Directing human neural stem/precursor cells into oligodendrocytes by overexpression of Olig2 transcription factor
Abstract
Multipotential neural stem/precursor cells of the central nervous system were extensively studied for their properties of generating myelinating oligodendrocytes both in vitro and in vivo upon engraftment in animal models of myelin disorders, such as leucodystrophy and multiple sclerosis. These studies provided proof-of-principle that efficient myelination can be achieved by cell transplantation. However, one major drawback of cell-based therapy of myelin diseases is the difficulty in generating oligodendrocytes efficiently from human fetal neural stem/precursor cells (hNPC). Here we explored whether overexpression of the basic helix-loop-helix (bHLH) transcription factor Olig2 in fetal hNPC could enhance the generation of oligodendrocytes both in vitro and in vivo. We report that transduction of hNPC with Olig2-encoding lentiviral vectors enhances their commitment toward an oligodendroglial fate. Moreover, Olig2-transduced hNPC, grafted into the dysmyelinated shiverer mouse brain, survived up to 9 weeks, migrated extensively, and differentiated into MBP+ myelinating oligodendrocytes. In contrast, control hNPC remained at a less mature stage and generated very few myelinating oligodendrocytes. Our study indicates that bHLH transcription factors, such as Olig2, are interesting targets for directing hNPC into myelinating oligodendrocytes. © 2009 Wiley-Liss, Inc.
White matter diseases such as leucodystrophy and multiple sclerosis manifest with a failure of white matter development or immune-mediated demyelination, leading to myelin lesions, oligodendrocyte loss, and axonal damage. More than 20 years ago, cell transplantation studies in demyelinated and dysmyelinated mouse models, such as the myelin basic protein (MBP)-deficient shiverer mouse, provided proof-of-principle that transplantation of myelin-producing cells is one of the most promising strategies for promoting myelin repair (for review see Baron-Van Evercooren and Blakemore, 2004; Goldman et al., 2008). Numerous studies with rodent cells indicated that myelination can be achieved by engraftment of embryonic or adult central nervous system (CNS) tissue (Lachapelle et al., 1983) and oligodendrocyte precursors (OPCs; Avellana-Adalid et al., 1996; Vitry et al., 1999) in the shiverer mutant mice. Moreover, initial data from Pfeiffer and colleagues demonstrated that transplanted A2B5+/O4− OPCs migrated extensively and produced large MBP+ myelin patches in the shiverer brain, whereas grafts of O4+/GalC− purified oligodendrocytes produced only scattered myelin (Warrington et al., 1993). These experiments revealed that the myelogenic capacity of oligodendroglial cells is critically dependent of their migratory properties, a feature of immature stages of the lineage. Later, uncommitted epidermal growth factor (EGF)- or/and basic fibroblast growth factor (bFGF)-responsive neural progenitors (Brüstle et al., 1997; Zhang et al., 1998; Yandava et al., 1999; Mitome et al., 2001), ES cells (Liu et al., 2000; Zhang et al., 2001; Billon et al., 2002; Glaser et al., 2005), and adult SVZ neural stem/precursor cells (Eftekharpour et al., 2007) were extensively studied for their ability to migrate and to generate myelinating oligodendrocytes in rodents.
However, in contrast to rodent cells, the differentiation of hNPC into oligodendrocytes is very low both in vitro (Murray and Dubois-Dalcq, 1997; Vescovi et al., 1999; Zhang and Duncan, 2000) and in vivo after transplantation into rodent CNS (Brüstle et al., 1997; Flax et al., 1998; Fricker et al., 1999; Tamaki et al., 2002). In fact, very few studies have reported myelin formation from fetal hNPC (Gumpel et al., 1987; Seilhean et al., 1996; Zhang and Duncan 2000; Martinez-Serrano et al., 2001). To improve the potential of hNPC to generate myelin in vivo, one strategy consisted of selecting progenitors already committed to the glial fate (Roy et al., 1999; Nunes et al., 2003; Windrem et al., 2004, 2008). Another strategy is to direct the commitment of hNPC toward the lineage of interest. In the present study, we tested whether overexpression of the basic helix-loop-helix (bHLH) transcription factor Olig2 could improve the ability of fetal hNPC to generate oligodendrocytes in vitro and in vivo after grafting. We report that transduction of hNPC with an Olig2-encoding lentivirus induces their commitment toward an oligodendroglial fate in vitro and significantly enhances the generation of myelinating oligodendrocytes in vivo after engraftment in the shiverer mouse brain.
MATERIALS AND METHODS
hNPC Cultures
hNPC were derived from ganglionic eminences (GE) of three human fetuses (from 6.5 to 8.5 gestational weeks, g.w), obtained from legal abortion and with informed consent of the patients. The whole GE was carefully dissected, dissociated in ATV (0.05% trypsin, 0.1 glucose, 0.02% EDTA; reagents from Sigma-Aldrich, St. Louis, MO), and grown as neurospheres in NEF medium composed of DMEM/F12 media supplemented with N2 (1%; Invitrogen, Cergy Pontoise, France), B27 (0.5%), HEPES (5 mM), glucose (6 mg/ml), and FGF2 and EGF (20 ng/ml each; Sigma-Aldrich). Neurospheres were passaged every 2–3 weeks and were kept in culture for 2 months. Cells were used at passage lower than 10. All procedures described above were approved by the French Biomedicine Agency and INSERM ethical committee and were in accordance with guidelines provided by European legislation.
Lentiviral Transduction
Olig2 cDNA (a gift from Dr. C. Stiles, Dana-Faber Cancer Institute, Harvard Medical School, Boston, MA) was cloned in a bicistronic lentiviral vector encoding enhanced green fluorescent protein (EGFP) under the control of the EF1-alpha promoter (Fig. 1; pWI lentiviral construct; kindly provided by Dr D. Trono, University of Lausanne). Viral stocks were obtained after triple transfection of 293T cells, with p8.7 (capsid plasmid), pMD.G plasmid encoding the VSV envelop proteins, and pWPI-EGFP or pWPI-Olig2 lentiviral vectors. After amplification in vitro, human fetal cells (5 × 105 cells) were transduced with lentiviral vectors encoding either EGFP alone (pWPI, control vector) or Olig2 and EGFP (pWPI-Olig2, test vector) with 100 ng of p24 (protocol available upon request).
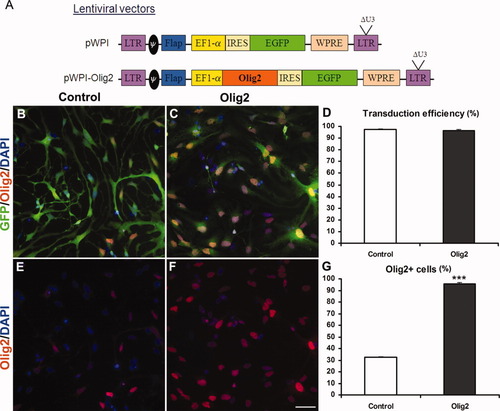
Lentiviral tranduction of Olig2 in fetal hNPC. Diagram of pWPI and pWI-Olig2 bicistronic lentiviral vectors used in this study (A). Coimmunolabeling for GFP and Olig2 of hNPC cultures, transduced with EGFP alone (B,E) and Olig2-expressing (C,F) lentiviruses. The transduction efficiency, expressed as the percentage of GFP+ cells over the total number of cells, was 97.5% ± 0.2% for the control and 96.3% ± 1.2% for the Olig2 lentivirus (D). Transduction with Olig2-expressing lentivirus leads to a threefold increase of Olig2+ cells compared with control (G). Student's t-test, ***P < 0.001. Scale bar = 50 μm.
In Vitro Characterization of hNPC and Differentiation Assays
Neurospheres were dissociated into single cells with ATV solution (0.05% trypsin, 0.1% glucose, 0.02% EDTA) and plated onto coverslips coated with polyornithine (100 μg/ml) and laminin (1 μg/ml; both from Sigma-Aldrich). For initial characterization, hNPC were kept in NEF medium for 7 days. For differentiation assays, hNPC were placed in a basal medium without EGF and FGF2 for 7 days. Cells were fixed for 20 min in 4% paraformaldehyde (PFA), washed in phosphate-buffered saline (PBS), and incubated with the following primary antibodies: rabbit polyclonal (1:100; Millipore, Molsheim, France) or chicken ployclonal anti-GFP (Aves Labs, Inc., Tigard, OR), anti-nestin (1/500; Millipore), A2B5 (1/10; ATCC, Manassas, VA), anti-Ki67 (1/100; BD Biosciences, Le Pont de Claix, France), anti-MAP5 (1/100; Sigma-Aldrich), anti-β3-tubulin (1/400; Sigma-Aldrich), anti-Olig2 (1/100; Millipore), and anti-GFAP (1/100; Millipore). Secondary antibodies used were FITC-, TRITC-, or Cy5-conjugated anti-rabbit, anti-chicken, or anti-mouse IgG (1/100; Dako, Trappes, France). Nuclei were stained by bisbenzimide (Hoechst 33342; Sigma-Aldrich).
Transplantation in the Shiverer Brain
pWPI and pWPI-Olig2 transduced human neurospheres were dissociated into single cell suspension of 100,000 cells/μl, and 5 × 105 cells were grafted in the newborn shiverer mouse brain (n = 15 for each condition) as previously described (Vitry et al., 1999). To avoid graft rejection, mothers received, until weaning, intraperitoneal daily injections of cyclosporine A (Sandimmun, 20 mg/kg; Novartis). After weaning, the grafted shiverer mice were kept under immunosuppressant treatment until sacrifice. Two days (n = 3 for each group) and 9 weeks (n = 12 for each group) after transplantation, mice were perfused with 4% (w/v) PFA in PBS through the right ventricle of the heart under terminal anesthesia. Brains were incubated overnight in 20% (w/v) sucrose in PBS, embedded in Cryomatrix compound (Thermo, Pittsburg, PA), and frozen in cold isopentane (–60°C). Horizontal serial sections (12 μm thick) were cut on a cryostat (Microm Microtech, HM560, Francheville, France) and stored at −20°C until use.
Immunohistochemical Analysis
Slides were incubated for 1 hr in blocking solution (PBS with 0.1% Triton X-100 and 10% normal goat serum; Sigma-Aldrich) and then with primary antibodies overnight at 4°C. The following primary antibodies were used to assess the differentiation of hNPC in the shiverer mouse brain: rabbit polyclonal human specific anti-nestin antibody (1/100; Covance, Princeton, NJ) for human neural stem cells and early progenitors; mouse anti-NeuN (1/100; Millipore) and antidoublecortin (DCX; 1/100; Santa Cruz Biotechnology, Santa-Cruz, CA) for neurons and neuroblasts, respectively; mouse anti-GFAP (1/100; Millipore) for astrocytes; mouse anti-NG2 (1/100; Millipore) for OPCs, rabbit anti-Olig2 (1/100; Millipore) and goat polyclonal anti-Sox10 (1/50; R&D Systems, Minneapolis, MN) for oligodendroglial cells; and mouse monoclonal anti-MBP (1/100; Millipore) for myelinating oligodendrocytes. In all immunohistochemical staining, grafted cells were detected with anti-human-specific nuclei (hNu; 1/100; Millipore). The expression of EGFP is not illustrated, and staining with cell-type-specific markers, revealed with AMCA-conjugated secondary antibodies, is shown in green in Figures 3-5.
Statistical Analysis
For the in vitro data, cells counts were performed in at least three independent experiments and are expressed as mean ± SEM. In grafting experiments, the number of MBP+ oligodendrocytes over the total number of hNu+ cells was quantified in five serial sections (100 μm apart) taken from three series of slides of pWI-EGFP (n = 12) and pWI-Olig2 (n = 12) grafted shiverer brains. Student's t-test and Mann-Whitney test were used for statistical analysis. P < 0.05 was considered statistically significant.
RESULTS
Immunocharacterization of Fetal hNPC in Neurosphere Cultures
Fetal hNPC were grown as floating neurospheres in a medium supplemented with FGF2 and EGF. Under these conditions, the vast majority of the cell population displayed an immature phenotype, as indicated by their widespread expression of the neural stem cell marker nestin (98.5% ± 0.3%) and by their expression of the proliferation marker Ki67 (45.3% ± 2.5%). A minority of committed astrocytes and neuroblasts was also present in these cultures, as indicated by the detection of GFAP+ (10.8% ± 0.6%) and β3-tubulin+ (2.0% ± 0.1%) cells. Furthermore, 3.4% ± 0.3% of the cells expressed A2B5, and 28.5% ± 1.0% were Olig2+, two markers expressed both by glial and neuronal precursors. O4+ oligodendrocytes were not detected under FGF2 and EGF conditions, indicating that committed oligodendrocytes were absent from these neurophere cultures.
Overexpression of Olig2 Enhances the Commitment of hNPC Toward an Oligodendroglial Lineage
To improve the generation of oligodendrocytes from hNPC, we first tested, in vitro, whether overexpression of Olig2 could favor their specification and/or differentiation into oligodendroglial cells. With this aim, we designed a bicistronic lentiviral vector encoding both Olig2 and EGFP under control of the ubiquitous EF1α promoter (pWPI-Olig2 vector; Fig. 1A). As a control, we used the same lentiviral vector encoding EGFP alone (pWPI vector). After amplification in vitro, fetal hNPC were transduced with each vector, and transduction efficiency was evaluated by counting the number of EGFP+ cells. One week after transduction with Olig2 or control lentiviruses, 97% ± 0.7% of the cells expressed EGFP Fig. 1B–D). Moreover, the number of Olig2+ cells was increased threefold after transduction with Olig2-encoding lentiviral vectors (95.6% ± 1.1% of Olig2+ cells; Fig. 1F,G) compared with control (32.5% ± 0.7% of Olig2+ cells; Fig. 1E,G). Furthermore, double immunocytochemistry confirmed that all human cells coexpressed EGFP and Olig2 (Fig. 1C,F) after transduction with Olig2-expressing lentiviruses. Finally, lentiviral transduction did not modify the phenotype of the hNPC, insofar as no major phenotypic difference was observed between hNPC transduced with EGFP alone and untransduced cells (data not shown).
We then tested whether Olig2 overexpression modified the differentiation potential of hNPC. Olig2- and control-transduced cells were induced to differentiate for 7 days, in basal medium deprived of FGF2 and EGF. Under these conditions, the number Ki67+ cells was unaffected after transduction with Olig2-encoding lentiviral vectors compared with the control group (Fig. 2A–C), indicating that Olig2 overexpression did affect the proliferation rate of hNPC. Furthermore, the number of MAP5+ neuroblasts (Fig. 2D–F) and GFAP+ astrocytes (data not shown) did not change between the two groups, whereas the proportion of A2B5+ cells underwent a twofold increase after transduction with the Olig2-encoding lentivirus (Fig. 2G–I). Interestingly, A2B5+ cells displayed the typical flat morphology of glial precursors, which was more pronounced after Olig2 transduction (Fig. 2G,H). To characterize A2B5+ cells further, we performed immunolabeling for GFAP, MAP5, and Olig1 (Supp. Info. Fig. 1). GFAP+ cells were rarely detected under either condition (less than 1%), and GFAP staining was never localized in A2B5+ cells with the typical fat morphology (Supp. Info. Fig. 1A–D). Moreover, A2B5+ cells did not express the neuroblast marker MAP5 (Supp. Info. Fig. 1E–H) and were stained for Olig1 (Supp. Info. Fig. 1I–L), after transduction with the control or Olig2-expressing lentiviruses. Altogether, our results suggested that newly generated A2B5+ cells correspond to oligodendrocyte precursors. However, fully committed O4+ oligodendrocytes were not detected under either condition, after 7 days of differentiation (data not shown).
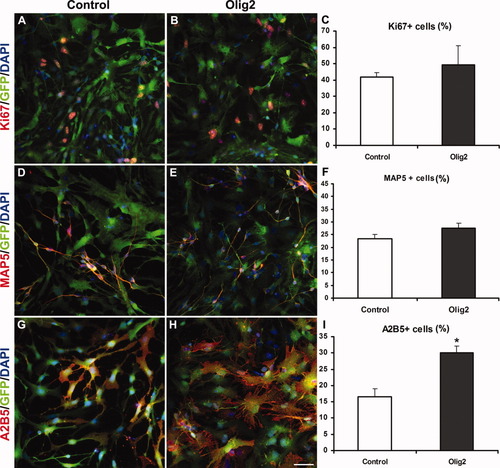
Olig2 overexpression drives the commitment of hNPC toward an oligodendroglial fate. A–C: Cell proliferation, assessed by Ki67 labelling, is unchanged in Olig2-transduced cultures (B,C) compared with control (A,C). Neuronal differentiation determined by MAP5 immunostaining remains also unaffected after lentiviral transduction with Olig2 (D–F), whereas the number of A2B5+ cells is significantly increased by twofold in Olig2-transduced cultures (H,I) compared with control (G,I). Student's t-test, *P < 0.05. Scale bar = 50 μm.
Overexpression of Olig2 in hNPC Increases the Generation of Myelinating Oligodendrocytes After Grafting Into the Shiverer Mouse Brain
To address whether Olig2 overexpression enhances the myelinating potential of hNPC in vivo, Olig2- and EGFP-transduced cells were grafted into the newborn shiverer mouse brain. Grafted mice (n = 15 for each experimental groups) were sacrificed 2 days (n = 3) and 9 weeks (n = 12) after transplantation. Because expression of EGFP is driven by an IRES sequence, expression of the reporter gene in the Olig2-transduced cells was weaker than in controls. Therefore, grafted cells were detected in the shiverer brain with a specific anti-human nuclei antibody. Two days after grafting, human cells were located mainly around the lateral ventricles, and few transplanted cells migrated in the corpus callosum, cingulum, and fimbria (data not shown). At this early time point, both control and Olig2-transduced hNPC were mainly nestin+/Ki67+ cells, consistent with their very immature phenotype.
Nine weeks after transplantation, hNu+ cells were detected in 80% of the grafted shiverer mouse brains, indicating that transduced cells survived and were not rejected from the host. Moreover, human cells migrated in the fimbria and corpus callosum and toward the contralateral hemisphere of the host brain (Fig. 3A). At this time point, the vast majority of the control and Olig2-transduced cells expressed nestin (Fig. 3B,C), suggesting that they still displayed a very immature phenotype. Double immunostaining confirmed that all grafted Olig2-transduced cells were stained for both hNu and Olig2 (Fig. 3E) compared with control (Fig. 3D). Interestingly, both control and Olig2-transduced hNPC generated Sox10+ oligodendroglial cells (Fig. 3F,G) and MBP+ oligodendrocytes (see Fig. 5A–D). Moreover, very few grafted hNPC differentiated into GFAP+ astrocytes (Fig. 4A,B) or DCX+ neuroblasts (Fig. 4C,D) in the two experimental groups.
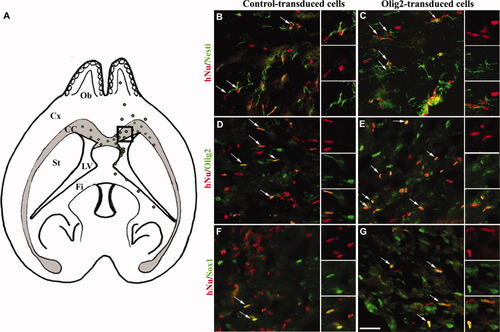
Immunocharacterization of transduced hNPC in the shiverer brain, 9 weeks after grafting. Schematic horizontal section of the shiverer mouse brain illustrating the distribution of transduced hNPC 9 weeks postgrafting (A). Double immunolabeling for hNu and nestin (B,C), Olig2 (D,E), and Sox10 (F,G) in control- and Olig2-transduced groups. Nine weeks after transplantation, hNPC stained with the anti-hNu antibody were mainly nestin+ in the corpus callosum (B,C). In the Olig2-transduced group, all hNu+ cells detected in the shiverer brain expressed Olig2 (E) compared with the control group (D). Under both conditions, few grafted cells differentiated into Sox10+ oligodendroglial cells (F,G). For each panel, arrows indicate double-stained grafted hNPC, shown in insets. Scale bar = 50 μm.
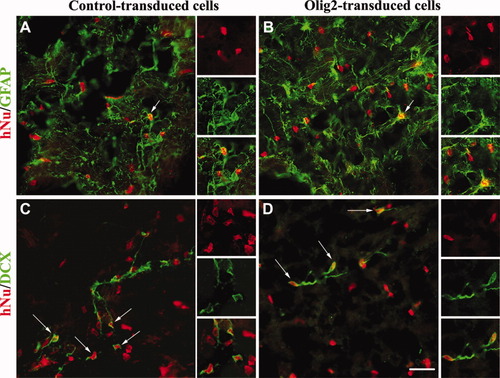
Differentiation potential of hNPC in astrocytes and neurons, 9 weeks after grafting. Few hNu+ NPC expressed the astroglial marker GFAP (A,B, arrow) or the neuroblast marker doublecortin (C,D, arrows) in the control and Olig2-transduced group. Insets illustrate grafted hNPC shown by the arrows. Scale bar = 50 μm.
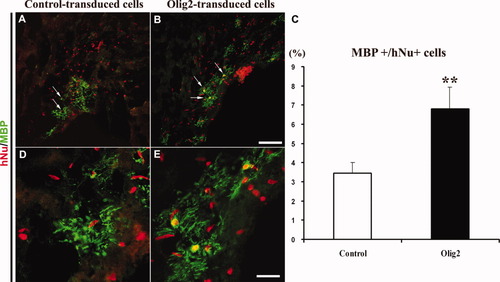
Olig2 transduction of fetal hNPC promotes the generation of MBP+ oligodendrocytes in the shiverer mouse brain. Control and Olig2-transduced cells were detected in the shiverer brain with the anti-hNu antibody (A,B). In both groups, grafted cells migrated in the corpus callosum and fimbria, where they differentiated into MBP+ oligodendrocytes. Arrows in A and B point to MBP+ oligodendrocytes, shown at higher magnification in D and E, respectively. Nine weeks after transplantation, quantification of MBP+ cells over the total number of hNu+ cells indicated that Olig2 transduction increased by twofold the generation of myelinating oligodendrocytes compared with control (C). Student's t-test, **P < 0.01. Scale bars = 200 μm in B; 50 μm in E.
To evaluate the effects of Olig2 overexpression on the myelinating potential of hNPC, we quantified the number of MBP+ cells over the total grafted hNu+ cells in both groups. MBP+/hNu+ cells were detected mostly in the corpus callosum (Fig. 5A,B) and fimbria, in close proximity to the lateral ventricle. Cell counts demonstrated a significant twofold increase in the number of MBP+ oligodendrocytes in the Olig2-transduced group (6.8% ± 1.1%; Fig. 5B,C,E) compared with control (3.4% ± 0.6%; Fig. 5A,D,C). Altogether our data indicate that overexpression of Olig2 increased the commitment of fetal hNPC toward an oligodendroglial fate and their ability to produce myelinating oligodendrocytes in vivo after engraftment in the shiverer mouse brain.
DISCUSSION
Cell transplantation-based strategies for demyelinating diseases using fetal hNPC are, in part, hampered by their poor ability to generate oligodendroglial cells both of high purity and at high yield. Previous studies indicated that glial precursor cells, FACS-purified or isolated on the basis of their expression of specific cell surface antigens, lead to extensive myelination upon grafting in dysmyelinating rodent mutants (Roy et al., 1999; Windrem et al., 2004, 2008). Here, we tested whether manipulating the expression level of Olig2 in hNPC could increase their ability to generate oligodendrocytes. Fetal hNPC transduced with an Olig2-encoding lentiviral vector generated an increased number of A2B5+ oligodendroglial precursors in vitro, whereas the generation of GFAP+ astrocytes and MAP5+ neuroblasts remained unchanged. Moreover, Olig2-transduced fetal hNPC grafted in the MBP-deficient shiverer mouse brain generated more efficiently myelinating oligodendrocytes than EGFP-transduced cells, indicating that Olig2 is a target of interest for enhancing hNPC-derived oligodendrocytes.
The specification of oligodendrocytes from ventricular neuroepithelial cells in human fetal development is still poorly understood. However, several lines of evidence suggest that the mechanisms of oligodendrogenesis within the developing CNS are conserved from chick to human. For instance, initial reports on the expression profile of OPC markers, such as PDGF-Rα, O4, CNPase, and PLP, indicated that human OPCs originate from restricted domains along the neuroaxis. In human fetal spinal cord, the first wave of OPCs arises from the precursor motoneuron domain at about 6.5 g.w. (Hajihosseini et al., 1996; Ligon et al., 2004; Jakovceski and Zecevic, 2005a), whereas, in the telencephalon, OPCs are present in the ganglionic eminences at about 15 g.w. (Back et al., 2001; Rakic and Zecevic, 2003; Jakovcevski and Zecevic 2005a). MBP is detected in the forebrain as early as 17 g.w., and 5 days later compact myelin is observed in the thalamus. As in rodents, Olig1 and Olig2 transcription factors are likely to be involved in human oligodendrocyte specification and differentiation. Both Olig1 and Olig2 are expressed before OPC markers, such as PDGF-Rα and NG2. At 5 g.w., Olig1 is expressed in the telencephalic radial glia, whereas Olig2 is expressed in OPCs as well as in migrating neuroblasts in more caudal regions (Jakovcevski and Zecevic 2005b). Moreover, as in rodents, Olig2 expression is maintained at all stages of human oligodendrocyte development (Lu et al., 2000; Jakovcevski and Zecevic 2005b). Interestingly, Olig2 is also expressed in a subpopulation of neural stem (type B) and transit-amplifying cells (type C) of the adult human subventricular zone (Sanai et al., 2004; Quinones-Hoja et al., 2006). However, the requirements for Olig2 in human oligodendrocyte specification and differentiation remain unclear. Our results provide evidence that overexpression of Olig2 directs the commitment of hNPC toward an oligodendroglial cell fate in vitro and promotes their ability to generate myelinating oligodendrocytes in vivo.
During development, oligodendrocyte specification requires combinatorial expression of several transcription factors, which act in synergy with Olig2 (Rowitch, 2004). In the chick spinal cord, coelectroporation of Olig2 together with Nkx2.2 promotes the generation of differentiated oligodendrocytes (Sun et al., 2001; Zhou et al., 2001). Moreover, combined expression of Olig2 and Mash-1 was shown to be necessary for oligodendrocyte specification and differentiation in the telencephalon and spinal cord (Parras et al., 2007; Sugimori et al., 2007). Interestingly, bHLH transcription factors, such as Mash1, are expressed in the fetal hNPC cultures used in this study (C.M. and B.N.-O., unpublished data). Thus, Olig2 transduction combined with the expression of other transcription factors in hNPC may account for the increase in the number of A2B5+ OPCs reported here. However, despite this increase, differentiated oligodendrocytes were not observed in Olig2-transducted cultures, indicating that overexpression of Olig2 alone did not accelerate the differentiation of hNPC along the oligodendroglial lineage. Thus, our data provide evidence for a functional role of Olig2 in the specification of oligodendrocytes from hNPC and therefore highlight further similarities between rodent and human NPC (Lu et al., 2002; Takebayashi et al., 2002; Zhou and Anderson, 2002).
In rodents, loss-of-function studies demonstrated that, together with a role in oligodendrocyte specification, Olig2 has a function in motoneuron and astrocyte specification (Lu et al., 2002; Takabayashi et al., 2002; Zhou and Anderson, 2002; Cai et al., 2007). In these latter cell types, transient expression of Olig2 is necessary for cell commitment, but down-regulation of Olig2 is required for their differentiation into mature neurons or astrocytes. In this respect, it was reported that sustained expression of Olig2 in motoneuron progenitors prevents their terminal differentiation by inhibiting neurogenin 2 (Lee et al., 2005). In our study, constitutive overexpression of Olig2 in hNPC may account for the absence of any noticeable effects on neuronal and astroglial cells reported here.
Our in vivo data indicated that lentiviral transduction of Olig2 increased the ability of fetal hNPC to generate MBP+ mature oligodendrocytes, upon transplantation into the shiverer mouse brain. These data are also in agreement with previous studies indicating that Olig2 overexpression in mouse embryonic neural stem cells induces their differentiation into mature oligodendrocytes in vitro and in vivo after grafting in chronically demyelinated lesions induced by a cuprizone diet (Copray et al., 2006). However, in our study, large myelin patches were not detected in either of the experimental groups at 9 weeks posttransplantation, suggesting that Olig2 overexpression in hNPC did not significantly modify their timing of differentiation. In fact, one major difference in oligodendrogenesis between rodents and human is the slow timing of differentiation of human cells (for review see Buchet and Baron-Van Evercooren, 2009). This notion is also supported by several lines of evidence, indicating that human glial-committed precursors take at least 12 weeks to give rise to extensive myelination after transplantation into the shiverer mouse brain (Windrem et al., 2004, 2008).
Cell therapies of genetically inherited dysmyelinated and demyelinated diseases still offer the most reliable approach for the replacement of oligodendrocyte and myelin regeneration (for review see Goldman et al., 2008). To this end, a variety of cell types from both the CNS and the PNS have been assessed as potential myelin-forming cell sources (for review see Baron-Van Evercooren and Blakemore, 2004). More recently, human glial progenitors, neural stem cells, and human ES-derived oligodendrocytes precursors were also shown to generate myelinating oligodendrocytes after transplantation in dysmyelinated mouse models (Goldman et al., 2008). These studies provided proof-of-principle demonstration of cell-based therapy for dysmyelinating diseases, such Pelizaeus-Merzbacher disease, hereditary spastic paraplegia, metabolic demyelination, and lysosomal storage disorders. However, the clinical translation of these experimental studies requires the amplification and generation of oligodendrocytes from hNPC with both high purity and high yield. In the present report, we provide compelling evidence that genetic manipulation of key transcription factors, such as Olig2, represents an interesting strategy for enhancing the generation of myelinating oligodendrocytes from human NPC and has a therapeutic relevance for myelin disorders.
Acknowledgements
We thank Corina Garcia, Violetta Zujovic, and Corinne Bachelin for helpful discussion and excellent technical advice on cell culture experiments.




