Clinical, electrophysiological, and biochemical markers of peripheral and central nervous system disease in canine globoid cell leukodystrophy (Krabbe's disease)
SIGNIFICANCE This study further characterizes the naturally occurring canine model of globoid cell leukodystrophy (GLD), or Krabbe's disease, and demonstrates that we can conduct identical electrodiagnostic and neuroimaging studies in GLD dogs with results analogous to those in human patients. Post-mortem histological evaluations verify clinical assessments and detail loss of myelin and accumulation of storage in the central and peripheral nervous systems. Furthermore, we establish the utility of a minimally invasive biochemical marker of disease. Thus, electrodiagnostic, imaging, and biochemical data will serve as secondary outcome measures in ongoing preclinical studies in the canine model of GLD and could benefit future clinical trials.
Abstract
Globoid cell leukodystrophy (GLD), or Krabbe's disease, is a debilitating and always fatal pediatric neurodegenerative disease caused by a mutation in the gene encoding the hydrolytic enzyme galactosylceramidase (GALC). In the absence of GALC, progressive loss of myelin and accumulation of a neurotoxic substrate lead to incapacitating loss of motor and cognitive function and death, typically by 2 years of age. Currently, there is no cure. Recent convincing evidence of the therapeutic potential of combining gene and cell therapies in the murine model of GLD has accelerated the requirement for validated markers of disease to evaluate therapeutic efficacy. Here we demonstrate clinically relevant and quantifiable measures of central (CNS) and peripheral (PNS) nervous system disease progression in the naturally occurring canine model of GLD. As measured by brainstem auditory-evoked response testing, GLD dogs demonstrated a significant increase in I-V interpeak latency and hearing threshold at all time points. Motor nerve conduction velocities (NCVs) in GLD dogs were significantly lower than normal by 12–16 weeks of age, and sensory NCV was significantly lower than normal by 8–12 weeks of age, serving as a sensitive indicator of peripheral nerve dysfunction. Post-mortem histological evaluations confirmed neuroimaging and electrodiagnostic assessments and detailed loss of myelin and accumulation of storage product in the CNS and the PNS. Additionally, cerebrospinal fluid psychosine concentrations were significantly elevated in GLD dogs, demonstrating potential as a biochemical marker of disease. These data demonstrate that CNS and PNS disease progression can be quantified over time in the canine model of GLD with tools identical to those used to assess human patients. © 2016 Wiley Periodicals, Inc.
Globoid cell leukodystrophy (GLD), also known as Krabbe's disease (KD), is a progressive and fatal neurodegenerative lysosomal storage disorder. This inherited disease is caused by deficient activity of galactosylceramidase (GALC), which is responsible for degrading myelin, specifically the myelin lipids galactosylceramide and galactosylsphingosine (psychosine). The absence of sufficient GALC activity results in galactolipid accumulation, oligodendrocyte and Schwann cell death, abnormal central (CNS) and peripheral (PNS) nervous system myelination, and infiltration of multinucleated macrophages (globoid cells; Wenger et al., 2015).
Symptoms in children emerge in the first year of life and include irritability, stiffness, loss of developmental milestones, and seizures. The disease rapidly progresses to loss of all body faculties and death, often by 2 years of age (Duffner et al., 2009, 2011). Currently, the only available therapeutic intervention for children with GLD is hematopoietic stem cell transplantation (HSCT) from donor umbilical cord blood or bone marrow. When employed early, HSCT has proved capable of slowing disease progression (Escolar et al., 2005); however, the quality of life in children is poor because of neurologic deterioration, resulting in loss of cognitive and motor functions (Aldenhoven and Kurtzberg, 2015).
Development of new and effective therapies for Krabbe's disease has been slow because of the dearth of experimental therapies that have been found to improve neurological disease substantially in animal models and the absence of validated surrogate markers of disease that can be monitored as secondary clinical endpoints. However, recent studies in the twitcher mouse, a murine model of KD, have shown that gene therapy with an adeno-associated virus vector carrying a wild-type copy of murine GALC cDNA synergizes with HSCT to improve motor deficits, survival time, and CNS pathology significantly (Lin et al., 2007; Reddy et al., 2011; Hawkins-Salsbury et al., 2015; Rafi et al., 2015). Although clearly provocative, differences in disease phenotype between murine and human KD and the small size and limited life span of the mouse hinder the translational utility of this model. In contrast, naturally occurring KD in dogs recapitulates the clinical, pathological, and biochemical abnormalities of human disease (Cozzi et al., 1998; Wenger et al., 1999; McGowan et al., 2000; Cantuti-Castelvetri et al., 2015). Additionally, the canine brain is similar in size compared with that of an infant, allowing for the implementation of HSCT and CNS gene transfer methods identical to those that would occur in infants and for the evaluation of the efficacy of these therapies with electrodiagnostic, imaging, and biochemical markers identical to those used in children.
We propose that the canine model of GLD is a critical intermediate step in advancing therapies from the twitcher mouse into the clinic. In this article, histological evaluations corroborate clinical assessments and detail loss of myelin and accumulation of storage product in the CNS and the PNS. Next, we demonstrate quantifiable measures of disease progression in canine GLD using electrodiagnostic and imaging tools identical to those used in pediatric patients. Finally, using high-performance liquid chromatography (HPLC)-coupled mass spectrometry, we establish significant elevations of psychosine in cerebrospinal fluid (CSF) of GLD dogs, suggesting that this as a sensitive biochemical marker of disease. These complementary clinical and biochemical markers of disease progression will be used in ongoing preclinical studies in the canine model of GLD and should benefit future investigations of new drug applications.
MATERIALS AND METHODS
Animals
Mixed-breed dogs were raised in the National Referral Center for Animal Models of Human Genetic Disease of the School of Veterinary Medicine of the University of Pennsylvania (NIH OD P40-10939) under National Institutes of Health and USDA guidelines for the care and use of animals in research. The experimental protocols were approved by the University's institutional animal care and use committee. Equal numbers of male and female dogs were used. Whole blood from dogs was tested for the GALC missense mutation with a TaqMan real-time polymerase chain reaction (PCR)-based DNA test to identify affected, normal, and heterozygote dogs. The custom TaqMan SNP genotyping assay included forward primer ACTGGCCTTACGTGAATCTTCAG and reverse primer GCTTGGCACCCACAATCC with VIC/NFQ as the reporter/quencher for allele 1 and FAM/NFQ for allele 2. The assay was performed with a TaqMan genotyping master mix (catalog No. 4371353; Life Technologies, Grand Island, NY) on an Applied Biosystems (Foster City, CA) 7500 platform. GLD dogs were euthanized at a humane endpoint (15.7 ± 4.8 weeks of age; mean ± SD; n = 19) defined by complete pelvic limb paralysis. Euthanasia was performed with an overdose of intravenous barbiturate. After having been sacrificed, animals were perfused through the left ventricle with 750 ml 0.9% cold saline, and tissues were collected.
Dogs were evaluated weekly via clinical neurological evaluations, approximately every 4 weeks (8 weeks to first day of 12 weeks, 12 weeks to first day of 16 weeks, and 16–20 weeks) for electrodiagnostic testing and at end-stage disease (pelvic limb paralysis) for psychosine, imaging, and histological evaluations.
Magnetic Resonance Imaging
Dogs were anesthetized with propofol, endotracheally intubated, and maintained on isoflurane anesthesia. Imaging was performed at 1.5 T with a dedicated research magnetic resonance imaging (MRI) scanner (Signa; GE Healthcare, Milwaukee, WI). The imaging session was performed as previously described (Cozzi et al., 1998). Briefly, an extremity coil was used in transmit–receive mode to acquire the images. Imaging sessions consisted of sagittal spin-echo imaging with long TR/TE, axial spin-echo imaging with short TR/TE, axial fast spin-echo imaging with long TR/TE sequences, and dorsal-plane axial conventional spin-echo imaging with short TR/TE. T2-weighted images were generated with TR = 6,000 msec, TE = 88 msec, 192 phase encode steps, and 256 data points. MRI analysis was conducted on four end-stage GLD-affected dogs (9.3–16.4 weeks of age) and two normal, age-matched control dogs. Representative images of three regions at the level of the caudate nucleus, midbrain, and cerebellum from one GLD-affected and one normal control dog are shown (see Fig. 1).
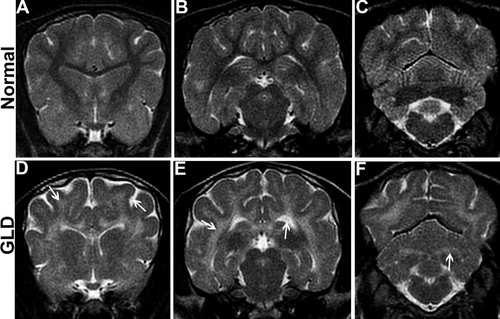
MRI. T2-weighted MRI of the brain shows bilaterally symmetrical increases in signal intensity of the corona radiata (D, arrow), corpus callosum, centrum semiovale, and internal capsule (E, arrow) in a GLD dog compared with a normal, age-matched control dog (A,B). Similarly, the cerebellar white matter (F, arrow) and optic radiations were found to be hyperintense in the GLD dog compared with a normal, age-matched control dog (C). Cerebral ventricles of the GLD dog were dilated (E, arrow) and sulci were widened, indicating cerebral atrophy (D, arrow).
Brainstem Auditory Evoked Response
Brainstem auditory evoked response (BAER) was conducted at 8–12 (normal n = 5, GLD n = 8), 12–16 (normal n = 6, GLD n = 6), and 16–20 (normal n = 5, GLD n = 4) weeks of age in left and right ears of normal and GLD dogs. Dogs were anesthetized with propofol, endotracheally intubated, and maintained on isoflurane anesthesia. BAER data were recorded on a Nicolet Viking Quest machine (Nicolet Biomedical, Madison, WI) with 12-mm, 29-gauge subdermal needle recording electrodes. The active electrode was placed in the skin over the osseous bulla of the stimulated ear, the reference electrode was placed in the skin over the vertex of the skull, and the ground electrode was placed in the skin over the contralateral osseous bulla. Alternating rarefaction and condensation clicks (0.1-msec duration) were delivered to the stimulated ear at 11.1 Hz with a 25-cm plastic tube connected to a plastic earpiece placed within the external ear canal. The filter settings for the amplifier were set to 20 Hz and 3 kHz. One thousand evoked responses were averaged for each tracing obtained. An amplifier sensitivity of 1 μV/cm was used to record the responses; the analysis time was 10 msec. Central conduction time was defined as the time between the first and the fifth peaks. Hearing threshold was defined as the sound intensity at which an evoked waveform was first visible.
Nerve Conduction Velocity
Nerve conduction velocity (NCV) determination was conducted at 8–12 (normal n = 5, GLD n = 8), 12–16 (normal n = 6, GLD n = 6), and 16–20 (normal n = 5, GLD n = 4) weeks of age in normal and GLD dogs. Dogs were anesthetized with propofol, endotracheally intubated, and maintained on isoflurane anesthesia. NCV was conducted with an electrodiagnostics machine (Nicolet Viking Quest; Nicolet Biomedical). Motor NCV in the tibial, sciatic, and ulnar nerves of the left limb was determined with 12-mm, 29-gauge subdermal needle recording electrodes placed in the interosseous muscle. The tibial nerve was electrically stimulated at the tarsus and stifle. The sciatic nerve was electrically stimulated at the stifle and at the level of the femoral head. The ulnar nerve was stimulated at the carpus and elbow. Nerves were stimulated with monopolar stimulating electrodes placed subcutaneously for a duration of 200 µsec. Stimulus intensity was increased until an M wave of maximal amplitude was obtained. Sensory NCV was determined for the superficial radial nerve. Subcutaneous recording electrodes were placed lateral to the superficial radial nerve at the level of the elbow and the skin over the dorsum of the paw and stimulated with subcutaneous monopolar stimulating electrodes for 200 µsec.
CSF Psychosine Concentrations
CSF psychosine levels were measured in GLD dogs (n = 5) at the late stage of disease progression (10.9–16.4 weeks of age) and compared with normal, age-matched control dogs (n = 5). CSF was collected from the cerebellomedullary cistern while animals were under anesthesia for NCV or immediately prior to euthanasia. Protein precipitation was performed to extract psychosine from 50 µl dog CSF. Deuterated galactosyl sphingosine (psychosine-d5, 1 ng/ml) was used as an internal standard and was added to the samples before extraction. Quality control (QC) samples were prepared by pooling the extracts from study samples. QC was performed every five-study sample to make sure that the analytical method and instrument response were constant. Data are reported as peak area ratio of analyte to internal standard.
Sample analysis was performed with a Shimadzu (Shimadzu Scientific, Somerset, NJ) 20AD HPLC system coupled to a triple quadrupole mass spectrometer (API 4000 QTrap; ABSciex, Framingham, MA) operated in MRM mode. The positive-ion ESI mode was used for detection of psychosine and deuterated psychosine-d5. Data processing was conducted in Analyst 1.5.1 (Applied Biosystems; http://sciex.com/products/software/analyst-software).
Histology
Perfused brains were fixed in 4% paraformaldehyde and paraffin embedded. Sections were cut at 5 μm, and slides were deparaffinized and rehydrated in a series of xylenes and ethanols (100%, 95%, and 70%). For myelin staining, slides were incubated in an eriochrome cyanine R solution (catalog No. 32752; Sigma, St. Louis, MO) with ferric chloride and sulfuric acid for 30 min at room temperature; rinsed in running water, followed by incubation in an iron (III) nitrate nonahydrate (catalog No. 216828; Sigma) differentiating solution for 2–5 min and rinsed in running water. Slides were counterstained with eosin, dehydrated in a series of ethanols and xylenes, and mounted. For periodic acid-Schiff (PAS) staining, slides were incubated in periodic acid solution (catalog No. 3951; Sigma) for 5 min at room temperature and rinsed with several changes of water, followed by incubation in Schiff's reagent (catalog No. 3952016; Sigma) for 15 min. Slides were rinsed in running water, dehydrated in a series of ethanols and xylenes, and mounted. Brain regions that were analyzed included sections at the level of the frontal lobe, caudate nucleus, thalamus, midbrain, occipital cortex, cerebellum, and brainstem of end stage from three GLD-affected (9.3–16.4 weeks of age) and two normal control dogs. Representative images of three regions at the level of the, caudate nucleus, midbrain, and cerebellum from one GLD-affected and one normal control are shown (see Figs. 2, 3.
Electron Microscopy
Samples of tibial, common peroneal, sciatic, ulnar, and superficial radial nerves were processed in a routine manner for single-nerve-fiber teasing and semithin/ultrathin sections as previously described (Vite et al., 2001). Nerves were fixed in universal fixative (37% formaldehyde aqueous solution; 100 ml 100% formalin, 880 ml distilled water, 2.7 g NaOH, 11.6 g NaH2PO4-H2O, and 20 ml 50% glutaraldehyde). Electron microscopy was conducted on ultrathin sections prepared from tibial, sciatic, ulnar, radial, and common peroneal nerves in a GLD dog at endpoint.
Statistical Analysis
Two-tailed, unpaired t-tests and figures were conducted in Prism 6 (RRID:SCR_002798; GraphPad Software, La Jolla, CA; http://www.graphpad.com).
RESULTS
CNS and PNS disease progression in the canine model of KD closely recapitulates human disease (Table 1). Affected dogs had a consistent age of onset of clinical signs, with pelvic limb ataxia, thoracic limb dysmetria, and head tremor beginning by 4–6 weeks of age; inability to walk without falling and urinary incontinence occurring by 12 weeks of age; and complete paralysis of the pelvic limbs warranting humane euthanasia occurring at 15.7 ± 4.8 weeks of age (mean ± SD, n = 19). A variable age of onset of increased tone to the pelvic limbs and decreased myotatic and withdrawal reflexes also occurred.
| Phenotype | Human patients | Murine model | Canine model |
|---|---|---|---|
| Irritability | + | − | − |
| Psychomotor regression | + | ? | ? |
| Cerebellar ataxia/intention tremor | + | + | + |
| Postural reaction deficits | + | + | + |
| Stiffness | + | + | + |
| Spastic paresis/paralysis | + | + | + |
| Hearing loss | + | − | + |
| Vision loss | + | − | + |
| Motor and sensory neuropathy | + | + | + |
| Autonomic nervous system dysfunction | + | + | + |
MRI
MRI of the brain of a GLD-affected dog showed T2-weighted bilaterally symmetrical increases in signal intensity of the corona radiata (Fig. 1D, arrow), corpus callosum, centrum semiovale, internal capsule (Fig. 1E, arrow), and cerebellar white matter (Fig. 1F, arrow) compared with a normal, age-matched control dog (Fig. 1A–C). Cerebral ventricles were dilated (Fig. 1E, arrow), and sulci were widened (Fig. 1D, arrow), indicating cerebral atrophy in GLD dogs. Gadolinium enhancement of the white matter was not seen in any of the dogs recently imaged, although it was previously described for a West Highland white terrier (Cozzi et al., 1998).
Brain Histopathology
In line with MRI findings, GLD-affected dogs showed severe loss of myelin, indicated by iron-eriochrome cyanine R histological stain. Loss of myelin was apparent in all sections of the brain compared with normal, age-matched control dogs. Representative images in Figure 2 include brain sections at the level of the caudate nucleus, cerebrum at the level of the midbrain, and cerebellum. Numerous white matter tracts, including the centrum semiovale, corpus callosum, fimbria hippocampi (Fig. 2, inset), and cerebellar white matter show little or no staining at end-stage disease. In contrast, subcortical u-fibers, also known as short association fibers, and the brainstem retained more myelin staining. U-fibers represent terminal zones of myelination, which are the last areas to myelinate in the normal brain and experience a slower rate of myelin turnover (Maricich et al., 2007), demonstrating that disparity in normal myelin metabolism may have consequences for disease pathogenesis. Loss of myelin strongly correlated with the presence of PAS-positive storage material (Fig. 3).
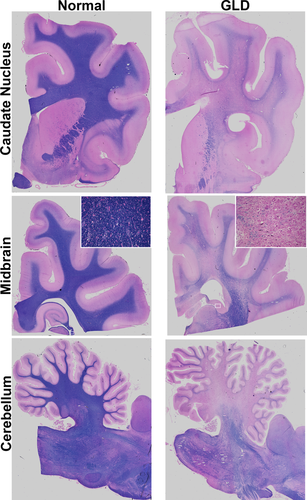
Myelination in normal and GLD dog brain. Iron-eriochrome cyanine R histological stain shows loss of myelin (purple stain) in a GLD dog at end stage (right) compared with a normal, age-matched control dog (left). Additionally, white mater regions are subjectively small in GLD dogs compared with normal control dogs. Cerebral hemisphere sections are shown at the level of the caudate nucleus (top), cerebrum at the level of the midbrain (middle), and cerebellum (bottom). Insets are × 25.
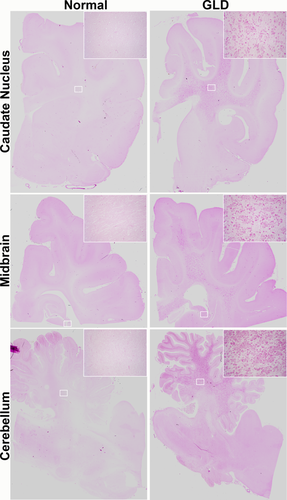
Storage material in normal and GLD dog brain. PAS histological stain shows accumulation of storage material in a GLD-affected dog at end stage (right) compared with a normal, age-matched control dog (left). Brain sections are shown at the level of the caudate nucleus (top), cerebrum at the level of the midbrain (middle), and cerebellum (bottom). Insets are × 25.
BAER Testing
BAER testing was conducted in GLD dogs to assess the effect of disease on the auditory pathway over time. In GLD dogs, there was a significant increase (P ≤ 0.001) in central conduction time, measured by the difference in peak I-V latencies in all age groups analyzed, 8–12, 12–16, and 16–20 weeks, compared with normal, age-matched control dogs (Fig. 4A). A significant increase in hearing threshold over normal was also identified in affected dogs at all time points evaluated (Fig. 4B). The slight reduction in threshold significance at the final time point (P ≤ 0.01) likely was due to the small number of affected animals that survived >16 weeks and still had recordable BAER (n = 4). The increase in central conduction time, increase in hearing threshold, and loss of waveform integrity on BAER are all consistent with auditory system demyelination in the GLD dog.
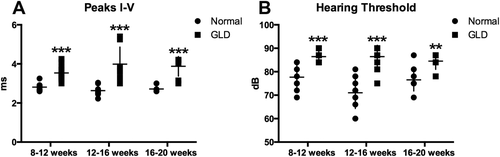
BAERs in normal and GLD dogs. BAER was conducted at 8–12 (normal n = 5, GLD n = 8), 12–16 (normal n = 6, GLD n = 6), and 16–20 (normal n = 5, GLD n = 4) weeks of age in left and right ears of normal (circles) and GLD (squares) dogs. A: Central conduction time, the time between the first and the fifth peak, was increased in affected dogs. B: Hearing threshold, defined as the sound intensity at which an evoked waveform was first visible, was increased in affected dogs. **P ≤ 0.01, ***P ≤ 0.001.
NCV Testing
NCVs were quantified in motor and sensory nerves of GLD dogs and normal, age-matched controls at three time points, 8–12, 12–16, and 16–20 weeks of age. In GLD dogs, motor NCVs of the tibial (Fig. 5A) and sciatic (Fig. 5B) nerves were significantly decreased by 12–16 weeks of age (P = 0.028, P = 0.004, respectively) and were also significantly decreased at 16–20 weeks of age for the sciatic nerve (P = 0.009). NCV of the ulnar nerve (Fig. 5C) was significantly reduced at 8–12 (P = 0.047) and 12–16 (P = 0.005) weeks of age. Significance was not achieved at the latest time point in the tibial and ulnar nerves, likely because of the low number of animals that survived beyond 16 weeks and still had recordable NCV (n = 4). Sensory NCV of the radial nerve (Fig. 5D) was the most dramatically decreased, with a P value of 0.0001 at both 8–12 and 12–16 weeks of age. Values trended toward significance at endpoint; however, statistics could not be calculated because of insufficient animal numbers (only two GLD dogs had recordable sensory NCV at 16–20 weeks; in the remaining dogs, no response could be evoked). The reduction in conduction velocity of motor and sensory nerves is consistent with severe demyelination of peripheral nerves in GLD dogs.
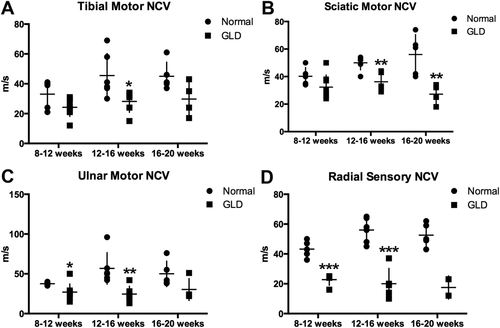
NCV in normal and GLD dogs. NCV was conducted at 8–12 (normal n = 5, GLD n = 8), 12–16 (normal n = 6, GLD n = 6), and 16–20 (normal n = 5, GLD n = 4) weeks of age in normal (circles) and GLD (squares) dogs. For pelvic limb motor NCV, the tibial nerve was stimulated at the tarsus and stifle (A), and the sciatic nerve was stimulated at the stifle and at the level of the femoral head (B); for the thoracic limb, the ulnar nerve was stimulated at the carpus and elbow (C); for sensory NCV, the radial nerve was stimulated at the level of the elbow (D). *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001.
Electron Microscopy of Peripheral Nerves
Analysis of ultrathin sections of peripheral nerves by electron microscopy revealed that storage inclusions and thinly myelinated fibers were a prominent feature in all nerves analyzed (tibial, sciatic, ulnar, radial, and common peroneal) in one GLD dog at end-stage disease. Storage inclusions were present in numerous Schwann cells (Fig. 6A) and in macrophages (Fig. 6B), most commonly located in the endoneurium with occasional presence in the perineurium. The inclusions were highly heterogeneous (Fig. 6A, arrow), with the most prominent and typical morphology consisting of straight, elongated, needle-like prismatic inclusions. In some cells, the prismatic inclusions were voluminous and arranged in different plans of section, giving them a wave-like curvilinear pattern (Fig. 6C, arrow). Other membrane-bound concentric or irregularly shaped inclusions as well as honeycomb, crystalloid structures frequently accompanied the characteristic prismatic inclusions. Myelin-like lamellar structures occasionally occurred with these inclusions (Fig. 6D, arrow). No inclusions were observed in unmyelinated fibers, which appeared normal. Thinly myelinated fibers were common in all nerves analyzed (Fig. 6E,F, arrows).
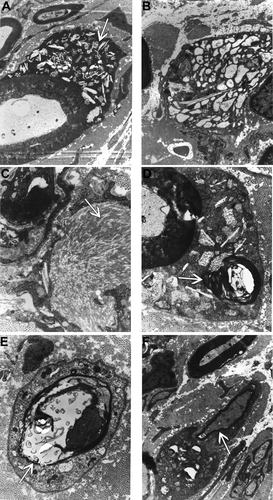
Electron micrographs of the peripheral nerve of a GLD dog at end stage demonstrating storage inclusion in Schwann cells (×19,500; A, arrow), storage inclusions in macrophages (×15,700; B), prismatic inclusions in a wave-like curvilinear pattern (×41,300; C, arrow), myelin-like lamellar structures within inclusions (×41,300; D, arrow), thinly myelinated fibers (×30,300; E, arrow), and thinly myelinated fibers (×19,500; F, arrow).
CSF Psychosine Concentrations
It has previously been shown that psychosine is dramatically increased in the white matter of GLD dog brains (Wenger et al., 1999). For this study, we quantified psychosine concentrations in CSF by HPLC-coupled mass spectrometry. CSF psychosine levels were significantly elevated (P = 0.004) in GLD dogs (mean 0.069 ± 0.036, n = 5) at late stage of disease progression (10.9–16.4 weeks of age) compared with normal, age-matched control dogs (n = 5; Fig. 7). The highest CSF psychosine concentration was found in the oldest GLD dog evaluated (16.4 weeks of age). This minimally invasive method of determining CNS-specific disease progression could serve as a valuable biomarker of disease progression; repeated measures over time are now underway.
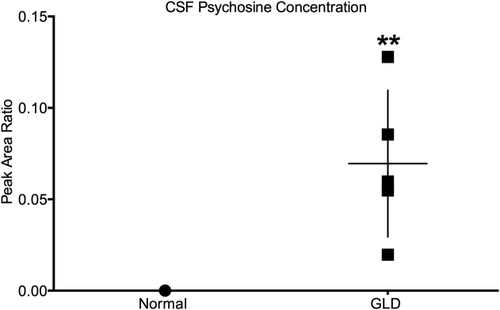
CSF psychosine concentration in normal and GLD-affected dogs. CSF psychosine concentrations determined by HPLC-coupled mass spectrometry and presented as peak area ratio for GLD-affected dogs (squares; n = 5; mean age 13.4 weeks, age range 10.9–16.4 weeks) compared with normal control dogs (circles; n = 5; mean age 18.0 weeks, age range 6.1–38.7 weeks). **P ≤ 0.01.
DISCUSSION
Canine GLD was first described in 1966 (Fletcher et al., 1966). In 1996, the disease-causing mutation in West Highland white and Cairn terriers was identified as an A-to-C transversion at cDNA position 473 in the GALC gene, resulting in the replacement of a hydrophobic tyrosine residue with a hydrophilic serine in the protein. Transient transfection of COS-1 cells with the mutant canine GALC cDNA revealed that the mutant protein is not functional (Victoria et al., 1996). Affected dogs, homozygous for the recessive mutation, can be identified at birth from whole blood with real-time PCR amplification of genomic DNA. In 2000, a breeding colony of canine GLD was established and has been maintained at the School of Veterinary Medicine at the University of Pennsylvania. Since then, KD has also been characterized in the Irish setter, in which it occurs because of an insertion mutation (McGraw and Carmichael, 2006); no colony of these dogs is currently established. In light of recent advancements demonstrating unprecedented therapeutic efficacy with a combination of bone marrow transplantation and gene therapy in the twitcher mouse (Lin et al., 2007; Reddy et al., 2011; Hawkins-Salsbury et al., 2015; Rafi et al., 2015), the canine model of GLD has renewed significance as an intermediate model in translation of therapeutics to the clinic. Here we further characterize the canine model and identify the onset of clinical signs by 4 weeks of age and a survival time to 15.7 ± 4.8 weeks of age (n = 19). Electrodiagnostic testing methods, routinely used in affected children, are reported longitudinally in the canine model of GLD, allowing a quantifiable means to track disease progression. Furthermore, we have determined that psychosine is elevated in the spinal fluid of affected dogs. Future studies will evaluate its use as a biomarker of disease severity or of therapeutic efficacy.
The clinical data in dogs compare well with those that have been published for human patients. In children, neurologic evaluations revealed that, in 41 patients, abnormal results on motor examination were identified, with 90% demonstrating increased muscle tone, typically in the extremities. Deep tendon reflexes were abnormal in the majority of children (71% decreased reflexes and increased in 19%), and clonus and plantar extensor responses were also common (Duffner et al., 2011). GLD is also naturally occurring in nonhuman primates. In a natural history study of GLD-affected rhesus macaques, the onset and progression of symptoms were highly variable, and survival ranged widely from 52 to 642 days (Borda et al., 2008). In this study, the canine model of GLD was found to have repeatable and predictable onset of clinical signs and limited range of survival.
Analysis of MRI scans from 39 early-infantile KD patients, those with symptom onset from 0 to 6 months of age, showed abnormalities in 38 of 39 cases. The most common irregularities were T2 signal intensity changes in the deep cerebral white matter periventricular/centrum semiovale (84.6%) and the dentate (84.6%), followed by cerebellar white matter (53.8%), thalamus (41.0%), and parietal-occipital regions (30.8%; Abdelhalim et al., 2014). Similar changes were found in our canine model of GLD, including T2 signal hyperintensities in numerous white matter tracts. In contrast, there were no remarkable findings on MRI of GLD-affected rhesus macaques (Borda et al., 2008).
BAER testing conducted in children with early-infantile KD revealed prolonged wave I-V interpeak latency in 88% (15/17) of children. Among children who were symptomatic at the time of testing, 100% (13) experienced increased central conduction time, and two of four presymptomatic children had similar abnormalities (Aldosari et al., 2004). Similarly, GLD dogs demonstrated a significant increase in I-V interpeak latency at all times analyzed, 8–12, 12–16, and 16–20 weeks of age. Because there is overlap in values between normal and GLD-affected dogs, BAER is not likely a sensitive measure for detecting improvements, especially in early disease stages following treatment. Instead BAER will be most useful as a repeated-measure analysis to study individual animals longitudinally.
Peripheral neuropathy has also been previously evaluated in children affected with early-infantile KD. Among 24 KD patients assessed, 23 had abnormal NCVs, with 82% showing deficient sensory and 82% showing slow motor conduction velocities. Abnormalities in motor conduction velocities were comparable in lower (79%) and upper (80%) extremities. Importantly, the severity of the demyelination on NCV studies correlated well with the clinical severity of the disease (Siddiqi et al., 2006). Similarly, conduction velocities of the median, ulnar, and tibial nerves were significantly slower in GLD-affected rhesus macaques compared with normal monkeys and also correlated with disease progression (Weimer et al., 2005). Comparably, motor NCVs of pelvic (tibial and sciatic nerves) and thoracic limbs (ulnar nerve) were a sensitive indicator of peripheral nerve dysfunction in the canine model of GLD, with all conduction velocities significantly lower than normal by 12–16 weeks of age. Decreases in sensory (radial) NCV achieved the greatest and earliest significance of 0.0001 by 8–12 weeks of age, and mean velocity continued to decrease with age. Unlike BAER, radial NCV resulted in no overlap between normal and GLD-affected dogs at any time point and is therefore expected to be a sensitive and robust means to determine therapeutic efficacy, even early in disease.
NCV studies in combination with neuroimaging studies have been proposed as the most sensitive measure to assess the severity of KD in human patients (Moser, 2006). Here we demonstrate that we can conduct identical electrodiagnositic and neuroimaging studies in the GLD dog with results analogous to those found in human patients. Post-mortem histological evaluations verify clinical assessments and detail loss of myelin and accumulation of storage product in the CNS and the PNS. Furthermore, we establish the utility of CSF psychosine as a potential biochemical marker of disease. Thus, electrodiagnostic, imaging, and biochemical data may serve as secondary clinical outcome measures in ongoing preclinical studies in the canine model of GLD and could benefit future human clinical trials for KD.
CONFLICT OF INTEREST STATEMENT
The authors have no identified conflicts of interest.
ROLE OF AUTHORS
All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: AMB, CHV. Acquisition of data: AMB, JHB, XJ, GPS, MLP, CAF, PAO, KGB, DSO, CHV. Analysis and interpretation of data: AMB, XJ, KGB, DSO, CHV. Drafting of the manuscript: AMB, CHV. Critical revision of the article for important intellectual content: AMB, JHB, PAO, KGB, DSO, CHV. Statistical analysis: AMB. Obtained funding: CHV. Administrative, technical, and material support: GPS, KGB, XJ. Study supervision: CHV.