Up-regulation of oligodendrocyte precursor cell αV integrin and its extracellular ligands during central nervous system remyelination
Abstract
To determine the role of extracellular matrix molecules and their integrin ligands in CNS remyelination, we have examined in experimentally induced focal demyelinated lesions the expression of the two classes of integrins implicated in oligodendrocyte development and myelination: α6 laminin-binding integrins and αV integrins that bind a range of extracellular matrix proteins containing the -Arg-Gly-Asp- (RGD) recognition sequence. Only αV integrins were up-regulated during remyelination, being expressed on oligodendrocyte precursor cells during their recruitment into the lesion. Next, therefore, we examined the expression of extracellular matrix ligands for αV integrins and documented increased expression of tenascin-C, tenascin-R, fibronectin, and vitronectin. Taken together with our previous discovery of high levels of expression of another αV ligand, osteopontin, during remyelination in these lesions, our findings suggest that αV integrins make an important contribution to successful repair in the CNS. © 2009 Wiley-Liss, Inc.
Repair by remyelinating oligodendrocytes is well recognized in the inflammatory demyelinating disease multiple sclerosis (MS), even in the presence of longstanding disease (Patrikios et al., 2006; Patani et al., 2007). However, repair fails in some patients, leading to persistent myelin loss (Franklin, 2002). This is thought to lead to the axon degeneration that causes chronic progressive disease as a consequence of the loss of protection from ongoing chronic inflammation and/or the loss of trophic support provided to the axon by the ensheathing oligodendrocyte (Nave and Trapp, 2008). Remyelinating cells are generated by oligodendrocyte precursor cells (OPCs) that migrate into the lesion, where they proliferate and differentiate into newly formed oligodendrocytes (Franklin and ffrench-Constant, 2008). Defining the molecular pathways that lead to successful repair by these oligodendrocyte precursor cells is therefore an important goal.
Just as in development, extracellular matrix (ECM) molecules are expressed at high levels in regeneration. Thus, for example, regeneration in the peripheral nervous system is associated with deposition of laminins and fibronectins by dedifferentiating Schwann cells in the region below the injury within which the distal axon degenerates prior to regrowth (Martini, 1994). These ECM molecules likely promote regeneration, as evidenced by enhanced peripheral nerve regrowth through implanted matrices containing laminins and fibronectins (Madison et al., 1988; Bailey et al., 1993; Whitworth et al., 1995) and the inhibitory effect of blocking antibodies on regrowth through sciatic nerve grafts (Wang et al., 1992). The principal receptors for ECM molecules are integrins, heterodimers of an alpha and a beta chain that together generate an extracellular ligand-binding site (Hynes, 1992). Ligand binding clusters the integrins, as a result of which the cytoplasmic domains of the integrins assemble a signalling complex that activates multiple downstream signalling pathways regulating migration, proliferation, and survival (Hynes, 2002), the processes essential for repair.
Experiments to identify the key integrin/ECM interactions that promote remyelination in MS lesions are complicated by the presence of inflammatory and regenerative processes occurring simultaneously in the tissue (Lassmann et al., 1997; Lucchinetti et al., 2000). It is therefore impossible to distinguish those changes that promote repair from those responsible for the destructive component of the inflammatory response. The same caveat applies to the study of animal models in which inflammation is used to model the disease as in experimental allergic encephalitis (EAE), the most commonly used model of the initial stages of MS (Gold et al., 2006). However, focal lesions generated by the injection of toxins such as ethidium bromide or lysolecithin overcome this problem, insofar as the initial damage caused by toxin injection is transient and is followed by a well-defined regenerative response tempo, which can be predicted based on the site of lesion and age of animal and which is not complicated by further destructive events (Blakemore and Franklin, 2008). Here, therefore, we have defined the pattern of integrin and ECM expression during remyelination in focal lesions to identify those associated with regeneration.
MATERIALS AND METHODS
Animals
Female Sprague Dawley rats aged 8–10 weeks were obtained from Harlan U.K. Animal care procedures were in accordance with the guidelines set by the European Council directives (86/609/EEC) and the Home Office, Animals Scientific Procedures Act (1986).
Induction of Demyelination in CNS White Matter
For expression of integrins and their ligands, demyelinating lesions were made in the caudal cerebellar peduncle of adult rats (Woodruff and Franklin, 1999). Demyelination was induced bilaterally by stereotaxic injection of 4 μl of 0.01% ethidium bromide (Sigma-Aldrich, Gillingham, United Kingdom) into the caudal cerebellar peduncles (CCPs) using a method previously described (Woodruff and Franklin, 1999). Briefly, rats were anesthetized with isoflurane and positioned in a rodent stereotaxic frame at a flat skull position. The following coordinates were used for the caudal cerebellar peduncle: –10.4 mm anteroposteriorly, –7.0 mm dorsoventrally, 2.6 mm laterally. Injection was carried out through a skull opening created with a dental drill, with a 10 μl Hamilton syringe at a rate of approximately 1 μl/min.
Animals were perfusion fixed with a 4% paraformaldehyde solution via the left ventricle for in situ hybridization (ISH) and immunohistochemistry (IHC). To generate ISH probes, demyelinating lesions were made in the spinal cord of adult rats, because lesions in this location can be readily located and dissected. With animals under halothane anesthesia, two foci of demyelination were created by direct injection of 1 μl 1% lysolecithin, dissolved in sterile phosphate-buffered saline (PBS), into both the dorsal and the ventral funiculi at the level of the first lumbar vertebra following laminectomy. The dissected fresh tissue was used for RNA isolation and probe synthesis (see below). For both procedures (spinal cord and brainstem lesions) the rats were kept in individual cages under standard conditions.
ISH With cRNA Probes
Animals were euthanized by intravenous injection with pentobarbital solution, and spinal cords were removed. A 2–3 mm-thick transverse slice of spinal cord containing the lesion sites was removed and immediately frozen in an isopentane bath on dry ice. The tissue was stored at –80°C until RNA isolation. Total RNA was extracted from frozen spinal cord using Trizol reagent (Invitrogen, Paisley, United Kingdom), following the manufacturer's instruction. Complimentary DNA was synthesized by using a Qiagen Omniscript RT-PCR Kit (Qiagen, Crawley, United Kingdom) and oligo-dT 15mer (Promega, Southampton, United Kingdom). The primers for RT-PCR were synthesized by TAG Newcastle Ltd. (now VH Bio Ltd., Gateshead, United Kingdom) using the sequences listed in Table I. Selected PCR products were subcloned in plasmid pCRII Topo using an Invitrogen PCR Topo Cloning Kit (Invitrogen), with SP6 and T7 flanking the PCR fragments. Complementary RNA probes were labelled with digoxigenin (DIG) of fluorescein (FITC) using a DIG RNA Labelling Kit (Roche, Lewes, United Kingdom), with appropriate RNA polymerase. The in situ hybridization procedure was carried out as described previously (Sim et al., 2002).
| Gene | Accession No. | Sequences | Size (bp) |
|---|---|---|---|
| Tenascin-C | NM_011607 | For 5′-GCTACCACAGAAGCTGAACC-3′ | 526 |
| Rev 5′-GATCGCTTTCTTCGAATCC-3′ | |||
| Tenascin-R | NM_013045 | For 5′-GATGCTCCCAAGAATTTGC-3′ | 578 |
| Rev 5′-GAGGTCACATGAGAAAAGTGC-3′ | |||
| Vitronectin | NM_019156 | For 5′-TGAGCTAGATGAAACAGC-3′ | 789 |
| Rev 5′-CCAGAGAAGAAATAGACG-3′ | |||
| Thrombospondin-1 | NM_011580 | For 5′-ACGATTCACTGGCTCACAGC-3′ | 705 |
| Rev 5′-TCACAAGTGTCCCCTATGAGG-3′ | |||
| Integrin α6 | XM_215984 | For 5′-TCACTTCCAGAAGTTCTTCC-3′ | 794 |
| Rev 5′-TTGCCATTGCTAATCTCC-3′ | |||
| Integrin αV | NM_001106549 | For 5′-TCCAAACTGGGAGTACAAGG-3′ | 720 |
| Rev 5′-TCTTGCTCTTCTTGAGGTGG-3′ |
Where two-color ISH was required for double labelling, the sections were hybridized with two riboprobes labelled with DIG and FITC and sequentially visualized with alkaline phosphatase (AP)-conjugated anti-DIG antibody, with substrate NBT/BCIP (Roche), which produces a dark blue deposit on targets, then AP-conjugated anti-FITC antibody, with INT/BCIP (Sigma-Aldrich), which produces magenta color. Prior to the second color reaction, the sections were incubated in maleic acid buffer at 65°C for 30 min to inactivate the AP activity used for the first color reaction.
Immunohistochemistry
IHC was performed on frozen sections of tissue perfused with 4% paraformaldehyde using standard protocols. For staining with antibody against transcription factor Nkx2.2, the sections were treated with an antigen retrieval procedure with 10 mM sodium citrate as described previously (Fancy et al., 2004). The antigens were visualized either with standard ABC methods (Vectalab, Peterborough, United Kingdom) or indirect immunofluorescence using Alexa 488- or 594-conjugated secondary antibodies purchased from Molecular Probes (Invitrogen). Images were acquired with a Nikon Optiphot-2 fluorescent microscope equipped with a digital camera or with a Zeiss Axiovision Imaging System. Monoclonal antibodies against tenascin C and tenascin R were products from R&D Systems (Abingdon, Oxon, United Kingdom); monoclonal antibody against Nkx2.2 was produced by the Developmental Studies Hybridoma Bank (DSHB, Iowa City, IA); polyclonal antibodies for glial fibrilary acidic protein (GFAP) and fibronectin were from DAKO (Ely, United Kingdom). Monoclonal antibodies against laminin EHS and vitronectin were from Sigma-Aldrich (Gillingham, United Kingdom); mouse monoclonal antibody against laminin α2 subunit (merosin) was from Chemicon (Southampton, United Kingdom); thrombospondin-1 antibody was from BD Transduction Laboratories (Lexington, KY); the monoclonal antibodies against neurofilament SMI 31 and SMI 32 were from Sternberger Monoclonal Inc. (Lutherville, MD).
RESULTS
OPCs Up-Regulate αV but Not α6 Integrin Following Toxin-Induced CNS Demyelination
We have shown previously that OPCs and oligodendrocytes express two families of integrins, those containing the α6 and αV chains. The former heterodimerize with β1 integrin to form laminin receptors, which contribute to the signalling events that promote survival of those newly formed oligodendrocytes that establish appropriate contacts with axons to be myelinated (Colognato et al., 2002). The latter heterodimerize with at least four different β subunits [β1, β3, β5, and β8 (Milner and ffrench-Constant, 1994; Milner et al., 1997)] that can bind a wide range of ECM ligands, including tenascins, fibronectins, vitronectin, and osteopontin (Marshall and Hart, 1996). Cell culture studies suggest roles for these integrin heterodimers in migration, proliferation, and differentiation (Milner et al., 1996; Blaschuk et al., 2000; Gudz et al., 2006). First, therefore, we defined whether one or both integrin families are implicated in remyelination by examining the expression of α6 and αV in OPCs as they enter the lesion, proliferate, and differentiate into myelin-forming oligodendrocytes. With ISH, the αV integrin subunit mRNA was not detectable in normal CNS white matter. However, after induction of demyelination by ethidium bromide (EB) injection into the caudal cerebellar peduncle, a well-characterized model of demyelination-remyelination, it was up-regulated as early as 3 days postlesion (DPL) within the area of the lesion (Fig. 1D–F). At this time, OPCs have begun to repopulate the lesion area (Sim et al., 2002; Fancy et al., 2004). αV mRNA continued to be expressed throughout remyelination, although the proportion of cells expressing the OPC marker Nkx2.2 (Watanabe et al., 2004; Fancy et al., 2004) that coexpress αV mRNA decreased at 10 DPL compared with 3 DPL, and overall levels were markedly reduced after 10 DPL, at which time OPC proliferation within the lesion is largely complete and differentiation is initiated (Fig. 1G–I). The individual αV mRNA-expressing cells colabelled with Nkx2.2 (Fig. 1G), confirming their identity and consistent with our prior in vitro findings that OPCs express αV integrins (Milner and ffrench-Constant, 1994; Milner et al., 1997). Low levels of αV integrin mRNA were also expressed by macrophages in the lesions (data not shown), as revealed by colabelling with osteopontin that is expressed exclusively by macrophages in early lesions (Zhao et al., 2008). In sharp contrast to the findings with αV, no expression of α6 mRNA was seen in OPCs or oligodendrocytes in the lesions at any time, although expression was seen in cells with a microglial morphology (data not shown). Together, these findings suggest that αV integrins play significant roles in remyelination, particularly at early stage of OPC lineage progression, whereas α6 integrins do not contribute to the regenerative events within the oligodendroglial lineage. Next, therefore, we examined the expression of candidate ECM ligands for these αV integrins, extending our earlier study on osteopontin (Zhao et al., 2008) by focusing on tenascins, fibronection, vitronection, and laminin.
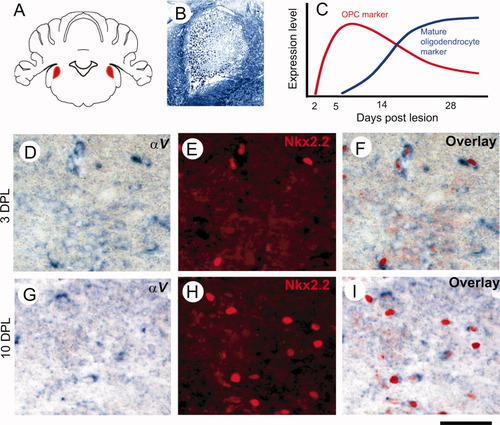
Expression of integrin αV subunit in oligodendrocyte progenitor cells. A: Diagram showing the location of the caudal cerebellar peduncle (CCP) in adult rat brain where bilateral demyelination lesions were induced (red). B: Typical CCP lesion at 10 days postlesion (DPL) stained with toluidine blue, illustrating the demyelinated area. C: Schematic profile of the expression of oligodendrocyte progenitor cells (OPC) markers and mature oligodendrocyte marker during remyelination in this model (see Sim et al., 2002). D–F and G–I are images of ethidium bromide CCP lesions at 3 and 10 DPL, double stained with integrin αV by ISH (D,G) and with Nkx2.2 immunofluorescent staining (E,H). The dark background of Nkx2.2 immunofluorescent in images in E,H was inverted into light background, and then the images were overlaid with corresponding αV ISH images (D,G). The merged images show cells with Nkx2.2-positive nuclei (a marker for OPCs) that strongly express αV mRNA. Scale bar = 500 μm for B; 30 μm for D–I. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Tenascin C Is Expressed Mainly by Reactive Astrocytes Whereas Tenascin R Is Expressed by Recruited OPCs Following Toxin-Induced CNS Demyelination
Tenascin C (TnC) is a glycoprotein ligand for αV integrin that has roles in embryonic development and adult tissue remodelling in the CNS (Jones and Jones, 2000). Analyses of TnC-null mice indicate that TnC is not essential for developmental myelination, although its role in remyelination has not been addressed (Kiernan et al., 1999). We were unable to detect TnC in normal, nonlesioned CNS white matter by either ISH or IHC (Fig. 2A,E). However, after EB-induced demyelination both TnC mRNA- and protein-expressing cells were detectable within the demyelinated area. These cells were most abundant at 3 DPL and had declined by 10 DPL (Fig. 2B–D,F). Combined TnC ISH and Nkx2.2 IHC indicated that the TnC-expressing cells were not OPCs (Fig. 2G) but instead were likely to be GFAP-positive astrocytes (Fig. 2H).
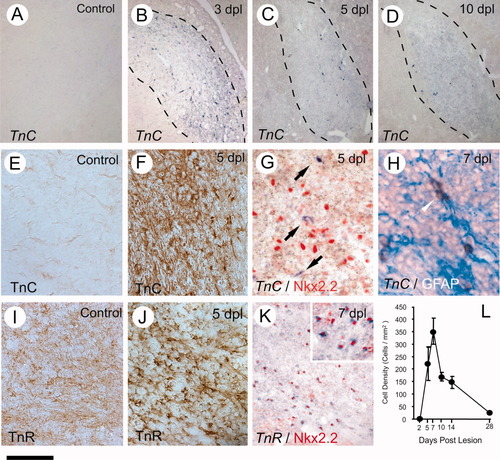
Expression of tenascin C (TnC) and tenascin R (TnR) following demyelination of rat caudal cerebellar peduncle (CCP) induced by ethidium bromide injection. A–D show ISH with TnC in unlesioned CCP and at 3, 5, and 10 days postlesion (DPL). The approximate lesioned area were marked by dotted lines on the images based on solochrome cyanine staining on adjacent sections. E and F illustrate immunostaining of TnC in unlesioned (E) and lesioned CCP (F) at 5 DPL, visualized by standard ABC method, which produces a brown precipitate, and counterstained with hematoxylin. Dual labelling of TnC mRNA by ISH and antibodies to detect the OPC marker Nkx2.2 or the astrocyte marker GFAP (pseudocolored) is shown in G and H, respectively. TnR mRNA was detected in lesioned CCP and colocalized with Nkx2.2 (K, inset shows higher magnification of the part of the same image, pseucolored in red). L: Graph showing temporal profile of the densities of TnR mRNA-expressing cells in the CCP lesion. I and J show the immunoreactive TnR visualized by standard ABC method in unlesioned and lesioned CCP, respectively, at 5 dpl. Scale bar = 400 μm for A–D; 40 μm for E,F,I–K; 20 μm for G,H and inset in K. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Tenascin R (TnR) has well-established roles in oligodendrocyte lineage cells, acting as an intrinsic autocrine regulator promoting oligodendrocyte differentiation (Schachner, 1994; Pesheva et al., 1997). Immunostaining revealed relatively low levels of TnR expression in normal, nonlesioned CCP. However, there was a substantial increase in expression following induction of demyelination (Fig. 2I,J). TnR mRNA was not detectable by ISH in normal white matter but was clearly evident at 5 DPL within the demyelinated area. The temporal profile of the densities of TnR-expressing cells resembled that previously described in this lesion model for platelet-derived growth factor receptor α (PDGFRα)-expressing OPCs (Sim et al., 2002), although overall densities were lower (Fig. 2L). Doubling labelling with the OPC marker Nkx2.2 (Arnett et al., 2004; Fancy et al., 2004) indicated that the TnR-expressing cells were OPCs (Fig. 2K). There was no colocalization of TnR mRNA with markers of either macrophages or astrocytes (data not shown).
Fibronectin and Vitronectin Are Both Present at Increased Levels Compared With Normal White Matter Following Demyelination
Fibronectin and vitronectin are potential adhesive substrates for cell migration following tissue injury via their specific αV integrin-binding domains. Both are present in plasma and can thus leak into injured tissue as well as being synthesized locally. IHC revealed distinct fibronectin staining around blood vessels and a more diffuse extracellular staining throughout the lesioned area at all survival times examined, suggesting that fibronectin may be predominantly plasma derived (Fig. 3A–C). Vitronectin IHC at 2 DPL revealed a distinct cellular pattern of expression in cells that were for the most part CD11b (OX-42)-positive microglia/macrophages (Fig. 3D,H). Vitronectin ISH revealed cells with morphologies similar to those of microglia/macrophages, suggesting that these cells are the principal source of this protein within lesions (Fig. 3G). The intensity of vitronectin IHC declined progressively at 7 and 14 DPL and at this last survival time was confined to large GFAP+ astrocytic processes at the lesion edge (Fig. 3I). Vitronectin mRNA could not be detected at 14 DPL. We also found low levels of expression of thrombospondin-1, an ECM molecule that stimulates migration of cultured OPCs (Scott-Drew and ffrench-Constant, 1997), by both IHC and ISH at 2 and 3 DPL (data not shown).
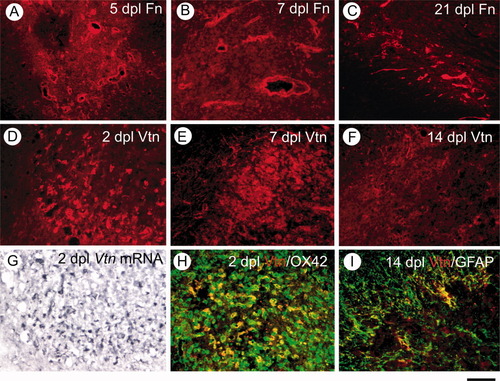
Detection of fibronectin (Fn) and vitronectin (Vtn) in demyelination of rat CCP induced by ethidium bromide. A–C: Immunofluorescent (Alexa 594, red) staining of Fn in lesioned CCP at 5, 7, and 21 DPL. D–F: Immunoreactive Vtn (red) in demyelinated CCP at 2, 7, and 14 days. G: ISH using DIG-labelled Vtn riboprobe at 2 DPL. H and I show merged double-immunofluorescence images of Vtn (red) in a CCP lesion with microglial/macrophage marker OX42 (H, green) or GFAP (I, green). Scale bar = 200 μm for A–F; 30 μm for G–I. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Laminin-α2 Is Expressed With an Axonal Pattern Within Demyelinating Lesions
Finally, we examined the expression of laminin, because axonally expressed laminin plays an important role in white matter development by amplifying growth factor signalling and promoting the survival of myelinating oligodendrocytes (Colognato et al., 2002). We found laminin-1 (containing the α1 laminin chain) expression mainly associated with structures likely to be blood vessels and no clear evidence of axonal staining (Fig. 4A–C). However, when longitudinal sections were stained with antibodies against the α2 subunit of laminin (a component of laminins 2, 4, and 12 often called merosin) at 14 DPL (when new myelin sheaths are being formed), a staining pattern was obtained similar to that seen with antibodies to SMI32 (a neurofilament marker), suggestive of axonal expression (Fig. 4D,E). (Colocalization with laminin-α2 and SMI32 was not possible because both were detected with mouse antibodies.) Laminin-α2 was not detected at earlier time points or in nonlesioned white matter.
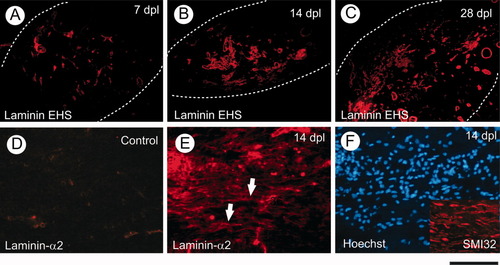
Laminins are up-regulated following demyelination induced by ethidium bromide injection into rat CCP. A–C illustrate the immunofluorescence of lesioned CCP with laminin EHS antibody. The lesioned areas are marked by dotted lines. Laminin α2 subunit (merosin) is not detectable in unlesioned CCP (D) but is up-regulated at 14 DPL following demyelination (E). D and E are images from longitudinal sections of white matter. F: The section adjacent to that shown in E, showing increased cellularity after demyelination as indicated by Hoechst 33258 staining. The inset in F shows the morphology of axons in the lesioned area stained with neurofilament antibody SMI32. Scale bar = 400 μm for A–C; 100 μm for D–F and inset in F. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
DISCUSSION
These results show that, of the two classes of integrins expressed on oligodendrocytes and their precursors during development (Milner and ffrench-Constant, 1994), only αV integrins are up-regulated during regeneration. At the same time, there is increased expression of at least five potential ligands for these αV integrins, TnC, TnR, fibronectin, and vitronectin (this study) and osteopontin (Zhao et al., 2008). In contrast, laminin-binding α6 integrins do not show detectable levels of expression during the remyelination process, and expression of the laminin α2 chain was observed only on axons in the final stages of remyelination. We conclude, therefore, that interactions between αV integrins and their ECM ligands will make the major contribution to integrin-mediated signalling during the initial stages of remyelination in the CNS. Laminins are recognized by both α6β1 integrin and nonintegrin receptors, including dystroglycan, during oligodendrocyte development (Colognato et al., 2007), and in the absence of detectable α6 expression during regeneration we conclude that the laminins observed on axons bind these other receptors.
Previous studies on αV integrins on oligodendrocytes and their precursors have documented that αV associates sequentially with three β subunits, β1, β3, and β5 (Milner et al., 1997). At the same time, high levels of αVβ8 are observed throughout development (Milner et al., 1997). Knockout studies using constitutive or conditional αV or β8 knockouts all reveal a cerebral hemorrhage phenotype showing a necessary role for the integrin in the formation of cerebral blood vessels (McCarty et al., 2002, 2005; Zhu et al., 2002). Interestingly, the conditional knockout studies show that αV function is required in the neural cells but not the endothelial cells (McCarty et al., 2005). Current hypotheses on the functions of αVβ1, αVβ3, and αVβ5 in oligodendrocyte precursors are based on cell culture studies using blocking antibodies, peptides, or overexpression strategies to show roles in migration, proliferation, and differentiation, respectively (Milner et al., 1996; Blaschuk et al., 2000). Moreover, these studies have shown that αV integrins can interact with AMPA receptors and growth factor receptors to regulate migration and proliferation, respectively (Baron et al., 2005; Gudz et al., 2006). However, no supporting knockout data are available to confirm a role for these αV integrins in development: β3, β5, or β3/β5 single- and double-knockout mice have not been reported to show any evidence of CNS developmental abnormalities. No specific studies of αVβ1 in myelination have been performed, because the β1 subunit knockout has an early embryonic lethal phenotype (Fassler and Meyer, 1995), and the reported oligodendrocyte-specific conditional knockout of this subunit excises the gene only after oligodendrocyte precursor migration and proliferation have been completed (Benninger et al., 2006). However, the absence of any neurological abnormalities before 2–3 months in mice in which αV has been removed from CNS neurons and glia by using a nestin-Cre line (that expresses the recombinase in neural stem cells) would suggest that myelination occurs normally without αV integrins (McCarty et al., 2005). Axonal degradation and demyelination are seen after this time, but this was interpreted as revealing a requirement for αVβ8 in long-term axonal survival, because the integrin is expressed on axons but not glia in the adult CNS (McCarty et al., 2005).
The observation that αV is expressed primarily on neurons in the adult CNS is also supported by our finding here that mRNA for αV is not detectable in white matter glia in the normal, undamaged CNS. As well, there are no reports of αV expression on oligodendrocytes during development in vivo, despite the cell culture studies described above. It would be premature to conclude that αV integrins do not play a role in oligodendrocyte development, insofar as a detailed analysis of myelination in the αV knockouts remains to be performed. By analogy with the α6β1 knockout studies, where increased levels of oligodendrocyte apoptosis resulting from a decrease in axon-dependent survival signalling did not prevent normal myelination (Benninger et al., 2006), αV-mediated effects may be compensated by changes in the subsequent pattern of development or by the inherent redundancy in a system in which significant overproduction of oligodendrocytes precedes myelination (Barres and Raff, 1994). However, when taken together with the essential roles of migration, proliferation, and differentiation in the recruitment into the lesion of oligodendrocyte precursors that allows remyelination in the adult CNS (Franklin and ffrench-Constant, 2008), our present findings do suggest strongly that αV integrins play a significant role in regeneration. The mechanisms uncovered by the cell culture studies may therefore be those activated during repair rather than those required for normal development, and further studies using the conditional knockout animals are required to test this hypothesis.
Significant increases in αV integrin expression have also been observed during regeneration in a number of non-neural tissues (AbiEzzi et al., 1995; Pilewski et al., 1997; Tsonis et al., 1997; Noszczyk et al., 2002). Taken together with our present data, this suggests that αV integrins may have a specific role in regenerative processes. This conclusion is interesting, in that it would predict that loss of αV ligands should lead to a failure of remyelination. However, we have shown that remyelination occurs normally in knockout mice lacking osteopontin (Zhao et al., 2008). This likely reflects the promiscuity of αV integrins, with their ability to bind multiple extracellular matrix proteins containing the tripeptide Arg-Gly-Asp (RGD) sequence (Marshall and Hart, 1996). Thus, although the affinity of the different αV heterodimers for the different matrix proteins may vary, each could generate the signalling required for regeneration, and loss of any one ligand will have no effect. More significant, however, might be the mistimed expression of one or more matrix proteins that, as we have articulated previously as the dysregulation hypothesis, might “jam” the regenerative process (Franklin, 2002; Franklin and ffrench-Constant, 2008). Further studies on the expression of αV integrin and its ligands in multiple sclerosis lesions will help to establish the contribution that such a mechanism might make to the failure of efficient repair and chronic progressive disease in this condition.




