brain regions such as the cortex, hippocampus, hypothalamus, and striatum, though in low abundance (Goebel & Poosch, 1999). The results of both quantitative analysis for mRNA and two knock-in mouse lines (GluN2C subunit-β-galactosidase knock-in mice and GluN2C subunit-EGFP/Cre knock-in mice) showed the expression of GluN2C in cerebral cortex (Goebel & Poosch, 1999; Karavanova et al., 2007; Ravikrishnan et al., 2018; Sun, Shipley, & Lidow, 2000). The main location of GluN2C in cortex is in the cell bodies of astrocytes (Alsaad et al., 2019; Ravikrishnan et al., 2018) and may be also in some neurons in the retrosplenial cortex (Karavanova et al., 2007). In the hippocampus, GluN2C shows higher expression in CA1 and subiculum, as compared to the dentate (Karavanova et al., 2007). Although mainly expressing in non-neuron cells (Alsaad et al., 2019; Karavanova et al., 2007; Ravikrishnan et al., 2018), especially the astrocytes, GluN2C is also expressed in the mossy fiber of granular cells in dentate gyrus and pyramidal neurons in CA3 region (Berg, Larsson, Morland, & Gundersen, 2013). In the suprachiasmatic nuclei (SCN), both mRNA analysis and whole-cell recordings indicate that GluN2C is expressed in neurons (Clark & Kofuji, 2010; O'Hara, Andretic, Heller, Carter, & Kilduff, 1995). In the amygdala, GluN2C showed more expression in non-neuronal cells (Alsaad et al., 2019; Palygin, Lalo, & Pankratov, 2011; Ravikrishnan et al., 2018). Additionally, GluN2C was expressed in the oligodendrocyte processes in the white matter and may be one of the most abundant NMDA receptors subunits (Burzomato, Frugier, Perez-Otano, Kittler, & Attwell, 2010; Karadottir, Cavelier, Bergersen, & Attwell, 2005; Salter & Fern, 2005). The GluN2C subunits in the above brain regions may be involved in the processes of circadian (Brancaccio, Patton, Chesham, Maywood, & Hastings, 2017), tuberous sclerosis complex- or focal cortical dysplasia-induced epilepsy (Lozovaya et al., 2014), depression (Karolewicz, Stockmeier, & Ordway, 2005), fear acquisition (Hillman, Gupta, Stairs, Buonanno, & Dravid, 2011; Ogden, Khatri, Traynelis, & Heldt, 2014), and cerebral ischemia (Chung et al., 2016; Doyle et al., 2018; Holmes et al., 2018; Kadotani et al., 1998) (Table 1).
4 ROLES OF GluN2C IN CEREBRAL ISCHEMIA
4.1 Changes in GluN2C expression after cerebral ischemia
Cerebral ischemia may induce the up-regulation of GluN2C expression in the ischemic tissues. In vitro reverse transcription-polymerase chain reaction (RT-PCR) results indicated that the mRNA expression level of GluN2C both in hippocampus and cortex significantly increased 45 min after oxygen-glucose deprivation (OGD) for 4 min in adult brain slices, and this phenomenon lasted more than three hours (Perez-Velazquez & Zhang, 1994). Using the same experimental method, Small et al. found that in the brain slice as a whole as well as CA1, CA3, and dentate gyrus regions, the mRNA expression of GluN2C significantly increased 90 min after a brief OGD, peaking up to 4 times the basic level, while the expression of GluN2A and GluN2B did not change (Small, Poulter, Buchan, & Morley, 1997). The above results were derived from conventional RT-PCR method, which can not accurately reflect the expression levels. Recently, by adopting quantitative RT-PCR and Western blot analysis, Chung et al. reported that OGD could induce the up-regulation of GluN2C expression (Control 1; OGD, 1.2 ± 0.1 fold expression) in the hippocampal synaptosomes, and similar results (Sham 1; transient global cerebral ischemia (tGCI), 1.8 ± 0.3 fold expression) could be observed in the hippocampus of animals with tGCI (Chung et al., 2016). However, by performing northern blot analysis, Kadotani et al. found that the amount of GluN2C mRNA in the cerebral cortex did not significantly change 2, 6, 12, and 18 hr after permanent middle cerebral artery occlusion (MCAO) (Kadotani et al., 1998). These inconsistent results in the expression levels of GluN2C may be due to the different degree of cerebral ischemia induced by different experimental conditions. Severe cerebral ischemia without reperfusion might prevent the process responsible for increased transcription expression of GluN2C.
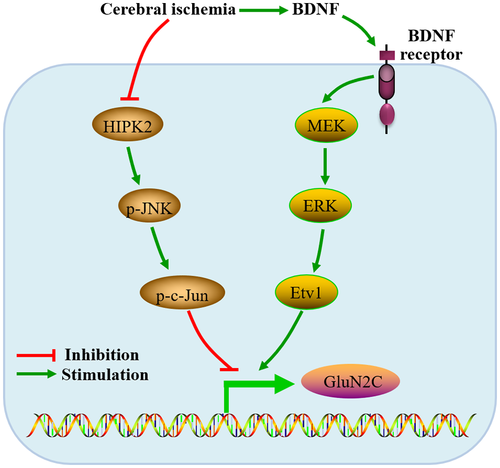
The underlying molecular mechanism enhancing GluN2C expression is not clear. Homeodomain interacting protein kinase 2 (HIPK2) plays important roles in many physiological and pathological processes such as neurogenesis and neurodegeneration (Lee et al., 2016). It was found that the expression of GluN2C was up-regulated in the cortex and substantia nigra of HIPK2 knockout mice, possibly due to the reducing JNK-c-Jun signaling (Shang, Zhang, & Huang, 2018). Studies have moreover demonstrated that hypoxia can trigger proteasome-dependent degradation of HIPK2, thereby inhibiting the role of HIPK2 (Moehlenbrink, Bitomsky, & Hofmann, 2010). Therefore, the up-regulation of GluN2C expression in cerebral ischemia may be related to the reducing HIPK2-JNK-c-Jun function (Figure 1). Another possible mechanism might be the upregulation in the function of brain-derived neurotrophic factor (BDNF)- mitogen-activated protein kinase/extracellular signal-regulated kinase kinase (MEK)-extracellular signal-regulated kinase (ERK) pathway (Abe, Okazawa, & Nakanishi, 2012) (Figure 1). Ischemia can induce the increase in BDNF expression (Tsukahara et al., 1994), subsequently promote the phosphorylation of Etv1 via the BDNF-MEK-ERK Cascade, and finally activate the promoter of the GluN2C gene via phosphorylated Etv1 (Abe et al., 2012).
4.2 Different roles of GluN2C expressed in different cell types in ischemic damage
4.2.1 Pro-survival effect of neuronal GluN2C
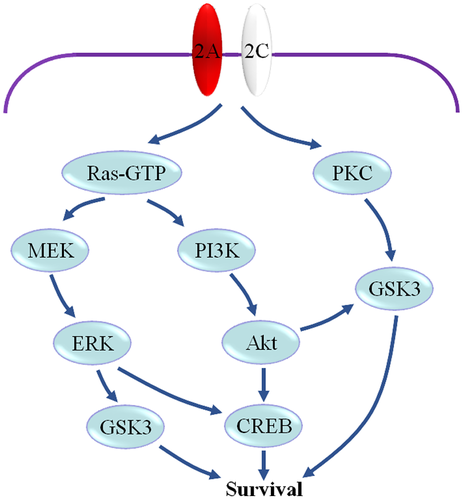
GluN2C shows the highest expression level in the cerebellar granular cells (Buller et al., 1994; Iijima, Abe, Okazawa, Moriyoshi, & Nakanishi, 2008), and moreover, they are expressed in the neurons of the mesencephalon (Allgaier, Scheibler, Muller, Feuerstein, & Illes, 1999), hippocampal CA3 area (Berg et al., 2013), substantia nigra (Ravikrishnan et al., 2018), SCN (Clark & Kofuji, 2010; O'Hara et al., 1995), globus pallidus, and olfactory bulb (Alsaad et al., 2019). It was found that over-expression of GluN2C in cerebellar granular cells or hippocampal neurons significantly reduced neuronal damage induced by NMDA, while over-expression of GluN2A or GluN2B produced opposite effects (Chen & Roche, 2009; Chung et al., 2016). Therefore, increased expression of GluN2C in neurons following ischemia may contribute to protecting neurons from ischemic damage. The underlying mechanism is not clear. One possible mechanism may be that the relative increase in the expression of GluN2C in neurons might induce the downregulation of Ca2+ influx, which are due to the characteristics of lower conductance and open probability, and finally relieve calcium overload in neurons after ischemia. An important evidence for this viewpoint is that compared with that of control group, GluN2C-overexpressing neurons display reduced intracellular Ca2+ influx following NMDA exposure (Chung et al., 2016). Another possible mechanism may be that GluN2C may cooperate with GluN2A to enhance some downstream pro-survival signaling, such as phosphatidylinositol 3-kinase (PI3K), glycogen synthase kinase-3 (GSK3) (Figure 2). Early research indicated that treatment of cultured cerebellar granule cells with 100 μM NMDA for 24 hr from day 4 to 5 in vitro resulted in protection of the cells from apoptosis (Bhave & Hoffman, 1997). The activation of NMDA receptors during this period can mediate several pro-survival signaling pathways, such as PI3K-Akt (Bhave, Ghoda, & Hoffman, 1999; Lafon-Cazal, Perez, Bockaert, & Marin, 2002; Xifro, Minano-Molina, Saura, & Rodriguez-Alvarez, 2014; Zhu, Lipsky, & Marini, 2002), MEK-ERK, and protein kinase C (PKC)-GSK3 (Ortega, Perez-Sen, Morente, Delicado, & Miras-Portugal, 2010). For the primary cultures of cerebellar granule cells, the expression of GluN2B subunit significantly decreases from day 2 to 4 in vitro, while GluN2A and GluN2C expression markedly increase during this period (Iijima et al., 2008). Therefore, it can be considered that GluN2A and GluN2C are the primary NMDA receptors subunits in the cultured cerebellar granule cells after day 4 in vitro and the pro-survival signaling might be mediated by these subunits. Recent data indicated that GluN2C subunits appear to be expressed in tetrameric assemblies with GluN2A in cerebellar granule cells and these triheteromeric GluN2C receptors have distinct properties which are different from diheteromeric NMDA receptors (Bhattacharya et al., 2018). In view of the above, we consider that the formation of triheteromeric GluN2A- and GluN2C-containing NMDA receptors may contribute to the transduction of downstream pro-survival signaling of NMDA receptors.
4.2.2 Pro-death effect of oligodendroglial GluN2C
By using immunohistochemistry and fluorescence polymerase chain reaction with reverse transcription methods, Salter et al. reported that GluN1, GluN2A, GluN2B, GluN2C, GluN2D, and GluN3A subunits showed clustered expression in oligodendroglial processes in cultured optic nerve (Salter & Fern, 2005). In the cerebellar white matter, GluN1, GluN2C, and GluN3 subunits showed more expression in oligodendrocytes than GluN2A, GluN2B, and GluN2D (Karadottir et al., 2005). Oligodendroglial NMDA receptors have the characteristics of weak Mg2+-block and lack of ifenprodil block, which suggests that oligodendroglial NMDA receptors may be GluN2C receptors (Karadottir et al., 2005). OGD could evoke a slowly developing inward current in precursor and mature oligodendrocytes, which can be reduced by NBQX and D-AP5 (Karadottir et al., 2005). Moreover, OGD could result in the rapid loss of oligodendroglial process in cultured optic nerve, which can be significantly prevented by MK801, but not NBQX (Salter & Fern, 2005). The above data indicated oligodendroglial GluN2C may be activated by ischemia and mediate the damage of oligodendroglial process in white matter. Recently, Doyle et al. confirmed this conclusion. They found that ischemic conditions triggered the activation of the myelinic GluN2C/2D-containing NMDA receptors, following the release of glutamate from the axonal vesicles into the peri-axonal space under the myelin sheath, thereby inducing myelin damage and functional deficit in the white matter of the brain (Doyle et al., 2018). Therefore, GluN2C receptors antagonists may become novel therapeutic drugs for preventing white matter damage after cerebral ischemia. In consideration of the pro-survival effect of neuronal GluN2C, selective GluN2C antagonists with frequency dependence, which can preferentially prevent over-activity while leaving some physiological functions intact (Wild et al., 2013), should be developed.
Myelinated oligodendrocytes provide the axons with lactate for metabolism. Saab et al. reported that NMDA receptors in myelin play an important role in maintaining the neuronal–axonal energy metabolism via the up-regulation of oligodendroglial glucose uptake (Saab et al., 2016). In view of the observation that GluN2C is one of the major NMDA receptor subunits in the myelin sheath of oligodendrocytes, GluN2C may have the additional role of providing nutritional support to neuronal axons.
4.2.3 Pro-death effect of astrocytic GluN2C
It was reported that in many brain regions such as the hippocampus, cerebral cortex, striatum, and amygdala, GluN2C showed more expression in non-neuronal cells, especially, the astrocytes (Alsaad et al., 2019; Palygin et al., 2011; Ravikrishnan et al., 2018). Astrocytic NMDA receptors show low sensitivity to magnesium blockade (Lalo, Pankratov, Kirchhoff, North, & Verkhratsky, 2006) and high sensitivity to GluN2C/D subunit-selective antagonist UBP141, which indicates the important roles of GluN2C in astrocytes (Palygin et al., 2011). Because among all NMDA receptor subunits, GluN1, GluN2C, and GluN3A show the highest expression in cortical astrocytes (Zhang et al., 2014), triheteromeric GluN1/GluN2C/GluN3A receptors may be the major type of astrocytic NMDA receptors (Alsaad et al., 2019). The astrocytic endfeet contain NMDA receptors enwrap the synaptic clefts, sense the glutamate concentration in the synapse, and mediate neuron-to-glia communication (Lalo et al., 2006). GluN2C receptors in astrocytes can be activated by the glutamate released from the synapses and lead to increased cytoplasmic calcium levels (Alsaad et al., 2019; Palygin, Lalo, Verkhratsky, & Pankratov, 2010). In response to elevated intracellular calcium, astrocytes may release more glutamate into the perisynaptic region and directly activate neuronal extrasynaptic glutamate receptors (Bezzi et al., 2004). Therefore, the activation of astrocytic GluN2C can enhance the release of glutamate from astrocytes and increased extracellular glutamate level may aggravate ischemic brain damage through over-activating some key pro-death glutamate receptors such as GluN2B receptors, AMPA receptors. In a word, there may exist a potential detrimental effect of astrocytic GluN2C to neuron survival during cerebral ischemia.
4.3 Roles of GluN2C in cerebral ischemia
The downregulation of GluN2C receptors may contribute to relieving MCAO-induced brain damage. Early research showed that at 24 hr after permanent MCAO in mice, the infarct volumes of GluN2C knockout mice were significantly smaller (50%–60% reduction) than those in the wild-type mice (Kadotani et al., 1998). Recently, Holmes et al. found no significant differences in both infarct size and neurological scores between wild-type and GluN2C knockout/nβ-galactosidase knock-in mice at 24 hr post-transient MCAO; however, the neuronal area in the penumbra of GluN2C knockout mice was significantly larger than the neuronal size in the WT penumbra, and the pyknotic nucleation in knockout mice was significantly decreased (Holmes et al., 2018). Moreover, at 72 hr post-transient MCAO, GluN2C knockout mice showed significantly lower average of neurological scores, weight loss, and brain edema (Holmes et al., 2018). Doyle et al. reported that 2 hr pretreatment with GluN2C/D-specific antagonist QNZ-46 prevented ischemia- or OGD-induced myelin injury, such as increased myelin thickness, extensive myelin decompaction, and bubbling (Doyle et al., 2018). Systemic i.p. injection of QNZ-46 2 hr before ischemia greatly reduced lesion volume, significantly increased external capsule axon integrity, and improved the performance in behavioral tests compared to vehicle-treated controls at 24 hr post-transient MCAO (Doyle et al., 2018). The possible mechanism for the pro-death effect of GluN2C may be that indirectly enhancing GluN2B function via promoting the phosphorylation of GluN2B at Tyr1336 (Holmes et al., 2018), decreasing nuclear localization of activated cyclic-AMP response element-binding protein (Holmes et al., 2018), and mediating ischemia-induced release of axonal vesicular glutamate into the peri-axonal space under the myelin sheath (Doyle et al., 2018).
On the contrary, the downregulation of GluN2C receptors may be harmful to hippocampal neuron survival after tGCI. Following 15 min tGCI and a 7 day reperfusion period, GluN2C knockout group had a significantly lower number of surviving neurons in the CA1 region and higher cognitive impairment in spatial working memory compared to wild-type tGCI group (Chung et al., 2016). The possible mechanism for this kind of pro-survival effect of GluN2C may be that GluN2C can modulate Ca2+ permeability and minimize receptor-mediated intracellular Ca2+ overload in neurons, and may be also related to some downstream pro-survival signaling pathways (Chung et al., 2016).
The above evidence shows that GluN2C inhibition or knockout can effectively alleviate the ischemic injury caused by MCAO (Doyle et al., 2018; Holmes et al., 2018; Kadotani et al., 1998) and, contrarily, can aggravate the damage to hippocampal CA1 circuit caused by tGCI (Chung et al., 2016). The aforementioned contradiction in data may be resulting from the differential roles of neuronal, oligodendroglial, and astrocytic GluN2C in ischemic damage. In the MCAO model, the pro-death effect of oligodendroglial and astrocytic GluN2C may be more predominant than the pro-survival effect of neuronal GluN2C. Whereas, in the tGCI model used by Chung and colleagues, the reverse was found true.
5 PERSPECTIVES
Significant evidence has proven that NMDA receptor-mediated calcium influx and the downstream pro-death signaling pathways induce ischemia-related damage. In addition to GluN2A and GluN2B, GluN2C exerts a strong interaction with the postsynaptic density (PSD)-95-like membrane-associated guanylate kinases (Cousins, Papadakis, Rutter, & Stephenson, 2008; Cui et al., 2007). However, the identity of the downstream signaling pathway mediated by GluN2C has not been reported yet. Another issue that needs to be addressed is the identity of the cell types in which GluN2C is up-regulated after a cerebral ischemia, which is an important foundation for clarifying its role in cerebral ischemia. Recent articles reported that GluN2C subunits in cerebellar granule cells mainly exist in the form of triheteromeric GluN1/GluN2A/GluN2C receptors (Bhattacharya et al., 2018). The elucidation of the subunit composition of GluN2C-containing NMDA receptors in other brain areas may contribute to understanding the roles of GluN2C in cerebral ischemia.
ACKNOWLEDGMENTS
This work was supported by the Natural Science Foundation of China (NSFC 81771265), the Natural Science Foundation of Hebei Province (H2017208063), and the project of Hebei hundred talents plan (E2018100009). The authors acknowledge support from the State Key Laboratory Breeding Base—Hebei Key Laboratory of Molecular Chemistry for Drug and Hebei Research Center of Pharmaceutical and Chemical Engineering.
CONFLICT OF INTEREST
All other authors declare no competing interests.
AUTHOR CONTRIBUTIONS
Writing – Original Draft, Y.D.; Writing – Review & Editing, Le.W., Y.H., Y.P.S., Long.W., Z.B.G., and Y.J.S.