Sex-dependent and differential responses to acute restraint stress of corticotropin-releasing factor–producing neurons in the rat paraventricular nucleus, central amygdala, and bed nucleus of the stria terminalis
Abstract
Male and female rodents respond differently to acute stress. We tested our hypothesis that this sex difference is based on differences in stress sensitivity of forebrain areas, by determining possible effects of a single acute psychogenic stressor (1-hr restraint stress) on neuronal gene expression (c-Fos and FosB immunoreactivities), storage of corticotropin-releasing factor (CRF) immunoreactivity, and CRF production (CRF mRNA in situ hybridization) as well as the expression of genes associated with epigenetic processes (quantitative RT-PCR) in the rat paraventricular nucleus (PVN), the oval and fusiform subdivisions of the bed nucleus of the stria terminalis (BSTov and BSTfu, respectively), and the central amygdala (CeA), in both males and females. Compared with females, male rats responded to the stressor with a stronger rise in corticosterone titer and a stronger increase in neuronal contents of c-Fos, CRF mRNA, and CREB-binding protein mRNA in the PVN. In the BSTov, females but not males showed an increase in c-Fos, whereas the CRF mRNA content was increased in males only. In the BSTfu, males and females showed similar stress-induced increases in c-Fos and FosB, whereas in the CeA, both sexes revealed similar increases in c-Fos and in CRF mRNA. We conclude that male and female rats differ in their reactivity to acute stress with respect to possibly epigenetically mediated (particularly in the PVN) neuronal gene expression and neuropeptide dynamics (PVN and BSTov) and that this difference may contribute to the sex dependence of the animal's physiological and behavioral responses to an acute stressor. © 2011 Wiley Periodicals, Inc.
Many psychopathologies are the result of chronic exposure to stressors, but acute stress may induce psychological and behavioral disorders too, in a sex-specific manner, as has been shown in the case of, e.g., posttraumatic stress disorder (Armario et al.,2008; Cook et al.,2009). However, compared with chronic stress, relatively little is known about the underlying mechanism(s) of sex-specific effects of acute stress on the mammalian brain. Rodent studies have suggested an involvement of the hypothalamo-pituitary adrenal (HPA) axis (Armario et al.,2008; Belda et al.,2008), because the main neuronal component of the HPA axis, the paraventricular nucleus (PVN), reacts to acute stressors such as restraint and cold stress by increasing the production of corticotropin-releasing factor (CRF) mRNA and the release of CRF peptide (Kalin et al.,1994; Hatalski et al.,1998; Cook,2004). The response of the HPA axis to acute stress seems to be sex-specific, because female rats show a higher corticosterone response than males (Lesniewska et al.,1990; Iwasaki-Sekino et al.,2009).
Various forebrain centers control HPA axis activity, of which the oval subdivision of the bed nucleus of the stria terminalis (BSTov) and the central amygdala (CeA; Herman et al.,1994, 2005) are of particular interest, because they 1) play a role in the control of mood (Walker et al.,2003; Walker and Davis,2008; Davis et al.,2010; Regev et al.,2010), 2) change their activity upon stress (Martinez et al.,1998), and 3) host the majority of the brain's CRF neurons (Merchenthaler et al.,1982; Swanson et al.,1983; Gray,1993; Morin et al.,1999). In addition to the BSTov, the fusiform subdivision of the BST (BSTfu) has been implicated in the regulation of the stress response because it has strong projections to the PVN and BSTov (Dong et al.,2001) and its lesioning decreases the HPA axis response to stress (Choi et al.,2007). Therefore, we hypothesized that acute stress would change the functioning of the PVN, BSTov/BSTfu, and/or CeA in a sex-specific manner and in this way would evoke a sex-specific stress response by the HPA axis. More specifically, these changes of these brain centers might be evident at the gene expression and secretory levels and might be mediated, at least in part, by epigenetic mechanisms.
Indeed, a single fear stimulus epigenetically changes BDNF gene expression in the hippocampus (Lubin et al.,2008), and epigenetic mechanisms such as histone acetylation seem to play a role in the behavioral responses to acute (and chronic) stress (Tsankova et al.,2006; Renthal et al.,2007; Lubin et al.,2008). Therefore, such epigenetic processes might also underlie acute stress-induced changes in the functioning of the PVN and limbic structures and, for that matter, in the (sex-dependent) character of the stress response.
To test these hypotheses, we have studied in the PVN, BSTov/fu, and CeA the effects of an acute psychogenic stress (restraint) on parameters for the neuronal expression of immediate early gene (IEG) products (c-Fos and FosB) and for the neuronal production capacity and storage of CRF (CRF mRNA and CRF peptide contents, respectively), by using in situ hybridization and immunocytochemistry. Subsequently, we correlated these data with changes in the amount of mRNAs of the epigenetic markers histone deacetylase (HDAC) 3, 4, and 5 and histone acetyltransferases (HATs) CREB-binding protein (CBP) and P300/CBP-associated factor (PCAF; Lee and Workman,2007; Morrison et al.,2007), by using quantitative (Q)-RT-PCR. Males as well as females were investigated, to test for sex specificity.
MATERIALS AND METHODS
Animals
Forty-eight Wistar-R Amsterdam rats (females 200–250 g, males 300–350 g) aged 14 weeks were housed on a 12/12-hr light/dark cycle (lights on 07:00 hr) at 23°C, with water and food ad libitum. Animals were divided into two groups, acutely stressed and control rats, each group consisting of 12 males and 12 females. For acute stress exposure, rats were placed in a cylindrical plastic restrainer (diameter 45 mm, length 200 mm; several ventilation holes), for 1 hr. Control animals were handled the same as challenged rats but were not stressed. One hour after the end of stressor exposure, rats were deeply anesthetized with Nembutal (100 mg/kg body weight; Sanofi-Synthélabo, Budapest, Hungary), and a blood sample was taken for corticosterone (CORT) assay. For the immunocytochemistry/in situ hybridization study, six animals per group were transcardially perfused with 50 ml 0.1 M sodium phosphate-buffered saline (PBS; pH 7.4), for 10 min, followed by 250 ml 4% ice-cold paraformaldehyde in PBS for 20 min. After decapitation, brains were dissected and postfixed in the paraformaldehyde fixative for 16 hr. For the Q-RT-PCR study, six other rats per group were decapitated, and after dissection their brains were frozen until further processing.
The character of the female stress response depends to some degree on the phase of the estrous cycle (Viau and Meaney,1991; Carey et al.,1995). To prevent handling-induced stress, we determined this phase (using vaginal smears) not during the experiment but directly after sacrificing the female rats, as described previously (Barha et al.,2010; Neufeld-Cohen et al.,2010; Verma et al.,2010). In the female experimental groups, all cycle phases appeared to occur, in a random-like fashion, and statistical analysis (see below) showed that outcomes of all parameters measured were normally distributed and had a variance not significantly greater than that of males, so we have considered the female group as a sexually homogeneous population. Obviously, this means that we cannot attribute a statistically significant difference between males and females to a particular phase of the female cycle. All studies were conducted in accordance with the Directive 86/609/EEC on the protection of Animals used for Experimental and other scientific purposes and the animal use guidelines of the Committee for Animal Resources of Pécs University, Hungary. Procedures were carried out at room temperature unless stated otherwise.
CORT Assay
For CORT radioimmunoassay, 5 μl blood serum was treated as described previously (Gaszner et al.,2004), using 3H-corticosterone (12,000 cpm; 90–120 Ci/mmol, NET-399; Perkin-Elmer, Boston, MA) and our CS-RCS-57 CORT antiserum (Jozsa et al.,2005). The inter-and intra-assay coefficients of variation were 9.2% and 6.4%, respectively, indicating the high reliability of the method.
Tissue Preparation
Fixed brains were transferred to 30% sucrose in PBS and, when completely submerged, frozen on dry ice. Twenty-five-micrometer coronal sections between Bregma–0.26 and–3.30 mm (Paxinos and Watson,1997) were cut on a freezing microtome (Microm, Walldorf, Germany) and saved in sterile antifreeze solution (0.05 M PBS, 30% ethylene glycol, 20% glycerol) at–20°C until further use.
Immunocytochemistry
After three 20-min rinses in 0.1 M PBS, sections were treated with 0.5% Triton X-100 (Sigma, Zwijndrecht, The Netherlands) in PBS, for 30 min, to enhance antigen penetration. After two 15-min rinses in PBS, sections were incubated in 1% H2O2 for 30 min, followed by two 15-min rinses in PBS. Pre-incubation in 2% normal donkey serum (Jackson Immunoresearch, West Grove, PA) in PBS-BTSA (PBS with 0.5% blocking reagent from the tyramide signal amplification kit; Perkin-Elmer) for 1 hr was followed by incubation in a primary antiserum in PBS-BTSA with 2% normal donkey serum for 16 hr. Primary antisera raised to c-Fos, FosB, and CRF are specified in Table I. After 2 × 15 min rinses in PBS, sections were incubated in biotinylated donkey anti-rabbit secondary antiserum (1:200; Jackson Immunoresearch), rinsed for 2 × 15 min in PBS, incubated with avidin-biotin-complex (1:200; Vectastain Elite ABC kit; Vector Laboratories, Burlingame, CA) for 1 hr, rinsed in PBS for 15 min, and rinsed in Tris buffer (pH 7.6) for 15 min. Immunostaining was visualized by incubation in 50 mg 3,3′-diaminobenzidine (DAB; D5637; Sigma) in 200 ml Tris buffer with 30 μl 35% H2O2 for 10 min. To intensify c-Fos and FosB immunostainings, sections were incubated in 7.5% ammonium nickel sulfate (BDH Laboratory Supplies, Poole, United Kingdom) in Tris buffer for 10 min. Finally, they were mounted on gelatin-coated glass slides, dried at 37°C for 16 hr, dehydrated, cleared with xylene, coverslipped with Entellan (Merck, Darmstadt, Germany), and studied with a DMRBE microscope connected to a CD 500 digital camera (Leica Microsystems, Wetzlar, Germany).
| Antiserum | Epitope | Dilution | Manufacturer,species, type, catalog No. | Specificity |
|---|---|---|---|---|
| c-Fos (sc-48) | N-terminus of human c-Fos | 1:2,000 | Santa Cruz Biotechnology (Santa Cruz, CA, rabbit, sc-52 | Gaszner et al.,2004, 2009 |
| FosB | Internal region of mouse FosB | 1:1,000 | Santa Cruz Biotechnology, rabbit, sc-48 | Wallace et al.,2008 |
| CRF | Human/rat CRF(1–41) | 1:2,000 | Kind gift from Dr W.W. Vale (The Salk Institute, La Jolla, CA), rabbit | Flak et al.,2009; Korosi et al.,2007; Sawchenko,1987 |
In Situ Hybridization
In situ hybridization of CRF mRNA was carried out using antisense and sense (control; no hybridization signal was seen) cRNA probes transcribed from CRF cDNA (kindly provided by Dr. W.W. Vale, La Jolla, CA) and labeled with digoxigenin (DIG)-11-UTP (Roche Molecular Biochemicals, Basel, Switzerland). Sections were rinsed in PBS for 4 × 15 min, fixed in 4% ice-cold paraformaldehyde for 30 min at 4°C, rinsed for 4 × 7 min in PBS, and preincubated in proteinase K medium containing 0.1 M Tris HCl, 0.05 M EDTA and 0.1 mg proteinase K (Invitrogen, Carlsbad, CA) for 10 min at 37°C. After rinsing in autoclaved MQ water, acetylation was performed with 0.25% acetic acid anhydride in 0.1 M triethanolamine buffer (pH 8.0) for 10 min, followed by rinsing in 2× concentrated standard saline citrate buffer (2× SSC; pH 7.0) for 5 min. Hybridization mixture (50% deionized formamide, 0.3 M NaCl, 0.001 M EDTA, Denhardt's solution, 10% dextran sulfate) together with 0.5 mg/ml tRNA and the mRNA DIG probe (∼ 40 ng/ml) were placed into a water bath for 5 min at 80°C and then on ice for another 5 min. Sections were incubated in hybridization solution for 16 hr at 58°C; rinsed for 4 × 7 min with 4× SSC; incubated for 30 min at 37°C in preheated RNAse medium (0.5 M NaCl, 0.01 M Tris HCl, 1 mM EDTA, pH 8.0) containing 0.01 mg/ml RNAse A (Roche), which had been added just before the start of incubation, and stringently rinsed in decreasing SSC concentrations (2×, 1×, 0.5×). Afterwards, the sections were incubated in 0.1× SSC for 30 min at 58°C. The alkaline phosphatase method with nitroblue tetrazolium chloride/5-bromo-4-chloro-3-indolyl phosphate and toluidine salt (NBT/BCIP; Roche) as a substrate was used for the detection of the DIG label. Briefly, after rinsing for 4 × 5 min with buffer A (0.1 M Tris HCl, 0.15 M NaCl, pH 7.5), sections were preincubated in buffer A containing 0.5% blocking agent (Roche) for 1 hr, followed by incubation in sheep anti-DIG-AP (1:5,000; Roche) in buffer A containing 0.5% blocking agent for 3 hr. Subsequently, sections were rinsed for 4 × 5 min in buffer A and 2 × 5 min in buffer B (0.1 M Tris HCl, 0.15 M NaCl, 0.05 M MgCl2; pH 9.5). After incubation in NBT/BCIP mixture consisting of 10 ml buffer B, 2.4 mg levamisole (Sigma), and 175 μl NBT/BCIP (Roche) in a light-tight box for 16 hr, the reaction was stopped by placing the sections in buffer C (0.1 M Tris HCl, 0.01 M EDTA; pH 8.0). Finally, they were processed and studied as for immunocytochemistry.
RNA Extraction and cDNA Synthesis
One-millimeter-thick coronal sections, cut with a razor blade between the cerebellum and the two hemispheres with a coronal brain matrix (15007; Ted Pella, Redding, CA), were placed on a chilled mat, and brain nuclei were punched out with a Harris Unicore Hole 1.0-mm puncher (Ted Pella). Separate punches were made of the PVN, CeA, and BST (containing both BSTov and BSTfu). Punches were collected in 500 μl ice-cold Trizol (Life Technologies, Paisley, United Kingdom) and homogenized by sonification. After chloroform extraction and isopropyl alcohol precipitation, RNA was dissolved in 30 μl RNAse-free, DEPC-treated MQ water. Total RNA was measured with an Eppendorf Biophotometer (Vaudaux-Eppendorf, Basel, Switzerland) and the RNA concentration with a NanoDrop 1000 spectrophotometer (Thermo Fisher Scientific, Wilmington, DE). The A260/280 ratio was always between 1.8 and 2.0, indicating high purity. To check for RNA integrity, 500 ng of RNA was loaded onto a 1% agarose gel with GelRed nucleic acid gel stain (Biotium, Hayward, CA), subjected to electrophoresis, and visualized by UV transillumination. No RNA degradation had occurred; 18S and 28S ribosomal RNA bands were always intact. First-strand cDNA synthesis was performed using 11 μg RNA dissolved in 11 μl RNAse-free DEPC containing 5 mU pd(N)6 random primers (Roche) at 70°C for 10 min, followed by double-strand synthesis in 1× strand buffer (Life Technologies) with 10 mM DTT, 20 U Rnasin (Promega, Madison, WI), 0.5 mM dNTPs (Roche), and 100 U reverse transcriptase (Superscript II; Life Technologies) for 75 min at 37°C and for 10 min at 95°C.
Quantitative RT-PCR
Quantitative RT-PCR was performed on the brain punches (see above) in a total volume of 25 μl buffer solution containing 5 μl of template cDNA, 12.5 μl SYBR Green Master Mix (Applied Biosystems, Foster City, CA), 1.5 μl DEPC-treated MQ water, and 15 pM of the forward and reverse primers. Primers were designed in Vector PrimerExpress software (Applied Biosystems) based on rat cDNA sequences, according to Table II. The cycling protocol was 95°C for 10 min followed by 40 reaction cycles at 95°C for 15 sec and at 60°C for 1 min, using a 7500 GeneAmp PCR system (Applied Biosystems). For each reaction, the cycle threshold (Ct) was determined, i.e., the number of cycles needed to detect fluorescence above the arbitrary threshold (0.8). At this threshold, Ct values are within the exponential phase of the amplification. Standard curves were included in duplicate with cDNA concentrations ranging from 6.25 to 100 ng cDNA per sample. Using these curves, in which every Ct value corresponds to a certain amount of cDNA, the quantity of cDNA was calculated for each sample with Applied Bioscience 7500 System Software. All data were normalized to 18S mRNA contents.
| Primer | 5′ → 3′ Sequence |
|---|---|
| HDAC 3 F | GCCAAGACCGTGGCGTATT |
| HDAC 3 R | GTCCAGCTCCATAGTGGAAGT |
| HDAC 4 F | CAATCCCACAGTCTCCGTGT |
| HDAC 4 R | CAGCACCCCACTAAGGTTCA |
| HDAC 5 F | TGTCACCGCCAGATGTTTTG |
| HDAC 5 R | TGAGCAGAGCCGAGACACAG |
| CBP F | GGACCTGGGATCTGCATGAA |
| CBP R | TCCAGCAGCCCCAAGAGA |
| PCAF F | CACGCTCAAGAACATCCTGCA |
| PCAF R | TCGCTGTAAGTCCGCCATGAATA |
| 18S F | GTAACCCGTTGAACCCCATT |
| 18S R | CCATCAATCGGTAGTAGCG |
- * F, forward; R, reverse; HDAC, histone deacetylase; CBP, CREB-binding protein; PCAF, P300/CBP-associated factor.
Image Analysis
CRF mRNA expression and immunoreactivity of CRF, c-Fos and FosB were assessed in five sections of the PVN and the CeA and in three sections of the BSTov and BSTfu, at the midlevel of each brain nucleus, interspaced by 125 μm. Digital images were taken with the Leica DMRBE microscope (at 1,200 × 1,600 dpi). Numbers of immunoreactive neurons were counted per section and then averaged over all sections. The specific signal density (SSD) of CRF staining per individual neuron was determined in Scion Image software (version 3.0b; NIH, Bethesda, MD), averaged over 10 randomly taken neurons, corrected for background staining in the same section outside the brain area, and finally averaged over all sections. In a similar way, the SSD of the area of CRF-immunopositive fibers in the BSTov, BSTfu, and CeA was determined.
Statistical Analysis
Each parameter is graphically represented as the mean and SEM of the animals in an experimental group. Means were analyzed by two-way ANOVA and, if a significant main effect (stress, sex) was found, by Fisher's post hoc test (Statistica, StatSoft, Tulsa, OK) after appropriate transformation of data on the basis of tests for normality (Shapiro and Wilk,1965) and Bartlett's test for the homogeneity of variance (Snedecor,1989). In the figures, statistical differences (α = 5%) are indicated only when they emerged from post hoc analysis of ANOVA main effects.
RESULTS
Corticosterone
For CORT, ANOVA revealed strong main effects of sex (F1,14 = 49.9, P < 0.00001) and stress (F1,14 = 41.1, P < 0.00005; Fig. 1). Post hoc analysis showed a clear sex difference for controls (males 0.05 ± 0.01 μmol/liter vs. females 0.23 ± 0.02 μmol/liter, P < 0.00005), which was maintained after stress (males 0.22 ± 0.02 μmol/liter vs. females 0.37 ± 0.02 μmol/liter, P < 0.001). However, compared with controls, stressed males had a much (4.2-fold) higher CORT titer (stressed 0.22 ± 0.02 μmol/liter vs. controls 0.05 ± 0.01 μmol/liter, P < 0.0005); stressed females showed an only 1.6-fold higher CORT titer (stressed 0.37 ± 0.02 μmol/liter vs. controls 0.23 ± 0.02 μmol/liter, P < 0.001).
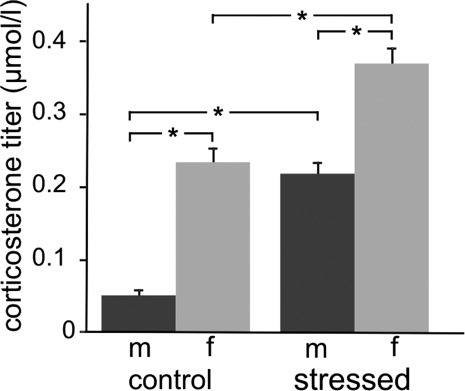
Corticosterone titers of control and stressed male (m) and female (f) rats. Means ± SEM, n = 6 per group. *P < 0.05 between groups.
Neuronal Activity Parameters
PVN
To determine the influence of acute stress on IEG expressions in the PVN, c-Fos and FosB were studied. Immunocytochemistry showed a strong staining of the nuclei of PVN neurons with the c-Fos antiserum (Fig. 2A), and morphometry revealed a main effect of stress on the number these immunoreactive neurons (F1,17 = 17.6, P < 0.001). Post hoc analysis demonstrated a higher number of such neurons after stress in both sexes, 12.8-fold higher in males (stressed 119.2 ± 31.6 vs. controls 9.3 ± 4.0, P < 0.0005) and 5.4-fold higher in females (stressed 62.8 ± 19.9 vs. controls 11.6 ± 4.9, P < 0.0005). The difference between males and females was not statistically significant. A stress effect was also present with regard to FosB immunoreactivity (F1,18 = 13.6, P < 0.005; Fig. 2B), which was due to the fact that stressed females showed more (4.8-fold) immunopositive neurons (29.2 ± 9.7) than control females (6.1 ± 2.5, P < 0.001). For the in situ hybridization of CRF mRNA (Fig. 2C), ANOVA revealed a stress effect for both the number (F1,18 = 4.8, P < 0.05) and the SSD (F1,17 = 5.6, P < 0.05) of CRF mRNA-positive neurons. Post hoc analysis showed that this effect was significant only in males, which, compared with controls, had 2.4-fold more CRF mRNA-containing neurons (stressed 31.5 ± 6.3 vs. controls 13.0 ± 4.5, P < 0.05) and had a 1.6-fold higher SSD (stressed 27.9 ± 3.6 vs. controls 17.2 ± 4.6, P < 0.05). As for CRF immunoreactivity (Fig. 2D), no stress or sex effects were seen. Taken together, c-Fos was increased in both sexes, whereas FosB was induced only in females. In males, the CRF mRNA content was higher after stress.
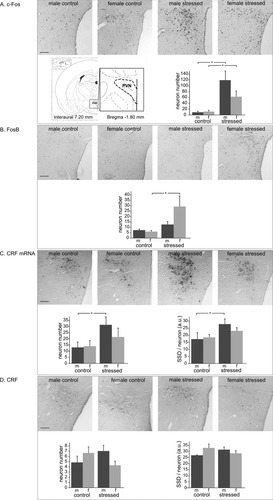
Paraventricular nucleus of the hypothalamus (PVN) in control and stressed male (m) and female (f) rats, with representative images; location (inset; based on Paxinos and Watson (1997); numbers per section of immunopositive neurons of c-Fos (A), FosB (B), CRF mRNA (C), and CRF (D); and specific signal density per neuron (SSD; in arbitrary units, a.u.; C,D). Means ± SEM, n = 6 per group. *P < 0.05. Scale bars = 100 μm.
BSTov
Immunocytochemistry of c-Fos (Fig. 3A) showed an effect of stress (F1,16 = 6.7, P < 0.05), and post hoc analysis revealed that stressed females had 2.6-fold more c-Fos-positive neurons than female controls (stressed 7.7 ± 1.5 vs. controls 3.0 ± 0.1, P < 0.01), whereas males did not show a significant stress effect. Stressed females had twice as many immunoreactive neurons as males (females 7.7 ± 1.5, males 3.9 ± 1.0, P < 0.05). As for immunocytochemistry of FosB (Fig. 3B), no differences between stressed and control animals or between sexes were found. The in situ hybridization study revealed that BSTov neurons were strongly positive for CRF mRNA (Fig. 3C), but no effect of stress or sex on their number was seen. In contrast, clear stress and sex effects were observed for the SSD of the CRF mRNA in individual neurons (F1,17 = 5.2, P < 0.05 and F1,17 = 4.8, P < 0.05, respectively). Post hoc analysis showed that stressed males had a 2.7-fold higher SSD than male controls (stressed 16.2 ± 3.5 vs. controls 6.0 ± 2.7, P < 0.05), whereas females differed from males in that they did not show a significant stress effect. The numbers of CRF-immunoreactive cells and the SSD of the individual CRF staining did not reveal significant stress or sex effects (Fig. 3D). The same held true for the SSD of the CRF immunoreactivity of the fiber network in the BSTov. Taken together, acute stress recruited more c-Fos-positive neurons in females and increased CRF mRNA content in males.
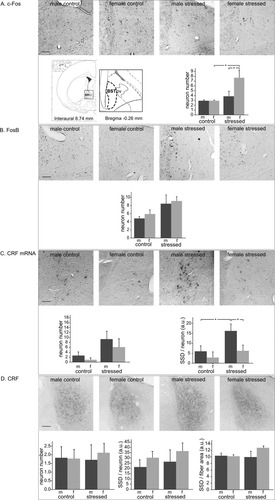
Oval bed nucleus of the stria terminalis (BSTov) in control and stressed male (m) and female (f) rats, with representative images; location (inset); numbers per section of immunopositive neurons of c-Fos (A), FosB (B), CRF mRNA (C), and CRF (D); and specific signal density (SSD; in arbitrary units, a.u.) per neuron (C,D) or fiber area (D). Means ± SEM, n = 6 per group. *P < 0.05. Scale bars = 50 μm.
BSTfu
In the BSTfu of control animals, no c-Fos-positive cells were detected (Fig. 4A). However, after stress, a high number of positive cells occurred, and the ANOVA revealed a clear stress effect (F1,17 = 34.1, P < 0.00005), which was not sex-specific because the numbers of c-Fos-positive cells were similar in males (22.7 ± 5.5) and females (17.0 ± 3.7). Also for FosB immunocytochemistry, an effect of stress was present (F1,18 = 47.9, P < 0.000005; Fig. 4B). Stressed males had 3.1-fold more FosB-positive cells than controls (stressed 6.0 ± 0.5 vs. controls 1.9 ± 0.8, P < 0.001), and a similar increase (3.7-fold) was seen in females (stressed 7.5 ± 0.6 vs. controls 2.0 ± 0.7, P < 0.00005). In situ hybridization showed a low number of CRF mRNA-containing neurons in controls, and no stress or sex effects were observed. Similarly, the SSD of CRF mRNA did not differ among groups (Fig. 4C). CRF immunoreactivity was only seen in fibers (Fig. 4D), and the SSD of CRF immunoreactivity was not significantly altered by stress. However, a sex effect was obvious (F1,16 = 7.8, P < 0.05), because post hoc analysis showed that in control females these fibers were more strongly stained than in control males (SSD females 2.19 ± 0.76 vs. SSD males 0.04 ± 0.04, P < 0.01). In conclusion, in both sexes, c-Fos and FosB were increased upon stress exposure, and there was a sex difference in the amount of CRF in fibers in nonstressed animals.
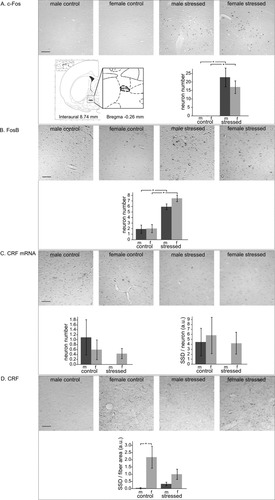
Fusiform bed nucleus of the stria terminalis (BSTfu) in control and stressed male (m) and female (f) rats, with representative images; location (inset); numbers per section of immunopositive neurons of c-Fos (A), FosB (B), CRF mRNA (C), and CRF (D); and specific signal density (SSD; in arbitrary units, a.u.) per neuron (C) or fiber area (D). Means ± SEM, n = 6 per group. *P < 0.05. Scale bars = 50 μm.
CeA
With immunocytochemistry, no c-Fos-positive cells were observed in the CeA of controls (Fig. 5A). In stressed animals, however, CeA neurons were recruited and a stress effect was obvious (F1,18 = 46.3, P < 0.00001), with similarly higher numbers of immunoreactive neuronal nuclei in males (17.0 ± 1.5) and in females (14.3 ± 4.0). No stress or sex effects were seen for FosB immunoreactivity (Fig. 5B). With in situ hybridization, control males showed a very low number of CRF mRNA-containing cells, whereas such neurons were clearly absent from females (Fig. 5C). Stressed animals, however, had very high numbers of CRF mRNA-positive neurons (males 20.8 ± 3.6, females 15.9 ± 2.2), and ANOVA revealed a clear stress effect (F1,14 = 34.1, P < 0.00005) but no difference between males and females (F1,14 = 2.9, P < 0.11). For the SSD of CRF mRNA, the ANOVA showed effects of stress (F1,14 = 25.0, P < 0.0005) and sex (F1,14 = 4.9, P < 0.05). Compared with controls, in both stressed males (stressed 16.7 ± 2.1 vs. controls 6.5 ± 3.8, P < 0.01) and stressed females (stressed 13.0 ± 1.8 vs. controls 0.0 ± 0.0, P < 0.005) the SSD was clearly higher. CRF-immunopositive neurons occurred in all experimental groups (Fig. 5D), but significant stress or sex effects on their numbers were not found. As for the SSD, a stress effect (ANOVA F1,18 = 14.4, P < 0.005) was observed only in males (stressed 37.6 ± 1.6 vs. controls 30.0 ± 1.0, P < 0.005). With the CRF staining intensity of the fibers in the CeA, a stress effect (ANOVA F1,18 = 9.7, P < 0.01) was seen in males, which had a 1.4-fold higher SSD than controls (stressed 15.4 ± 1.4 vs. controls 10.9 ± 0.3, P < 0.0005). In summary, in both males and females, c-Fos and CRF mRNA contents were increased upon stress exposure, whereas stress resulted in more CRF peptide in males only.
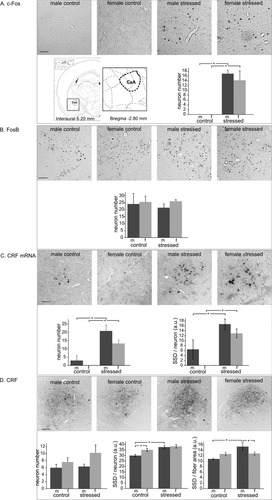
Central amygdala (CeA) in control and stressed male (m) and female (f) rats, with representative images; location (inset); numbers per section of immunopositive neurons of c-Fos (A), FosB (B), CRF mRNA (C), and CRF (D); and specific signal density (SSD; in arbitrary units, a.u.) per neuron (C,D) or fiber area (D). Means ± SEM, n = 6 per group. *P < 0.05. Scale bars = 50 μm
Epigenetic Activity Parameters
PVN
To assess whether the acute restraint stressor would act via an epigenetic mechanism, enzymes involved in histone acetylation and deacetylation were studied by Q-RT-PCR. Clear Q-RT-PCR signals were found for the mRNA amounts of HDAC 3, 4, and 5 and of PCAF, but the ANOVA did not show effects of stress or sex (Table III). For CBP mRNA, however, a stress effect (F1,19 = 6.8, P < 0.05) and a sex effect (F1,19 = 7.0, P < 0.05) were found. Post hoc analysis showed that stressed males had 1.5-fold more CBP mRNA than control males (stressed 1.16 ± 0.11 vs. controls 0.78 ± 0.18, P < 0.05). Interestingly, females did not show a significant stress effect.
| Brain centre | Enzyme | Male control | Male stressed | Female control | Female stressed |
|---|---|---|---|---|---|
| PVN | HDAC 3 | 1.67 ± 0.19 | 2.05 ± 0.42 | 2.07 ± 0.35 | 3.39 ± 1.15 |
| HDAC 4 | 2.06 ± 0.27 | 3.45 ± 0.87 | 2.34 ± 0.38 | 6.25 ± 2.43 | |
| HDAC 5 | 1.41 ± 0.17 | 2.15 ± 0.27 | 1.85 ± 0.21 | 3.22 ± 0.91 | |
| CBP | 0.78 ± 0.18a,* | 1.16 ± 0.11 | 0.54 ± 0.02a* | 0.78 ± 0.14a* | |
| PCAF | 0.79 ± 0.08 | 1.07 ± 0.12 | 0.82 ± 0.14 | 0.96 ± 0.18 | |
| BST | HDAC 3 | 1.61 ± 0.10 | 1.51 ± 0.23 | 1.36 ± 0.16 | 1.28 ± 0.26 |
| HDAC 4 | 5.15 ± 1.12 | 4.14 ± 0.49 | 4.12 ± 0.64 | 6.77 ± 1.68 | |
| HDAC 5 | 1.49 ± 0.17 | 1.52 ± 0.16 | 1.30 ± 0.10 | 1.46 ± 0.26 | |
| CBP | 0.63 ± 0.13 | 0.74 ± 0.21 | 0.47 ± 0.05 | 0.61 ± 0.10 | |
| PCAF | 0.52 ± 0.09 | 0.54 ± 0.11 | 0.43 ± 0.05 | 0.41 ± 0.06 | |
| CeA | HDAC 3 | 1.68 ± 0.23 | 1.54 ± .029 | 1.85 ± 0.19 | 1.58 ± 0.24 |
| HDAC 4 | 1.62 ± 0.20 | 1.65 ± 0.09 | 1.72 ± 0.06 | 2.27 ± 0.47 | |
| HDAC 5 | 1.57 ± 0.21 | 1.23 ± 0.21 | 2.26 ± 0.58 | 1.43 ± 0.12 | |
| CBP | 0.76 ± 0.12 | 1.12 ± 0.23 | 0.65 ± 0.06 | 0.90 ± 0.20 | |
| PCAF | 0.92 ± 0.14 | 0.75 ± 0.19 | 0.50 ± 0.07b* | 0.57 ± 0.04 |
- † Means ± SEM, n = 6.
- * P < 0.05; a: significant with stressed males, b: significant with control males.
BST
The Q-RT-PCR data regard the whole BST. We did not find any stress or sex effect for any of the epigenetic enzyme mRNAs studied (Table III).
CeA
No differences were found in the expression of HDACs, PCAF, and CBP after acute stress. However, PCAF showed a clear sex effect (F1,19 = 6.0, P < 0.05), because control males had more mRNA than control females (males 0.9 ± 0.1 vs. females 0.5 ± 0.1; P < 0.05; Table III).
DISCUSSION
It is well established that chronic stress activates the HPA axis and associated brain centers involved in the stress response in a sex-specific way (Duncko et al.,2001; Dalla et al.,2005; Goel and Bale,2010). In contrast, evidence for such an action of acute stress is scarce and largely limited to the PVN (Lesniewska et al.,1990; Iwasaki-Sekino et al.,2009). In addition, little is known about the possible sex-specific cellular responses to acute stress of stress regulatory centers such as the BSTov, BSTfu, and CeA. The present study is the first to support the hypothesis that an acute stressor can sex-specifically evoke a stress response in the rat brain, by changing the expression of CRF and IEGs, as well as the dynamics of production and sequestering of CRF peptide, by the PVN, BSTov/BSTfu, and CeA. Interestingly, our data point to the possibility that stress-induced changes in gene expression in the PVN could be partially mediated by epigenetic mechanisms. The results show that all these nuclei react to an acute restraint stress with clear cellular responses, and these responses are sex-specific in the PVN and the BSTov. These conclusions are discussed below.
Significance of Parameters
We have studied various parameters to obtain an impression of the differential involvement of IEGs in the neuronal response to an acute stressor (Imaki et al.,1996); two IEGs have been investigated. Among these, c-Fos is generally induced more rapidly and more transiently by stress than FosB. Therefore, by measuring both IEGs instead of only one, differential aspects of IEG induction can be investigated (Perrotti et al.,2004). Upon IEG induction, sets of other genes become expressed, including those involved in neuronal secretory activity. We have focused on the expression of the main stress neuropeptide gene, CRF, using in situ hybridization. Finally, to obtain an impression of stress-induced CRF peptide dynamics, we assessed the amount of stored CRF peptide by immunocytochemistry, at the level of a nucleus (number of immunopositive neurons) as well as at the level of individual neurons (SSD). The CRF mRNA and peptide dynamics were studied 2 hr after the start of the stress exposure. As reviewed by Watts (2005), already about 1 hr after stress new CRF mRNA has been transcribed in the PVN, and 30 min later the newly translated CRF peptide is packed into secretory vesicles to be released from the axon terminals 30 min later. This time course means that in our study, upon acute stress, newly synthesized CRF could already have been exported from the cell bodies to the axon terminals to be released. This notion underscores the relevance of the 2-hr sample point and the parameters used to assess CRF transcription and CRF translation, storage, and release. As for the latter process, our measurements do not give direct information about the secretion rate of CRF. However, the rate of CRF secretion can be estimated by relating the degree of CRF mRNA (measure for CRF production) to the degree CRF immunoreactivity (measure for CRF storage).
Below we interrelate the outcomes of all parameters studied, to obtain an impression of the series of events that takes place in a brain nucleus upon acute stress exposure, from IEG induction, via CRF production and storage, to CRF secretion. In this respect, it should be considered that, in the PVN, c-Fos data can be directly related to the CRF mRNA and CRF peptide data, because stress-induced c-Fos strongly colocalizes with CRF in PVN neurons (Loughlin et al.,2006). In the BST and CeA, however, c-Fos induction upon stress occurs mainly in non-CRF, enkephalin-producing neurons (Day et al.,1999; Loughlin et al.,2006), so our Fos data provide information on acute stress-induced neuronal responses in these nuclei.
The PVN
The PVN is a main player in the HPA axis response to acute stress in males (Chowdhury et al.,2000; Figueiredo et al.,2002; Ito et al.,2009) as well as in females (Figueiredo et al.,2002). For males, we found a stress-induced activation of the HPA axis as evidenced by a strong increase in CORT titer. This stress-induced activation is also reflected at the level of PVN neurons by a strong increase in the number of neurons revealing c-Fos and by their increased CRF mRNA content. This result indicates that restraint stress induces the expression of IEGs and CRF to increase the neuron's capacity to produce CRF peptide. Stress-induced activation of the gene expression machinery is also indicated by the finding of stress-induced increase in the amount of CBP, suggesting the involvement of an epigenetic process (see also below).
Previously, it was found that acute stress does not change CRF immunoreactivity in the PVN (Chappell et al.,1986), and this result has been confirmed in the present study. When we combine this observation with the increase in CRF mRNA found in male rats, it appears likely that in activated PVN neurons CRF production and CRF export from the cell body occur with similarly increased speeds, leaving the net amount of CRF inside the cell body unchanged. Increased transport of CRF toward the axons would imply that restraint stress stimulates CRF secretion. This notion is supported by the fact that acute stress exposure increases CRF release in the PVN in the sheep (Cook,2004). An acute stress-induced release of CRF is also in line with the observed increase in CORT release in males. In the female PVN, the stress-induced increase in c-Fos is less strong (7-fold) than that in males (14-fold), and, in contrast to the case in males, no effect of the stressor on neuronal CRF mRNA content was seen. This suggests that the CRF neurons in the PVN of females are less strongly stimulated than in males, leading to less stimulation of CRF release. This sex difference is reflected by the smaller CORT change in stressed females (1.6-fold increase) than in stressed males (4.2-fold increase; see also Belz et al.,2003).
In contrast to our results with restraint stress, foot shock does increase the amount of CRF mRNA in the female rat PVN (Iwasaki-Sekino et al.,2009). Therefore, the sex-specific response of the rodent PVN to acute stress appears to be stressor-specific.
The BSTov
The BSTov is well known for its role in the regulation of mood (Walker et al.,2009; Davis et al.,2010; Regev et al.,2010), but it may also play a role in the stress response (Martinez et al.,1998; Walker et al.,2003; Herman et al.,2005; Choi et al.,2007). This idea is supported by the present study, which reveals clear, sex-specific effects of restraint stress on IEGs and on CRF dynamics in this nucleus. Upon stress, males did not show a stress effect on IEGs, but their CRF mRNA content was increased. Stress did not significantly change the amount of CRF stored in neurons, so, following the argumentation given above for CRF dynamics in the PVN, restraint stress might stimulate CRF release from the BSTov after equally stimulating CRF production and CRF export from the cell body, leaving the net amount of CRF stored in the neuron constant. Interestingly, females revealed a completely different picture; the parameters for CRF dynamics (CRF mRNA and CRF peptide contents) did not show a significant stress effect, but c-Fos increased. This might mean that in the BSTov not CRF but other genes downstream of c-Fos are activated by this stressor. Indeed, it was shown in the BST that c-Fos was colocalized not with CRF after acute stress but with met-enkephalin (Day et al.,1999; Kozicz,2002). In this respect, it is also interesting that the BSTov is larger in males than in females (Zhou et al.,1995) and contains a dense network of axon terminals immunoreactive for vasoactive intestinal polypeptide that reveal clear sexual dimorphism (Zhou et al.,1995). Moreover, CRF neurons in the BSTov receive input from fibers immunoreactive to pituitary adenylate cyclase-activating polypeptide (PACAP; Kozicz et al.,1997) and express the PAC1 receptor (Vaudry et al.,2009). Interestingly, polymorphisms in the PACAP and PAC1 receptor gene are associated with increased vulnerability to PTSD in females but not in males (Ressler et al.,2011). Overall, we suggest that a sexual dimorphism in VIP/PACAP in the BSTov could play a role in the presently demonstrated sex-dependent response of CRF neurons in this nucleus to acute stress and, consequently, in stress-induced disorders such as PTSD.
The BSTfu
In addition to the BSTov, the BSTfu also seems to play a role in the stress response, insofar as it contains CRF (Phelix and Paull,1990; Kozicz et al.,1997; Kozicz and Arimura,2001) and has strong projections to both the PVN and the CeA (Dong et al.,2001), and its lesioning attenuates the stress-induced corticosterone response (Choi et al.,2007). Here, we present circumstantial evidence for a role of the BSTfu in the acute stress response; we found that restraint stress increases the amounts of c-Fos and FosB. However, no stress effects on CRF dynamics, at either the mRNA or the peptide level, were seen. This suggests that the IEG responses concern non-CRF neurons, which is in accordance with the almost complete absence of coexistence of c-Fos and CRF in this nucleus (Day et al.,1999). In this respect it is relevant that the BSTfu contains glutamatergic neurons (Csaki et al.,2000; Forray and Gysling,2004) and met-enkephalin-producing neurons (Kozicz,2002), which might well be involved in a BSTfu stress response.
Finally, we have found a clear sex dependence for the CRF immunoreactivity of fibers in this nucleus, which was stronger in females than in males. Functional interpretation of this result in terms of regulation of CRF neuronal activity in the BSTfu awaits identification of the origin of these fibers.
The CeA
Until now, stimulation of the CeA by an acute stressor (single defeat: Martinez et al.,1998; restraint stress: Dayas et al.,2001; Ito et al.,2009) had been described only for male rats. We show that restraint stress also activates the CeA in female rats, as appears from the stress-induced increase in numbers of c-Fos and CRF mRNA-containing neurons. The neuronal storage of CRF was only slightly (males) or was not (females) increased upon stress, so CRF release seems to be increased at the same rate as CRF production. Because stress exposure induces c-Fos not in CRF-containing neurons but in enkephalin-containing cells in the CeA (Day et al.,1999; Loughlin et al.,2006), the present increase in the number of c-Fos-positive neurons may concern a non-CRF neuronal population in this nucleus. We have found a clear stimulatory effect of stress on CRF mRNA, indicating that restraint stress does lead to a secretory response by the CRF neurons. In contrast to the BSTov, this response can be observed in both males and females, indicating the absence of sex dependence. Finally, the functional significance of the sex-related difference in the CRF staining intensity of the CeA fiber network, along with the question of whether these fibers belong to CRF cell bodies in the CeA or are afferents from CRF neurons running to other brain areas (e.g. the BSTov), deserves future attention.
Epigenetic Processes
We have sought to provide the first evidence that epigenetic mechanism(s) may be involved in the mediation of acute stress-induced changes in neuronal activity and, in particular, in gene expression. We found an indication for such an involvement in the PVN. It appears that restraint stress induces the expression of the CBP gene in this nucleus. CBP is assumed to be involved in long-term epigenetic changes in gene expression (Wang et al.,2010). Consequently, the observed stress-induced change in CBP mRNA in the PVN might be an early sign of major changes (reprogramming) in the stress adaptation system that could account for a failure to maintain homeostatic equilibrium in stress. In this view, the increase in CBP mRNA might allow increased histone acetylation and, consequently, induce the CRF mRNA transcription that we observed in the male rat PVN upon restraint stress. These increases in CBP and CRF mRNAs were not seen in female rats, so our finding is a first indication that epigenetic changes can mediate acute stress-induced gene expressions in the PVN that are sex-specific.
It should, however, be considered that there are other, nonepigenetic mechanisms by which CBP can control transcription, such as by binding to the transcriptional machinery (Karamouzis et al.,2007). Therefore, further experiments are needed to conclude definitely that acute stress acts via histone acetylation on CRF transcription. Such experiments might concern the determination of the effect of acute stress on the degree of acetylation of CRF and on the amount of CBP bound to this gene. The absence of any stress effect on epigenetic enzyme mRNAs in the other brain centers studied suggests that acute restraint stress does not recruit epigenetic mechanisms.
CONCLUSIONS
We have supported our hypothesis that an acute stressor can sex-specifically evoke stress response (CORT) and induce changes in the expression of CRF and IEGs as well as the dynamics of CRF peptide in the PVN, BSTov, BSTfu, and CeA. We have also shown that acute stress may recruit epigenetic mechanisms involving histone acetylation in the PVN. Finally, we have provided evidence that all centers differ from each other in one or more aspects of their stress response (amounts of IEGs and/or CRF mRNA and CRF peptide). Taken together, these results support the idea that each of the brain centers responds to the acute stress in its own distinct fashion and that, in this way, these brain areas control/modify, in a coordinated manner, the animal's stress response. This would allow mental and body responses necessary for successful stress adaptation. Apparently, males and females differ from each other in some components of this response, particularly at the level of the PVN and BSTov. Such differences may contribute to the sex-specificity of the animal's physiological and behavioral responses to an acute stressor.
Our data also provide initial evidence that sex-specific response(s) to acute stress may be mediated, at least in the PVN, by an epigenetic mechanism. Because epigenetic changes in response to significant life events might underlie the development of brain diseases such as PTSD (Yehuda and Bierer,2009), which occurs more frequently in females than in males (Gill et al.,2005), the present data may provide a basis for understanding how a strong, acute psychogenic stressor would evoke epigenetic changes contributing to the pathogenesis of such diseases.




