Lack of aspartoacylase activity disrupts survival and differentiation of neural progenitors and oligodendrocytes in a mouse model of Canavan disease
Abstract
Loss of the oligodendrocyte (OL)-specific enzyme aspartoacylase (ASPA) from gene mutation results in the sponginess and loss of white matter (WM) in Canavan disease (CD). This study addresses the fate of OLs during the pathophysiology of CD in an adult ASPA knockout (KO) mouse strain. Massive arrays of neural stem/progenitor cells, immunopositive for PSA-NCAM, nestin, vimentin, and NG2, were observed within the severely affected spongy WM of the KO mouse brain. In these mice, G1→S cell cycle progression was confirmed by an increase in cdk2-kinase activity, a reduction in mitotic inhibitors p21Cip1 and p27Kip1, and an increase in bromodeoxyuridine (BrdU) incorporation. Highly acetylated nuclear histones H2B and H3 were detected in adult KO mouse WM, suggesting the existence of noncompact chromatin as seen during early development. Costaining for BrdU- or Ki67-positive cells with markers for neural progenitors confirmed a continuous generation of OL lineage cells in KO WM. We observed a severe reduction in 21.5- and 18.5-kDa myelin basic protein and PLP/DM20 proteolipid proteins combined with a decrease in myelinated fibers and a perinuclear retention of myelin protein staining, indicating impairment in protein trafficking. Death of OLs, neurons, and astrocytes was identified in every region of the KO brain. Immature OLs constituted the largest population of dying cells, particularly in WM. We also report an early expression of full-length ASPA mRNA in normal mouse brain at embryonic day 12.5, when OL progenitors first appear during development. These findings support involvement of ASPA in CNS development and function. © 2009 Wiley-Liss, Inc.
During development, oligodendrocyte (OL) progenitors (OLPs) undergo a series of highly regulated processes involving extensive migration, proliferation, and differentiation before emerging as mature myelinating cells (Pfeiffer et al., 1993). Several studies have also confirmed proliferation of mitotic OLPs in the normal adult human and rodents as an endogenous source of cells for myelin repair (Tramontin et al., 2003; Nait-Oumesmar et al., 2008). Although proliferation of OLPs occurs widely in CNS and in lesions of multiple sclerosis, the OLPs fail to differentiate (Scolding et al., 1998). These OLPs undergo apoptosis, probably as a result of conditions described as inflammation or genetic or epigenetic modifications. To date, the potential affect of ASPA mutation on OL proliferation, survival, and maturation remains poorly understood in the childhood leukodystrophy model of Canavan disease (CD). Recent studies have identified OLs to be the site of expression and function of ASPA that hydrolyzes N-acetyl-L-aspartate to release acetate, an important component of certain fatty acids in myelin. Early observations led to the association of CD with a progressive destruction of the myelin sheath and the loss of white matter (WM) tracts as a result of dysfunction in ASPA enzyme and a buildup of its substrate, NAA, in plasma and urine (D'Adamo et al., 1973; Kaul et al., 1991). The buildup of NAA is described to act as a water pump, whereby NAA can sequester water molecules in the region of degenerated WM tissue (Baslow, 2003), to give rise to the spongy morphology seen in the WM of CD patients and in the ASPA knockout mouse brain (for review see Kumar et al., 2006a).
The cellular localization of NAA and ASPA enzyme in the CNS has been a focus of investigation. Although NAA is produced and is localized primarily in neurons (Moffett et al., 1991), a high concentration of NAA was also reported in primary cultures of bipolar OLPs and immature OLs. However, NAA was not detected in mature OLs or astrocytes, which suggests a role for ASPA in immature OLs (Urenjak et al., 1992; Bhakoo and Pearce, 2000). In rat cortical cultures, the presence of ASPA enzyme activity (Baslow et al., 1999; Bhakoo et al., 2001; Kumar et al., 2006b) and expression of ASPA mRNA as well as ASPA protein have been reported in nonmyelinating OLS (Kumar et al., 2006b). However, ASPA activity continues to be present in adultmyelin. Ledeen and his coworkers reported an association of a high concentration of ASPA enzyme activity within the myelin subfraction of cerebral hemispheres and brainstem of normal rat. Although a direct uptake of NAA by OL has not been reported, axonal transport of [14C]NAA following its intraocular injection and incorporation into myelin lipid was demonstrated (Chakraborty et al., 2001). These observations would suggest impairment in myelin maintenance in the absence of NAA-derived acetate in adults. However, the consequences of developmental absence of ASPA on myelin synthesis and maturation of OLs remain to be elucidated.
During our initial stages of investigation, we observed the presence of highly proliferating neural stem progenitor cells in the adult ASPA mutant mouse brain. The presence of undifferentiated progenitors during CD pathophysiology suggests alteration in cell cycle regulation. Furthermore, a nuclear localization of ASPA has been observed, in addition to the cytoplasm (Madhavarao et al., 2004; Hershfield et al., 2006), and ASPA enzyme is a deacetylase. In this context, histone acetylation is involved in chromatin regulation for its role in DNA replication and transcription. Although acetylated histone exists in OLPs, differentiation of OLs critically depends on deacetylation (Marin-Husstege et al., 2002). Here we report on novel findings about the fate of neural progenitors and OLs in the pathophysiology of CD.
MATERIALS AND METHODS
Immunohistochemical Analysis of ASPA and Expression of Cell-Specific Markers
All animal handling and treatments were performed according to the regulations of the University of California Animal Research Committee. Six-month-old mice (referred to as adult throughout the study) from ASPA knockout strain (–/–; Matalon et al., 2000) and age-matched WT (+/+) as controls were used as described for each experiment. The animals were anesthetized and perfused with PBS, followed by fresh 4% paraformaldehyde. Brains were dissected and postfixed in the same fixative overnight at 4°C and cryoprotected in 20% sucrose. ASPA WT and KO brain tissue sections were processed for immunocytochemistry as described by Kumar et al. (2007). Antibodies against early OLPs, mature OLs, and astrocytes and neurons were used overnight at 4°C at predetermined dilutions. Antibodies against cell-specific markers for early progenitors and immature and mature OLs were as follows: mouse anti-PSA-NCAM (1:100; Chemicon, Temecula, CA); mouse antinestin (1:200; Chemicon); mouse antivimentin (1:100; Lab Vision; clone V9); mouse anti-Nkx2.2 (1:50; Developmental Studies Hybridoma Bank); rabbit anti-NG2 (1:150; Chemicon); mouse anti-GD3 (1:200; Calbiochem, La Jolla, CA); goat anti-Olig 2 (1:200; Santa Cruz Biotechnology, Santa Cruz, CA); rabbit anti-CNP (1:200; SMI91; Covance, Berkeley, CA); rabbit anti-MBP (1:200; Chemicon); mouse anti-PLP/DM20 (1:200; Chemicon); rabbit anti-GSTpi (1:800; Stressgen, Victoria, British Columbia, Canada); and rabbit anti-ASPA (1:500; Dr. R. Matalon; Surendran et al., 2005); for an astrocyte marker rabbit anti-GFAP (1:500; Chemicon), for a neuronal marker mouse anti-NeuN (1:200; Chemicon), and for cell proliferation mouse anti-BrdU (1:200; Dako, Carpinteria, CA) and mouse anti-Ki67 (1:250; Novacostra, Boston, MA) were used. For detection of immunofluorescence, incubations were carried out with appropriate secondary antibody labeled with Alexa green 488 (Molecular Probes, Eugene, OR) and Cy3 (Sigma, St. Louis, MO). The probed tissue sections were viewed on an Olympus Provis microscope equipped with Hamamatsu C7780 camera or by a Zeiss Confocal Laser Microscope 510 Meta.
BrdU Incorporation in Mouse Brain
Two intraperitoneal injections of 75 mg/kg body weight of BrdU (Roche, Germany) were performed in mice 6 hr apart. Eighteen hours following the last BrdU administration, the brain was fixed, cryoprotected, and processed for sectioning as described above. Antigen retrieval was carried out for 30 min with 2 N HCl and neutralized with sodium borate buffer, pH 8.5, before processing for BrdU immunostaining in a manner similar to that described above.
TUNEL Assay for Detection of In Situ Apoptotic Cell Death
Tissue sections used for the study were prepared as described above. Apoptosis assay was performed by labeling 3′-end nicked genomic DNA with terminal deoxytransferase and incorporation of fluorescence-labeled deoxynucleotide using a kit (BD Biosciences, Mountain View, CA) by the accompanying protocol. For combination of TUNEL and immunostaining, TUNEL was performed first, followed by the antibody incubation as described above.
Nuclear Histone Extraction and Western Blotting
The region of cortical WM tissue containing corpus callosum/lateral ventricle was cut out under dissection microscope from adult WT and KO brain, and nuclear extracts were prepared from fresh tissue using the nuclear extract kit from Active Motif (Carlsbad, CA). Thirty micrograms of the nuclear extract was immunoprecipitated with antiacetyl lysine antibody (Upstate Biotechnology, Lake Placid, NY) at 4°C as previously described (Marin-Husstege et al., 2002). Immunoprecipitates were separated by electrophoresis on SDS-PAGE, and the protein blots were probed with antibodies against histone H2A (H-124), H2B (FL126), H3 (FL-136), H4 (F-9; Santa Cruz Biotechnology) and actin (Sigma). The acetylated histone bands were detected by chemiluminescence reagent (Santa Cruz Biotechnology), and band density was normalized to actin and data quantified.
Detection of cdk2 Activity in Brain Tissue
The cyclin-dependent kinase 2-associated kinase activity was determined in cerebral WM tissue lysate prepared in a buffer containing 50 mM HEPES, pH 7.4, 250 mM NaCl, 5 mM EDTA, 0.5% NP-40, 10% Na4P2O4, 1 mM Na3VO4, 4 μM NaF, and protease inhibitor cocktail (Sigma) as described previously (Ghiani and Gallo, 2001). Tissue lysate containing 50 μg protein was immunoprecipitated with 10 μl (2 μg) rabbit polyclonal antibody against cdk2 (M2; Santa Cruz Biotechnology) in 1 ml for 2 hr at 4°C, and cdk2 antigen-antibody complex was incubated with 20 μl protein A-agarose beads (Santa Cruz Biotechnology) for 1 hr. The beads were washed three times in cell lysis buffer and twice with kinase assay buffer (50 mM HEPES, pH 7.4, 50 mM MgCl2, and 1 mM DTT), and the immune complexes bound to beads were collected by centrifugation. The kinase reaction was carried out at 30°C for 30 min in a 20 μl volume, containing beads in 1× kinase buffer, 2 μg histone H1 (Upstate Biotechnology) as substrate, 20 μM ATP, and 2 μCi [γ-32P]ATP (Dupont NEN, Boston, MA). The reaction was stopped by addition of 20 μl 2× SDS-sample buffer and heating at 95°C for 3 min. The proteins were separated by electrophoresis on a 4–20% SDS gel and visualized by autoradiography. To detect cell cycle inhibitors, Western blots of tissue lysates were probed with rabbit anti-p21Cip1 (C-19; 1:200) and mouse anti-p27Kip1 (F-8; 1:200; Santa Cruz Biotechnology).
Data Analysis
For quantitative analysis of proliferation and cell death, BrdU+ or TUNEL+ cells costaining for cell-specific markers were counted randomly in 20 fields in the region of corpus callosum, rostral migratory stream, and lower cortex, and the average cell count was reported. Data are presented as means ± SD and analyzed by t-test for independent samples or two-way ANOVA followed by Tukey post hoc test. P < 0.05 was taken to indicate statistically significant differences between WT and KO samples.
RNA Preparation and Detection of Myelin Gene Expression and Transcription Factors by RT-PCR
For detection of PLP/DM20 and MBP gene expressions, total RNA was isolated from adult WT and ASPA KO cortex by RNeasy Lipid Tissue Mini Kit (Qiagen, Valencia, CA). For detection of full-length ASPA gene expression, RNA from embryo (E12.5–18.5) brain and postnatal cortical tissues (P0 and P30) were used. First-strand synthesis was performed using DNase-treated total RNA template and oligo(dT)18 primer with M-MuLV reverse transcriptase (Ambion, Austin, TX). DNase treatment was carried out using a Turbo DNA-Free Kit (Ambion), followed by column purification of RNA with an RNeasy Kit (Qiagen) as per the manufacturer's protocol. Corresponding cDNAs were PCR amplified using the following primers: ASPA full length (939 bp), forward 5′-ATGACCTCTTGTGTTGCTAAAG-3′ and reverse 5′-TTAGTG CAAAGTGGAGCG-3′, tm 56°; GAPDH, 132 bp, forward 5′-AACTTTGGCATTGTGGAAGG-3′ and reverse 5′-GGA TGCAGGGATGATGTTCT-3′, tm 60°, PLP/DM20 forward 5′-GCTAATTGAGACCTATTTCTCC-3′ and reverse 5′-AGCAATAAACAGGTGGAAGGTC-3′ (Jalabi et al., 2003); and MBP exons 1–7; forward 5′-CCGGAGGCCTGGATGT GATG-3′ and reverse 5′-GAGGGTACCGCTCTGCGACT-3′ (Mathisen et al., 1993). The band sizes were identified by gel electrophoresis, and the PCR product was ligated into TOPO cloning vector (Invitrogen, Carlsbad, CA). The ligated DNAs were cloned and identified by sequencing.
RESULTS
Early Developmental Expression of ASPA Gene in WT Brains
By utilizing a full-length primer for ASPA, we detected expression of ASPA transcripts by RT-PCR in WT brain during early (E12.5) to late (E18.5) gestation of embryos and in newborns to postnatal day 30 (Fig. 1). The sequencing of PCR fragments confirmed the presence of a full-length ASPA.
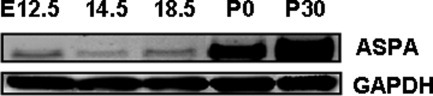
Detection of early developmental expression of full-length ASPA mRNA in WT mouse brain by RT-PCR. Total RNAs from embryonic and postnatal brain tissues were reverse transcribed, and cDNA was PCR amplified with primers for ASPA and a housekeeping gene, GAPDH, as described in Materials and Methods. The amplified DNAs were cloned, and sequence homologies were determined. ASPA gene expression was seen as early as embryonic day 12.5 and continued to increase throughout embryonic to postnatal development.
Status of ASPA in WM Tract of WT and ASPA KO Mice
Because this study focuses on glial cells during pathophysiology of CD, we examined expression of the oligodendroglial cell markers CNP and ASPA in adult ASPA KO, with age-matched WT brains as control. ASPA immunofluorescence colocalizes with CNP in corpus callosum above the lateral ventricle (Fig. 2A,C,E) and cerebellum (Fig. 2B,D,F) WM region at the base of rostral lobules I and II in WT. These structures were not intact in ASPA KO brain because of degeneration of WM (Fig. 2G–J). Although no ASPA immunostaining was detected in the same regions of cerebellum (Fig. 2G) or corpus callosum (Fig. 2I), large numbers of CNP-positive cells were visible within spongy WM (Fig. 2H,J). This demonstrates the presence of OL lineage cells in WM regions during the progression of the disease. This led to further characterization of several early and late markers for OL in ASPA KO brains.
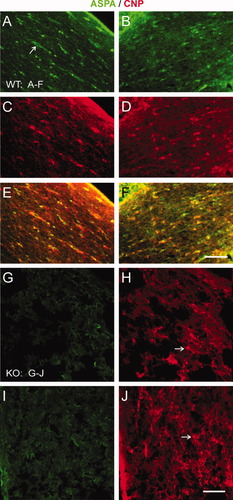
Status of ASPA in WM tracts of adult WT and KO mouse brain. Double immunostaining of ASPA (green; A,B) with an OL marker, CNP (red; C,D), readily colocalizes (E,F) in corpus callosum above the lateral ventricle (A,C,E) and cerebellum at base of rostral lobules I and II (B,D,F) of WT mouse brain. Lack of ASPA immunostaining (G,I) is evident in ASPA KO cerebellum (G) and corpus callosum (I), whereas CNP staining in cerebellum (H, arrow) and in corpus callosum (J) can be seen in these spongy WM tissues. Scale bars = 50 μm in F (applies to A–F); 20 μm in J (applies to G–J).
Presence of Early Neural and OLPs in Adult ASPA KO Brain
Neural cell marker analysis of adult WT mouse brain revealed little or no significant immunostaining for early neural progenitors such as polysialic acid (PSA) linked to neural cell adhesion molecule (NCAM), nestin, or a developmentally regulated intermediate filament protein, vimentin (Fig. 3A,B,E), in the subventricular zone (SVZ). The age-matched ASPA KO tissues revealed an array of immunopositive cells for undifferentiated neural cell markers, PSA-NCAM, nestin (Fig. 3C,D), and vimentin, (Fig. 3F–H). Cells expressing these markers were identified in corpus callosum and SVZ of ASPA KO tissue with spongy morphology, a hallmark of CD as described by Matalon and Michals-Matalon (2000). The expression of vimentin could be seen radiating from the corpus callosum area to the cortex (Fig. 3F) in cells with bipolar morphology as evident at high magnification (Fig. 3H). Immunostaining for NG2 (Fig. 3I), a cell membrane-associated chondroitin sulfate proteoglycan, colocalized with gangliosides GD3 (Fig. 3J) that are found associated with OLs during early development (Ellison and de Vellis, 1995). The presence of numerous cells with properties of neural stem/progenitor cells in the adult KO mouse brain suggested to us the persistence of active mitosis in the SVZ.
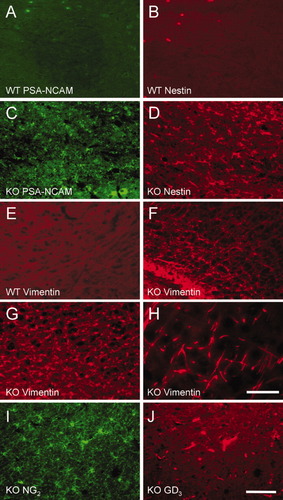
Detection of neural stem/progenitors in ASPA KO adult mouse brain. Only a few PSA-NCAM (A)-, nestin (B)-, and vimentin (E)-immunopositive cells are observed in adult WT corpus callosum and SVZ. However, in the KO brain, significant numbers of PSA-NCAM+ (C)-, nestin+ (D)-, and vimentin (F–H)-immunopositive cells are present in the region of corpus callosum and lower cortex. The densely positive vimentin cells radiate from the corpus callosum to the cortical region (F). Higher magnification of cortical area adjacent to corpus callosum shows a bipolar morphology of vimentin-positive cells (H). Staining of NG2 (I) and GD3 (J) reveals the presence of progenitor OLs in the corpus callosum/cortex of ASPA KO brain (n = 3). Scale bars = 50 μm in J (applies to A–G,I,J); 20 μm in H.
Detection of Active Mitosis in Adult ASPA KO Brain
During proliferation, the binding of the cell cycle regulatory kinase cyclin-dependent kinase 2 (cdk2) with cyclin E increases kinase activity, promoting progenitor proliferation at the G1→S transition phase (Ghiani and Gallo, 2001). Inhibition of cyclin E-Cdk2 complex by the mitotic inhibitor p27Kip1 plays an important role in cessation of OL proliferation and the transition from G1 to G0, leading to its differentiation for myelination (Casaccia-Bonnefil et al., 1997). To ascertain the possibility of active mitosis in the absence of ASPA enzyme, several tests were performed. Cdk2-associated kinase activity was determined in the extracts prepared from cerebral WM of adult KO mice (Fig. 4). Relative to WT, a significant increase in cdk2 kinase activity was evident in the KO mouse tissue. In the KO tissue, cell cycling inhibitors p21Cip1 and p27Kip1 were significantly reduced, signifying impairment in cell cycle arrest. BrdU incorporation study in adult WT and KO mouse brains was carried out to detect cell proliferation. In the WT brain tissue, a few, sparse BrdU+ cells could be detected (Fig. 5A). In contrast, the ASPA KO mouse brain displayed an intense BrdU immunostaining (DAB) in corpus callosum (Fig. 5B), lateral ventricle area (Fig. 5C), rostral migratory stream (Fig. 5D), cortex (Fig. 5E), and cerebellum WM (Fig. 5F). These areas all displayed a distinct spongy structure. These observations are consistent with an increase in proliferation of cells in KO mice.
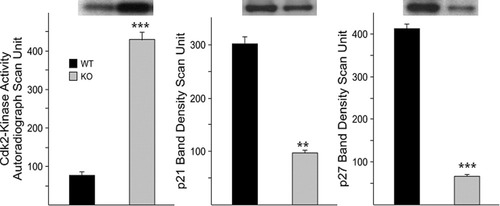
Evidence for failure in cell cycle arrest in ASPA KO cerebral WM tissue. Cdk2-associated kinase activity was determined in anti-cdk2 antibody immunoprecipitates of tissue lysate from WT and KO mice, in the presence of [γ32P]ATP and Histone H1 as substrate. Relative to WT, the kinase activity is highly significant (P < 0.0001) in the KO tissue. Western blots of WM tissue lysate probed with antibody against p21Cip1 and p27Kip1 mitotic inhibitors revealed a significant reduction in p21Cip1 (**P < 0.005) and p27Kip1 (***P < 0.0001) in the KO (n = 4).
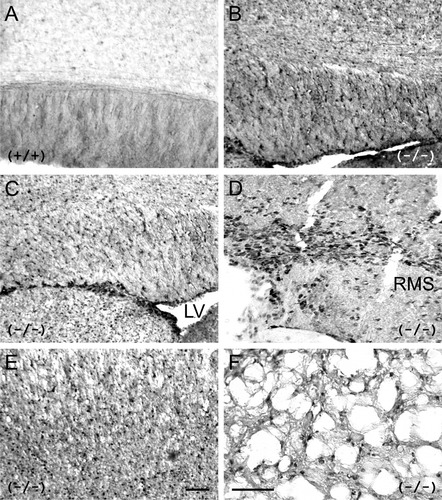
BrdU incorporation and DNA synthesis in WT and ASPA KO mouse brain. Immunostaining of BrdU-injected adult WT mice brain tissue shows a few BrdU-positive cells in the WT (A), and a significant number of BrdU-positive cells can be seen in different regions of age-matched ASPA KO brain, such as corpus callosum (B), lateral ventricle area (LV; C), rostral migratory stream (RMS; D), cortex (E), and cerebellum with the spongy WM (F; n = 3). Scale bars = 10 μm in E (applies to A–E); 20 μm in F.
Cell-Specific Distribution of Proliferating Cells in ASPA KO Brain
Increased proliferation and presence of progenitors in the SVZ of the mutant mouse brain prompted us to examine the specific lineage distribution of proliferating cell types. Coimmunostaining of cell proliferation markers BrdU and Ki67 with Olig2 and NG2 revealed proliferation of OLs predominantly in the rostral migratory stream and corpus callosum (Fig. 6A–C). In view of the presence of stem cell-like progenitors in adult KO mouse brains, we examined SVZ-derived cells for GFAP and proliferation markers. This was of importance based on the finding that adult SVZ-derived GFAP+ multipotent progenitors exist and can participate in neurogenesis in the adult brain (Garcia et al., 2004). In ASPA KO brains, a few cells were detected that were copositive for Ki67+/GFAP+ (Fig. 6D) and BrdU+/GFAP+ (image not shown). The quantification of WT and KO BrdU+ cells confirmed a significant increase in BrdU+ cells in the KO tissue (P < 0.002) relative to WT (Fig. 6E). The most significant increase in BrdU+/Olig2+ (P < 0.0003) and BrdU+/NG2+ (P < 0.0002) cells in KO tissue showed OLPs as the highly proliferating cells in the ASPA mutant SVZ. Though also significant, BrdU+/GFAP+ (P < 0.03) cells represented fewer proliferating multipotent GFAP-expressing progenitors relative to Olig2- and NG2-expressing cells.
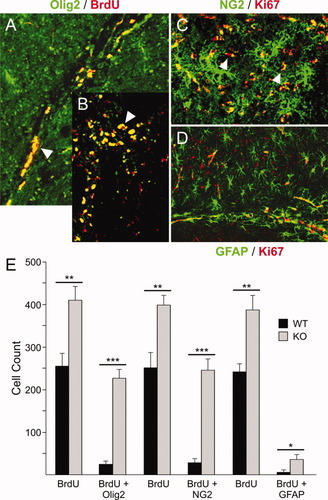
Colocalization of proliferating markers in adult WT and KO mouse brain. Nuclear immunostaining (red) for BrdU or Ki67+ are visualized with OLP markers Olig 2, and NG2 (green). BrdU+/Olig2+ cells are seen in the rostral migratory stream (A,B). Predominant Ki67+ nuclear immunostain can be seen in proliferating cells copositive with cytosolic NG2 (C). A few proliferating Ki67+/GFAP+ are seen in SVZ (D; n = 4). Cell counts and quantification of proliferating neural cell populations in ASPA KO brain, were determined as described under Methods. A significant increase in BrdU incorporation was observed in the KO brains as compared with WT (**P < 0.002), replicated three times with each group of markers. The analysis of BrdU+/Olig2+ (***P < 0.0003) and BrdU+/NG2+ (***P < 0.0002) cell counts revealed OLs as most of the proliferating cells in the adult ASPA mutant brain. Although the proliferation of BrdU+/GFAP+ (*P < 0.03) cells was also significant in the KO tissue over WT, these adult SVZ-derived multipotent GFAP+ proliferating cells known for adult neurogenesis (Garcia et al., 2004) were not as predominant as Olig2+ and NG2+ cells (n = 4).
Histone Modification in ASPA Mutant WM Tissue
Because both cytosolic and nuclear localization of ASPA immunostaining has been reported in rodent OLs (Hershfield et al., 2006), and considering the deacetylation property of ASPA enzyme, we analyzed levels of histone acetylation in the WM tissue of WT and KO. Western blots of antiacetyl lysine immunoprecipitates from WT and KO tissue were probed with antibody for histone H2A, H2B, H3, and H4. The percentage relative acetylation of histone H2B and H3 in the absence of ASPA activity remained at an elevated level compared with the WT (Fig. 7). This suggests a direct or indirect effect of ASPA in nuclear function, which requires further investigation at early ages of OL development as well as histone HDAC activities.
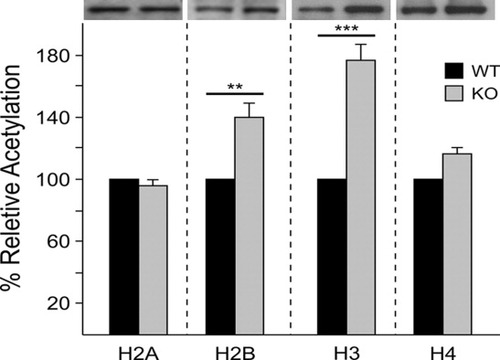
Increased histone H2B and H3 acetylated levels in adult ASPA KO WM tissue. Antiacetyl lysine immunoprecipitates of nuclear extracts from adult WT and KO tissue were analyzed by Western blot. Protein blots were independently probed with histone antibodies as shown, and the densitometry scan of each band was normalized to actin. Results are means of three independent experiments, and the significance value for histone H2B (**P < 0.005) and H3 (***P < 0.0003) are based on the percentage relative acetylated histone in WT vs. KO tissue.
Myelin Markers in ASPA Mouse Brain
The adult WT, myelinated cerebellar fibers were immunopositive for MBP (Fig. 8A) and PLP (Fig. 8C). However, immunostaining for myelin markers in the ASPA KO brain revealed a severe reduction in MBP (Fig. 8B) and PLP (Fig. 8D) in fibers. Instead, a perinuclear/cytosolic staining was seen in OL cell bodies The immunostaining for CNP, a predominantly cytosolic enzyme expressed from immature to mature stages in OLs, was visualized in age-matched WT (Fig. 8A) and KO (Fig. 8B) mice. Furthermore, the immunostaining for NG2 was not prominent in the WT cerebellum (Fig. 8C) but was clearly detectable in the KO cerebellum (Fig. 8D). The symptoms of WM degeneration and spongy disruption of tissue structure observed here is the main feature of CD. Interestingly, Western blot analysis of ASPA KO revealed that CNP, which appears in OL from the premyelinating stage onward, was not altered in cortical and cerebellar WM tissue protein band (Fig. 8E), whereas levels of mature myelin proteins, MBP and PLP, were impaired. In the ASPA KO tissue, the 21.5- and specifically 18-kDa more basic isoforms of MBP proteins were hardly expressed. The 14-kDa MBP band was present at a lower level. A significant reduction in the level of PLP/DM20 proteins was also noted in ASPA KO tissue on Western analysis. On the other hand, gene expression of MBP and PLP/DM20 was confirmed in KO tissue by RT-PCR amplification and gene sequencing data (Fig. 8F). The presence of RNA and the absence of proteins may reflect a posttranscriptional regulation of myelin proteins in the KO mouse.
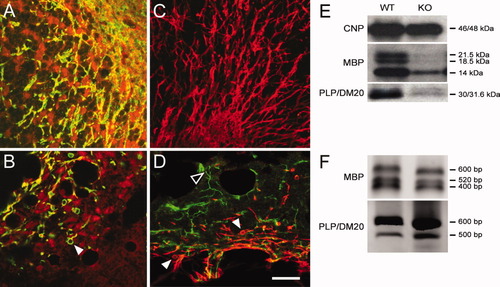
Impairment in MBP and PLP/DM20 immunostaining in the adult ASPA KO mice cerebellum WM. Double immunostaining of WT mouse brain for MBP (green) with CNP (red; A) and PLP (red; C) displayed myelinated fibers without significant staining for NG2 (green; C). In age-matched KO brain, a disruption and lack of myelin staining were observed. A perinuclear/cytosolic staining of MBP and PLP (B,D) could be seen. Most importantly, a strong immunostaining for CNP (B) was visible, signifying the presence of immature OLs. Many distinct NG2+ progenitor cells occurred within the same region where lack of PLP (D) immunostaining could be noted. Western blot analysis of normal and ASPA KO cortical WM tissue as described in Materials and Methods shows the presence of MBP, PLP/DM20, and CNP protein bands in WT tissue (E). An absence of mature MBP and PLP/MD20 protein bands is observed in KO, whereas no significant difference can be seen in the levels of CNP to suggest presence of immature OLs. The mRNA template-dependent RT-PCR amplification of DNA, followed by cloning and sequencing, confirmed the transcription of all mRNA isoform of MBP and PLP (F). Scale bar = 20 μm.
Apoptosis of CNS Cells in ASPA KO Mouse Brain
A highly organized programmed cell death was observed by TUNEL assay throughout different regions of the adult KO mouse brain (Fig. 9). Compared with that in the age-matched WT (Fig. 9A), a high level of DNA fragmentation was observed in cortex, lateral ventricle region, hippocampus, and striatum of KO brains (Fig. 9B–E). A higher magnification of the bracketed regions in Figure 9C–E shows a TUNEL-positive nucleus (Fig. 9C1,D1), and a few TUNEL-positive large nuclei were detected in the striatum (Fig. 9E1).
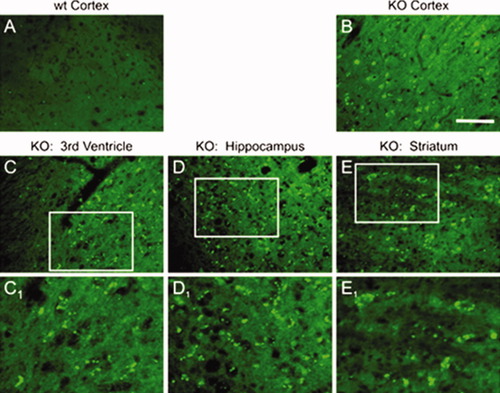
In situ detection of apoptosis in ASPA WT and KO brains. Incorporation of bright green fluorescent-labeled dUTP at the 3′-end of nicked DNA sites were performed by deoxynucleotidyl transferase in tissue sections of adult WT and KO brain. In WT (A) tissue, little or no significant TUNEL staining could be seen, whereas, in KO tissue, some cells appear to be undergoing apoptosis in cortex (B), third ventricle region/caudate putamen (C), hippocampus/dentate gyrus (D), and striatum (E). A further enlargement of areas from C–E is displayed in (C1–E1) that elaborates death of cells with smaller (C1 and D1) to larger nucleus as seen in E1 (n = 4). Scale bar = 20 μm for A–E.
Analysis of Apoptotic Cells for Lineage Specificity
To delineate the lineage of apoptotic cells, a combination of TUNEL assay and immunostaining of cell-specific markers was performed. We used GSTpi in combination with TUNEL to identify OLs. This was based on an earlier study in which colocalization of immunostaining of GSTpi with the mature and immature markers of OLs had revealed a nuclear localization of GSTpi in the immature and cytosolic in mature OLs (Tamura et al., 2007). With antibody for cell-specific markers, such as GSTpi for OLs, GFAP for astrocytes, and NeuN for neurons, we determined the specificity of cells prone to apoptosis in ASPA KO cortical WM (Fig. 10). In WT brain, an intense GSTpi cytosolic immunostaining of mature OL was detected, without significant costaining with TUNEL (Fig. 10A). However, in the KO tissue, a prominent nuclear immunostaing for GSTpi was detected (Fig. 10B), which strongly suggests KO OLs to be immature OLs, resembling a pattern of GSTpi localization that has been described for identification of immature vs. mature OLs (Tamura et al., 2007). Most importantly, large numbers of nuclear GSTpi+ cells were TUNEL+ (Fig. 10B), with fragmented nuclei seen at higher magnification (Fig. 10C), which confirms death of immature OLs in adult KO WM. Death of astrocytes in KO corpus callosum was confirm by GFAP+/TUNEL+ immunostaining (Fig. 10D,E). Likewise, death of NeuN+/TUNEL+ neurons (Fig. 10F) and TUNEL+ nuclear disruption (Fig. 10F′) were detected in the KO tissue.
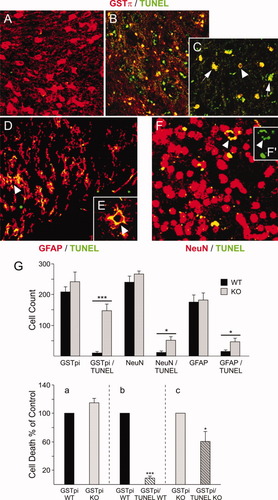
Determination of lineage specificity of apoptotic cells in ASPA KO brain. Antibody against cell-specific markers, such as GSTpi, to differentiate between mature and immature OLs (Tamura et al., 2007), NeuN for neurons, and GFAP for astrocytes were each used in red and combined with TUNEL (green) to determine cell death. A normal cytosolic immunostaing of GSTpi can be seen in mature OLs of WT corpus callosum/splenium, with a few examples of TUNEL costaining with GSTpi to account for a low level of apoptosis in the WT tissue (A). Within the same regions in KO tissue, only nuclear staining of GSTpi is visible, which suggests the presence of immature pre-OLs and that a number of these are copositive for TUNEL (B). At higher magnification, GSTpi+/TUNEL+ nuclear staining and nuclear abnormalities (C; arrowheads) are seen in the midst of a few nuclear GSTpi+ progenitor cells (C; arrow). GFAP (red) or NeuN (red) combined with TUNEL (green) identifies death of astrocytes (D,E) and neurons (F,F′) in the corpus callosum region above the lateral ventricle. Immunostaining of GFAP+ astrocytes is identified in the corpus callosum of KO brain along with TUNEL+ cells (D, arrowhead; ×20). A nuclear blebbing and DNA labeling can be identified in the outer periphery of astrocyte nucleus (E) at higher magnification (×63). A similar nuclear disruption can also be identified for neurons copositive for NeuN and TUNEL (F, arrowhead; ×20) and TUNEL+ alone (F′; ×63; n = 4). Quantification of lineage-specific apoptotic cells from WT and KO tissue was performed as described in Materials and Methods (G). GSTpi-, NeuN-, and GFAP-immunopositive cells were analyzed against TUNEL copositive cells representing cell death (top). The cell counts for GSTpi, NeuN, and GFAP between WT and KO were not significantly different. Overall, small numbers of TUNEL-positive cells were detected in the WT tissue, although the death of OLs (GSTpi+/TUNEL+) in KO tissue was highly significant (***P < 0.0002). Also, whereas fewer TUNEL+/GFAP+ and TUNEL+/NeuN+, apoptotic astrocytes and neurons were present in WT, in KO significant numbers of apoptotic astrocytes (*P < 0.05) and neurons (*P < 0.05) were detected. Analysis of total cell death between cytosolic GSTpi+, mature OLs in WT, vs. nuclear GSTpi+ immature OLs in KO (a), revealed no significant difference. Relative to total WT cytosolic GSTpi+ mature OL cells, the GSTpi+/TUNEL+ mature apoptotic OLs were significantly low (***P < 0.0001), which suggests that only a small percentage of mature OLs undergoes apoptosis in the WT animals (b). Conversely, a large percentage of GSTpi+ immature cells was also TUNEL+ in KO (*P < 0.05; c). Collectively, death of all cell types was confirmed in the KO tissue, and the number of OL cell death was highly significant.
Total cell counts for GSTpi+, GFAP+, and NeuN+ cells from WT and KO were not significantly different (Fig. 10G, top). On the other hand, relative to WT, death of TUNEL+/GSTpi+ KO OL was highly significant. Even though it was at a lower percentile, death of neurons and astrocytes was also significant. Because GSTpi staining was distinctly cytosolic in WT (mature OLs) and nuclear in KO (immature OLs), a separate analysis was performed to quantify the mature vs. immature OL cell death (Fig. 10G, bottom panels a–c). Relative to mature WT GSTpi+ OLs, immature GSTpi+ KO OLs were not significantly different (Fig. 10G, a). Also, only a small percentage of mature WT OLs was apoptotic with a high significance value (Fig. 10G, b). Conversely, a large percent of immature OLs was apoptotic in KO (Fig. 10G, c).
DISCUSSION
Mutations in the ASPA gene are the known cause of severe loss of WM in CD, a childhood leukodystrophy. Our observations strongly suggest disruption in cell cycle regulation, acetylated state of nuclear histones, continuous neurogenesis of neural/stem cell-like progenitors, lack of or severe reduction of certain myelin proteins, and massive death of OLP in a mouse model of CD in which the ASPA gene has been knocked out (Matalon et al., 2000). We report here for the first time that, in normal mouse brain, ASPA gene expression occurs as early as E12.5 and continues to increase throughout embryonic to postnatal development. Thus far, the role of ASPA in the specification of neural stem cells to the OL lineage cells has not been addressed. This concept arises from our striking observations from immunohistochemical study of neural cell populations in the corpus callosum and SVZ of adult KO mouse brain during the late stage of disease. Adult ASPA KO brains display an array of cells expressing markers for neural stem/progenitors, such as PSA-NCAM, nestin, vimentin, NG2, and GD3. In adult brain, PSA-NCAM-expressing progenitors can give rise to cells of neuronal lineage and are thought to be important in repair of the CNS (Zhang et al., 2004; Nait-Oumesmar et al., 2007). Proliferating NG2-expressing cells have been characterized as OLPs (Dawson et al., 2003), and, although early postnatal SVZ-derived NG2+ cells have been described as neuronal precursors that may give rise to different CNS lineage cells in vitro and in vivo (Belachew et al., 2003; Aguirre and Gallo, 2004; Aguirre et al., 2004), recent reports for adult brain do not support NG2 as precursors for neurons (Buffo et al., 2008; Zhu et al., 2008). GD3 gangliosides are found associated with OLs during early development (Ellison and de Vellis, 1995). In view of these reports, our observation provides evidence for the presence of a higher order of proliferating stem cell-like/early progenitors in the absence of ASPA activity. The presence of early neural cells in large numbers is a direct result of continuous cellular proliferation. Increase in proliferation was confirmed by BrdU incorporation studies. A significant increase in cdk2-dependent kinase activity responsible for G1→S transition was also observed in the KO tissue. Furthermore, reductions in levels of cell cycle inhibitor p21Cip1 and more importantly of p27Kip1, normally required for G1→G0 transition of OLs, were also detected for ASPA mutants WM. The role of p27Kip1 in cell cycle transition and progression toward differentiation was supported by an earlier study in which ectopic expression of p27Kip1 in OLPs cells caused cell cycle arrest (Tikoo et al., 1998), and another study demonstrated that an increase in p27Kip1 enhanced MBP promoter activity (Miskimins et al., 2002). In yet another study, the presence of a large number of BrdU+/NG2+ proliferating OLPs was seen in p27-null mutant mouse spinal cord compared with WT to suggest a role of p27Kip1 in regulation of the G1→G0 phase (Crockett et al., 2005).
It is not known whether ASPA normally down-regulates cell cycle progression and promotes OLP differentiation at the onset of myelination. Our results strongly suggest that lack of ASPA activity coincides with loss of cell cycle arrest and increase in proliferation of neural progenitors. In the ASPA KO, BrdU and Ki67 coimmunostaining with makers for glial cells demonstrated increased proliferation in the SVZ, a region known for proliferation of progenitors that migrate and differentiate into neural cell types throughout adult life. A large pool of these proliferating cells belongs to the OLP lineage, as evident by a ninefold increase in BrdU+/Olig2+, BrdU+/NG2+, and Ki67+/NG2+ cells in the KO tissue during the pathophysiology of disease. Though at much higher levels, our observation corroborates recent finding in multiple sclerosis in which a two- to threefold increase in cell density and proliferation of early glial progenitors was seen in the subventricular region of patients (Nait-Oumesmar et al., 2007) as well as in rodent models (Nait-Oumesmar et al., 2008). Interestingly, an earlier brain tissue histology report of a 4-year-old CD patient described an increased density of OLs (Mirimanoff, 1976), and yet another report described loss of OLs (Adachi et al., 1966). These reports were based on staining tissues with various dyes. Utilizing TUNEL assay combined with cell- and lineage-specific marker antibodies, we detected program cell death of various neural cell types. The death of immature OLs was most significant based on TUNEL-copositive cells with nuclear staining for GSTpi, a characteristic of immature OLs, as opposed to cytosolic staining for GSTpi, which identifies differentiated OLs (Tamura et al., 2007), whereas TUNEL-copositive NeuN or GFAP cells constituted a small population. Thus, a concurrent neurogenesis of OLPs and apoptosis of immature OLs occur in the absence of ASPA enzyme activity.
Because OLs are known to play a major role in fatty acid biosynthesis required for myelin assembly, a decline in acetyl groups in the absence of ASPA enzyme activity is considered a possible major cause of CD. Lipid analysis of 17-day-old ASPA KO brains compared with WT mouse brains showed a 21–38% reduction in nonpolar and polar lipids, critical for myelin synthesis, whereas other lipids were not altered significantly (Madhavarao et al., 2005). In a recent study, reduction in cerebrosides and sulfatides with unsubstituted fatty acids in myelin was reported at 28 days in the tremor rat, a model of CD. Similar changes were also found in 7-month-old tremor rats, whereas phosphatidylethanolamine, one of the more abundant lipid groups of myelin, showed insignificant change at either age. However, phosphatidylcholine was significantly increased at 28 days, which returned to normal at 7 months (Wang et al., 2008). A decline in cerebroside and sulfatide levels was also reported in lipid contents of WM from human CD patients (Madhavarao et al., 2005); however, the observed reduction in lipid levels might not directly correlate with the severity of clinical outcome.
Analysis of myelin proteins in adult KO mouse WM tissue revealed a marked reduction of myelin proteins, 21.5- and 18-kDa MBP, and PLP/DM20, relative to WT. In the KO WM, MBP and PLP/DM20 immunostaining revealed absence of myelinated axon tracts in corpus callosum, cortex, and cerebellum. This relates to loss of mature OLs, failure of maturation of OLs, or a combination of both. Detection of perinuclear/cytosolic staining of MBP and PLP in the OL cell body of ASPA KO can be a result of impairment in protein trafficking resulting from accumulation of misfolded proteins and its retention in the endoplasmic reticulum of OLs, leading to cell death (Gow et al., 1994, 1998). Our RT-PCR amplification study confirmed that transcription of MBP and PLP/DM20 does occur in ASPA KO brain. However, it is not known whether these messages accumulate at the cytosolic level for translation or are turned over as we reported for PLP mutation in which other members of myelin gene, such as MBP protein, were absent, and, even though the transcription of MBP was not impaired, mRNA was not detected by Northern blot analysis, suggesting a posttranscriptional regulation of myelin genes (Kumar et al., 1988, 1990). Similar regulation of myelin genes is evident in other myelin mutants, such as jimpy, quaking, and shiverer, affecting synthesis and stability of myelin proteins, leading to hypomyelination (Sorg et al., 1986). Our observation in ASPA mutants, i.e., active transcription of all myelin isoforms and lack of proteins, strongly suggests an epigenetic regulation of myelin genes in ASPA mutants that requires further investigation.
A direct or indirect nuclear role of ASPA in OLs differentiation is possible based on our results of acetylated histone H2B and H3 levels observed in the absence of ASPA activity. Proliferating OLPs maintain histone acetylation, whereas differentiation of OLs into myelinating cells critically depends on deacetylation (Marin-Husstege et al., 2002). Working with neonatal OLPs in culture to maintain proliferation in the presence of growth-promoting factors and subsequently enter differentiation upon mitogen withdrawal as described previously (Gard and Pfeiffer, 1993; Temple and Raff, 1985), Casaccia-Bonnefil and colleagues demonstrated that histone deacetylation occurs during a specific temporal window between proliferation and onset of differentiation of OLs. A decline in acetylated lysine residues of histone H3 and high-mobility group proteins (HMGN-1) supported the presence of HDAC activity during lineage progression and differentiation of OL during early development (Marin-Husstege et al., 2002). Also, in vivo timing of OLP differentiation into myelin-forming cells was found to be dependent on histone deacetylase activity localized within the developing corpus callosum (Shen et al., 2005). Insofar as acetylated histones can modify proteins and alter functions, such as protein–DNA interaction and protein–protein interaction and their stability, the epigenetic modifications may underlie impairment in the absence of ASPA, during both early development and the late chronic stage of demyelination. Future deacetylation studies in ASPA mutants should provide useful information for understanding impairment in OL differentiation in CD. These findings are important to consider for the development of future therapy for CD.
Acknowledgements
The authors thank Poonam Sachan for her technical assistance and Donna Crandall, IDDRC Media Core, for her contributions to preparation of illustrations for publication.




