N-cadherin mediates interaction between precursor cells in the subventricular zone and regulates further differentiation
Abstract
Neurogenesis and cell differentiation in the brain continues throughout life. In the subventricular zone and rostral migratory stream, precursor cells contact each other. Cell–cell interactions mediated via adhesion molecules are no doubt involved in establishing and maintaining the neurogenic ability of these cells. Here, we demonstrate that N-cadherin plays important roles in forming cell clusters and in regulating cell differentiation. N-cadherin is abundantly expressed in chain migrating cells in the subventricular zone and rostral migratory stream but is down-regulated after cells exit these regions. We also show that neurosphere formation is inhibited via suppression of N-cadherin function and that N-cadherin expression is decreased after induction of neurosphere differentiation. Furthermore, we demonstrate that functional blockade of N-cadherin can enhance glial cell differentiation in explant cultures of precursors from the subventricular zone. © 2009 Wiley-Liss, Inc.
Multipotential precursor cells continue to generate neurons and glia in the adult subventricular zone (SVZ) (Weiss et al., 1996a, b). In the SVZ, specialized microenvironments support the self-renewal of precursor cells and their production of differentiated cells (Doetsch, 2003). Adhesive interactions between cells and local signals are the main elements for controlling precursor cell proliferation and differentiation. Adhesive interactions mediate both cell-to-cell and cell-to-substratum contacts, and are crucial for providing the specialized microenvironments for the precursor cells.
In the SVZ, precursor cells form cell clusters. The precursor cells further migrate from the SVZ to the olfactory bulb through the rostral migratory stream (RMS), maintaining contact with each other and so forming a chain migration (Lois et al., 1996). In these environments where they are in close contact with each other, there are very few fully differentiated neurons and glial cells present. It is highly likely that cell–cell contact between precursors may regulate cell differentiation in vivo. Electron microscopic analysis has shown that adhesive junction–like structures exist between clustered cells in the SVZ and RMS (Doetsch et al., 1997; Chazal et al., 2000). Polysialylated neural cell adhesion molecule (PSA-NCAM) is highly expressed in precursor cells and is considered to be an essential adhesion molecule that mediates the formation of cell clusters in the SVZ and RMS (Tomasiewicz et al., 1993; Cremer et al., 1994). However, even in NCAM-deficit mice, the RMS is not completely disturbed and cell clusters still form (Chazal et al., 2000). Moreover, adhesive junction–like structures known to be mediated by cadherin are observed between clustered cells in NCAM-deficit RMS. These observations suggested to us the presence of cell adhesion molecules other than NCAM, which might be involved in cell–cell contact between the precursor cells.
Precursor cells isolated from the SVZ can be maintained in vitro where they proliferate and form floating cell clusters (neurospheres) (Reynolds et al., 1992; Weiss et al., 1996b). Cell–cell interactions between neighboring cells in neurospheres are essential for the self-renewal and multipotency of the neurospheres, and it has been shown that Notch signaling is involved in the process (Hitoshi et al., 2002). Notch signaling can work only between cells in close contact, and therefore, a cell adhesion system between precursor cells is required to form such specialized microenvironments. It has been reported that in addition to PSA-NCAM, N-cadherin is abundantly expressed in neurospheres (Lobo et al., 2003). N-cadherin belongs to the classical cadherin family. Cadherins are Ca2+-dependent homophilic cell adhesion molecules (Takeichi, 1990). In the adult brain, N-cadherin is localized at certain central nervous system synapses (Fannon and Colman, 1996; Uchida et al., 1996). N-cadherin has been shown to play important roles in the formation and function of synapses (Benson and Tanaka, 1998; Tanaka et al., 2000; Bozdagi et al., 2001; Okamura et al., 2004). Because N-cadherin can form junctional structures between neuronal cells and is expressed in neurospheres, we thought that it may play essential roles in forming cell clusters in neurospheres and in the SVZ and RMS in situ.
It is possible that N-cadherin may mediate homophilic cell adhesion in neurospheres, and we tested this idea as described as below. Here, we report that N-cadherin mediates cluster formation of precursor cells and regulates differentiation. First, we show that N-cadherin is expressed in clustered cells in the adult neurogenic region, but not in cells exiting this region. Our in vitro studies show that N-cadherin mediates neurosphere formation and that it is down-regulated during neurosphere differentiation. Moreover, we demonstrate that functional blockade of N-cadherin enhances glial cell differentiation.
MATERIALS AND METHODS
Immunohistochemistry
Adult Swiss Webster mice (2–3 months old) were perfused with 4% paraformaldehyde (PFA)/phosphate-buffered saline (PBS), and brains were immersed in the same fixative at 4°C overnight. Coronal or sagittal sections (14 μm) were cut by cryostat after cryoprotection with 30% sucrose. Sections were washed with 0.1% Triton X-100 in Tris-buffered saline (T-TBS) and blocked with 10% normal goat serum in T-TBS at room temperature (RT) for 1 hr. Subsequently, they were incubated with primary antibodies, including affinity purified N-cadherin antibody raised against the cytoplasmic region (anti-NcadICD, Tanaka et al., 2000), PSA-NCAM (1:100, BD Biosciences), class III β-tubulin (clone Tuj1, 1:500, Research Diagnostic), RIP (1:200, Developmental Studies Hybridoma Bank), S-100β (1:400, Sigma), and IQGAP1 (1:200, Santa Cruz Biotechnology) at 4°C overnight. For N-cadherin immunostaining, ABC Elite kit (Vector) was used after incubation with biotinylated rabbit IgG antibody (1:500, Vector). Signals were detected by diaminobenzidine–peroxidase reaction. For immunofluorescence, secondary antibodies conjugated with fluorophores (Jackson ImmunoResearch or Molecular Probes) were used. For labeling of 5-bromo-2′-deoxy-uridine (BrdU, Roche), mice injected with BrdU (50 mg/kg i.p. for each time) three times a day for 3 days. They were killed on the day after the last injection and processed as above. Frozen sections were incubated with 2 N HCl at 37°C for 30 min followed by immunodetection of BrdU-labeled cells with BrdU antibody (1:100, Harlan Sera-labo). Bright field images were obtained through Olympus upright microscope equipped with CCD camera, and fluorescent images were observed by confocal microscopy (Leica). Images were processed with Adobe Photoshop software.
Preparation of Neurospheres
Neurospheres were generated as described (Weiss et al., 1996a) with slight modifications. E16 mouse brains were dissected in Dulbecco's modified Eagle medium (DMEM)/F-12 (Invitrogen). Striatal tissues were collected in DMEM/F12 and triturated by fire-polished Pasteur pipettes. Dissociated cells were collected by centrifugation and resuspended in growth medium, DMEM/F-12 contained B-27 supplements, penicillin–streptomycin, 25 mM D-glucose (Sigma), 2 μg/ml heparin (Sigma), 20 ng/ml basic fibroblast growth factor (FGF, PeproTech) and 1,000 U/ml mouse leukemia inhibitory factor (LIF, Chemicon). The cell suspension was filtered through a 70-μm cell strainer (Falcon) to remove tissue aggregates. A total of 1–5 × 105 cells/ml were plated in 60-mm petri dishes. Seven days after incubation at 37°C without a medium change, neurospheres were observed in the culture. Floating neurospheres were dissociated by passing through fire-polished Pasteur pipette replated on 60-mm dishes in the same medium. Secondary neurospheres obtained in these cultures were used for experiments. Seven days after secondary plating, neurospheres were fixed with 4% PFA/PBS for immunohistochemistry.
To analyze the differentiation profiles, neurospheres were plated on Matrigel in the differentiation medium (DMEM/F-12 containing B-27 supplements, penicillin–streptomycin, and additional 25 mM D-glucose). Neurospheres were collected at 0, 4, 24, and 48 hr after plating for preparation of protein samples for Western blot testing.
Western Blot
Neurospheres were homogenized with sodium dodecyl sulfate (SDS) sample buffer. Samples were incubated at 4°C overnight, boiled for 10 min, and centrifuged at 15,000 rpm for 30 min at 4°C. Supernatants were subjected to SDS–polyacrylamide gel electrophoresis (SDS-PAGE), and separated proteins were transferred to nitrocellulose membrane. The membrane was blocked with 5% skim milk/PBS containing 0.1% Triton X-100 for 1 hr at RT and incubated with primary antibodies overnight at 4°C. Membranes were washed and incubated with peroxidase-conjugated secondary antibody (Amersham). After washing, bound antibodies were visualized by a chemiluminescence system (PerkinElmer Life Sciences).
Generation of Antibody Against N-cadherin Extracellular Domain
Procedures have been previously described (Shan et al., 2004). In brief, we generated a fusion protein of mouse N-cadherin extracellular domain (most of the EC1–4 domains) and Fc region of human IgG. Purification of N-cadherin–Fc fusion protein was carried out as described by using DE52 followed by protein A column chromatography (Sakurai et al., 1998). Purified fusion protein was used as an immunogen to obtain an antibody against extracellular domains of N-cadherin (MT79). IgG fractions were purified from serum with the Immunopure IgG Protein G purification kit (Pierce) for function-blocking experiments.
Antibody Treatment of Neurospheres
Dissociated primary neurospheres were seeded to generate secondary neurospheres in the growth medium in the presence of 500 μg/ml of MT79 or normal IgG. Cultures were analyzed by size on day 7 after seeding. All clumped floating cells were collected on day 7, dissociated, and replated in the fresh growth medium without antibodies. The numbers of tertiary neurospheres were counted on day 5 after explating.
SVZ Explant Culture
SVZ explant culture was carried out as previously described (Wichterle et al., 1997; Chazal et al., 2000). P3–5 mice were decapitated, and brain slices (300 μm) were prepared with a tissue chopper (McIlwain). Brain slices were placed in Neurobasal medium (Invitrogen) and small pieces (100–400 μm in diameter) of the anterior part of the SVZ were excised with a 22G needle under a dissecting microscope. Cover glasses placed in four-well dishes were coated with Matrigel (1:3 dilution, Becton Dickinson) at RT for 30 min. Explants were plated on the coated coverslips and cultured at 37°C in serum-free medium (NB/B-27) that contained Neurobasal (Invitrogen), B-27 supplement (1:50, Invitrogen), L-glutamine (0.29 mg/ml, Invitrogen), and penicillin–streptomycin (1:200, Invitrogen).
Antibody Treatment in the SVZ Explant Culture
Purified IgG fraction of MT79 was dialyzed against Neurobasal medium and filtered. SDS-PAGE followed by Coomassie brilliant blue (FisherBiotech) staining was performed to estimate the protein concentration of antibody. SVZ explant cultures were prepared as above, and 500 μg/ml of MT79 IgG or rabbit normal IgG in Neurobasal was added 4 hr after plating. Before adding antibody solution, the same amount of medium was removed. Cultures were fixed with 4% PFA 60 hr after plating and subjected to immunocytochemistry and quantitative analysis.
RESULTS
N-cadherin Expression in the Adult SVZ and RMS
First, we analyzed N-cadherin expression patterns in the adult mouse brains in situ by immunohistochemistry with an N-cadherin antibody. The N-cadherin signal was broadly distributed throughout the gray matter. In particular, high signals were detected in synapse-rich regions, such as the mossy fiber terminals of the hippocampal CA3 region and the glomeruli of the olfactory bulb. In contrast, the white matter expressed much lower levels of N-cadherin. N-cadherin was also highly expressed in the SVZ and RMS of adult brains adult brains as visualized in sagittal sections (Fig. 1). These regions contain several types of cells, including multipotential cells, neuroblasts, and glial cells (Doetsch et al., 1997). To clarify which cell types were N-cadherin positive, we performed double staining with several cell markers (Fig. 2). In the SVZ (Fig. 2A–C), N-cadherin signals were virtually colocalized with that of PSA-NCAM-positive cells. PSA-NCAM is expressed in neuroblasts (Miragall et al., 1990; Seki and Arai, 1993) and certain precursor cells (Ben-Hur et al., 1998; Vitry et al., 2001; Balenci et al., 2007). β-Tubulin type III (Tuj1), a neuronal marker, was abundant in the SVZ neuroblasts and colocalized with N-cadherin-positive cells in the SVZ (Fig. 2D–F). In the striatal neurons, N-cadherin was expressed but much less abundantly than in the SVZ cells. RIP-positive cells that are in the oligodendrocyte lineage (Friedman et al., 1989) were not observed in the SVZ, but were present in the surrounding area of the SVZ (Fig. 2G,I). Unlike the cells in the SVZ cells, they expressed little N-cadherin and formed a clear border with the N-cadherin-positive region. S-100β labeled ependymal cells, but not SVZ cells (Chiasson et al., 1999), and S-100β-labeled ependymal cells were clearly distinguished from N-cadherin-expressed cells in the SVZ (Fig. 2J–L). Similarly, in the RMS, N-cadherin colocalized with PSA-NCAM (data not shown).
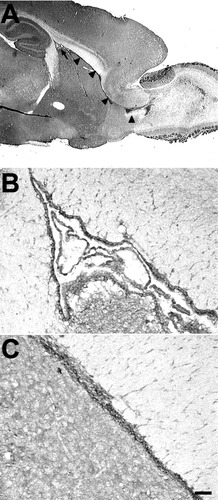
N-cadherin expression in the SVZ and RMS of adult mouse brain. A: In the adult mouse brain, N-cadherin is expressed throughout the gray matter but is less expressed in the white matter. The SVZ (arrow) and RMS (arrowhead) are regions with the highest N-cadherin expression. B,C: N-cadherin is expressed in the cell bodies aggregated in chainlike structures. Expression level is similar throughout the SVZ and RMS. Scale bar = 1 mm in A, 0.1 mm in B, C.
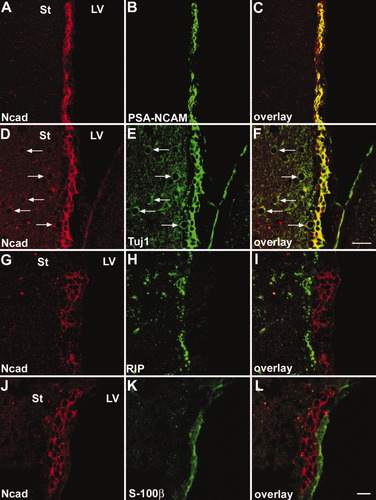
Neuronal precursor cells express N-cadherin in the SVZ. Double immunohistochemistries showed localization of N-cadherin and different neuronal markers in the coronal section of adult mouse brain. A–C: N-cadherin colocalizes mostly with PSA-NCAM. D–F: Tuj1-positive neuroblasts also express N-cadherin. Mature neurons in the striatum express less N-cadherin than neuroblasts in the SVZ (arrows). G–I: There are few RIP-positive cells in the SVZ. RIP-positive cells at the edge of the striatum are distinct from the SVZ. J–K: S-100β is predominantly expressed in the ependymal cells. N-cadherin does not colocalize with S-100β. St, striatum; LV, lateral ventricle. Scale bars = 20 μm in A–F, 10 μm G–L.
In order to distinguish the different cell types that are N-cadherin positive in SVZ, we have colabeled with PSA-NCAM because positive cells are known to be multipotent. We also compared N-cadherin expression patterns with that of IQGAP1, a marker of transit-amplifying precursor cells (Balenci et al., 2007). We found that N-cadherin is partially colocalized with PSA-NCAM at the same sites of cell–cell contacts and exhibited similar expression patterns with the IQGAP1 in the anterior horn of the SVZ (Fig. 3), suggesting that N-cadherin is not only expressed in neuroblasts, but also in the transit-amplifying precursor cells.
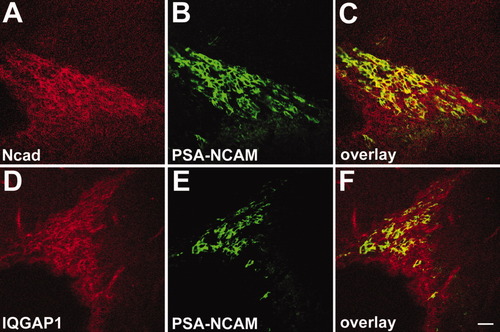
Precursor cells express N-cadherin in the anterior horn of the SVZ (Sagittal section). A–C: Double staining with N-cadherin and PSA-NCAM. D–F: Double staining with IQGAP1 with PSA-NCAM. The expression pattern of N-cadherin is similar to that of IQGAP1, the marker of transit-amplifying precursor cells. Scale bar = 20 μm.
To follow the cells that were born in the SVZ or RMS, we pulse-labeled proliferative cells by BrdU. Two groups of BrdU-positive cells were found in or around regions of the SVZ (Fig. 4A) and RMS (Fig. 4B); one with high levels of N-cadherin was located in the SVZ and RMS, another without N-cadherin expression was found in cells migrating out of these regions (Fig 4, asterisks).
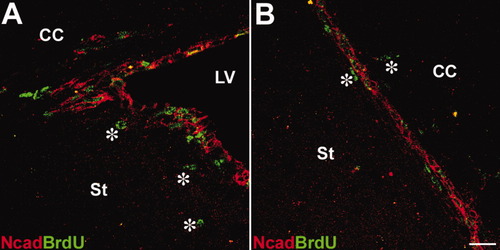
N-cadherin is down-regulated in the postmitotic cells that exit the SVZ or RMS. A: Nuclei of postmitotic cells are labeled BrdU. BrdU-positive cells are found to express N-cadherin as well in the SVZ. In contrast, BrdU-positive cells that exited the SVZ or RMS are losing the expression of N-cadherin (*). B: Similar results were obtained in the RMS (*). Scale bar = 20 μm. St: striatum, LV: lateral ventricle, CC: corpus callosum.
N-cadherin Is Expressed in Neurospheres and Down-regulated During Differentiation
In order to examine the roles N-cadherin plays in the differentiation of precursors in the SVZ and RMS, we isolated precursor cells from these regions and cultured them in vitro. Our results show that N-cadherin is abundantly expressed in floating neurospheres (Fig. 5A,B). In contrast, once neurospheres were induced to differentiate after plating in a differentiation-inducing environment, cells migrated out of the neurospheres, and N-cadherin and β-catenin expression was dramatically down-regulated (Fig. 5C,D). Our data reveal that levels of N-cadherin expression might be correlated with precursor cell differentiation in the neurospheres.
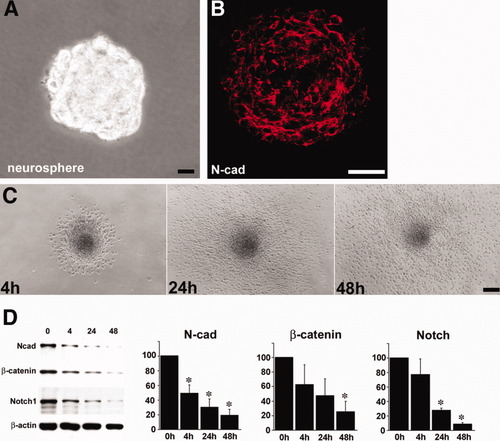
N-cadherin expression in neurospheres and down-regulation during differentiation. A: Cells tightly bind each other in the neurosphere. B: N-cadherin is abundant at the cell–cell contact sites in the neurosphere. C: Single neurosphere is observed at 4 hr, 24 hr and 48 hr after plating. Cells rapidly migrate out of the plated neurosphere. D: N-cadherin and β-catenin are significantly (P < 0.01) decreased in parallel with activated-Notch1 during differentiation of neurosphere. Statistical analysis included one-way analysis of variance, followed by Sheffé's post hoc test. Data are mean ± SE. Scale bars = 50 μm.
Perturbation of N-cadherin Function Inhibits Neurosphere Formation
To assess N-cadherin function in the formation of neurospheres, we applied anti-N-cadherin extracellular domain serum MT79 to the neurosphere culture. Adhesion assay experiments (Tamura et al., 1998; Shan et al., 2000) revealed that treatment with MT79 can inhibit N-cadherin-mediated cell adhesion (data not shown). MT79 or IgG was put in the medium at the beginning of secondary neurosphere culture. Neurospheres treated with MT79 were smaller in size than those subjected to IgG treatment (Fig. 6A–C). The numbers of smaller neurospheres (30–90 μm in diameter) in MT79-treated cultures were increased compared with IgG-treated (IgG, 28.0 ± 3.3%; MT79, 55.9 ± 3.3%; mean ± standard error [SE], P < 0.05). In contrast, the numbers of large (150–210 μm) and extra-large spheres (210-μm in diameter) were greatly reduced in MT79-treated groups than that in IgG-treated groups (IgG, 22.0 ± 2.6%; MT79, 8.0 ± 2.1%; mean ± SE, P < 0.05, IgG, 14.9 ± 2.0%; MT79, 1.0 ± 0.7%; mean ± SE, P < 0.05). After passage, we counted the number of tertiary spheres. Our data showed that the numbers of spheres are more reduced as the cells are blocked by MT79 antibody (Fig. 6D). These results reveal that N-cadherin seems to be required for the formation of neurospheres.
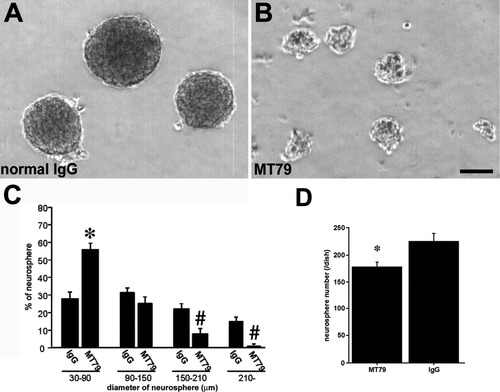
N-cadherin antibody (MT79) perturbs formation of neurosphere. A,B: Neurospheres are formed equally portions at the diameter from 30 to 210 μm in the presence of normal rabbit IgG, whereas, neurospheres are predominate at the diameter from 30 to 90 μm in the MT79 treated culture. Scale bar = 100 μm. C: The number of small neurospheres (30–90 μm in diameter) was significantly (*P < 0.05) higher in MT79-treated culture than that in IgG-treated. In contrast, the number of spheres with the large diameter (150–210 μm and 210 μm in diameter) was significantly (#P < 0.05) higher in IgG-treated group than that in MT79-treated. D: the numbers of spheres (tertiary neurosphere) were greatly reduced as the cells treated with MT79 at the beginning of secondary neurosphere culture. Statistical analysis is performed by nonparametric analysis (Mann-Whitney U-test). Data are mean ± SE.
Functional Blocking of N-cadherin Promotes Differentiation of SVZ Cells Into Glial Cells
To further analyze N-cadherin function in the SVZ, we used an in vitro culture system that has been used previously to analyze chainlike migration (Wichterle et al., 1997). Explants of SVZ regions prepared from P3–5 mice were cultured in Matrigel. After 48 hr, massive cell migration was observed (Fig. 7). In the culture, we first analyzed N-cadherin expression profile together with several cell markers to see which cell types were N-cadherin positive. N-cadherin colocalized with PSA-NCAM and Tuj1 that were expressed in chainlike migrating cells (Fig. 7A–F). This result revealed that N-cadherin expression patterns in the culture were similar to data obtained in vivo studies, suggesting that N-cadherin might be involved in retaining cells in the SVZ.
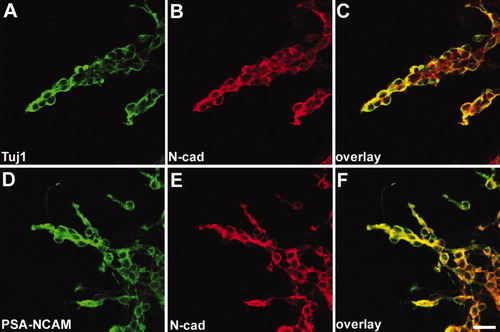
N-cadherin is expressed in the chainlike migrating precursor cells in the SVZ explant culture. N-cadherin started to express at 48 hr after the SVZ plating. A–C, N-cadherin is expressed in the chainlike migrating cells, which are Tuj1 positive. D–F, PSA-NCAM-positive precursor cells are also expressed N-cadherin. Scale bar = 20 μm.
Next, we asked whether perturbation of N-cadherin function could change the differentiation patterns of the SVZ cells by treating cultures with N-cadherin antibodies. In control cultures, cells migrated out of the explants in chainlike spines. In this situation, a few O4 (oligodendrocyte marker)-positive cells were observed outside the explants (Fig. 8B). In the explants, O4-positive cells appeared as fine dots. These cells did not have any particularly distinctive morphology. However, in cultures treated with MT79, the number of O4-positive cells migrating out of the explants increased significantly (34.9-fold increase). Moreover, these O4-positive cells were multipolar (Fig. 8A,C), the typical shape of mature oligodendrocytes. This finding supports the idea that disruption of N-cadherin-mediated cell–cell interactions between precursor cells promotes oligodendrocyte differentiation.
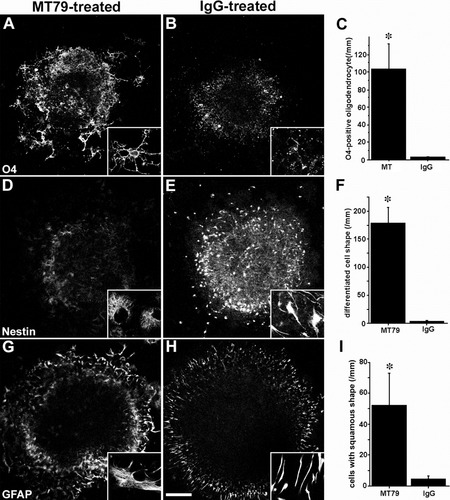
Functional blocking of N-cadherin promotes differentiation of glial cells in the SVZ explant culture. All observation was performed 60 hr after the SVZ plating. A,B: In the MT79-treated culture, multipolar O4-positive cells are observed. C: The number of these cells significantly (*P < 0.05) increases when compared with the IgG-treated cells. D,E: Nestin immunoreactivity is decreased in the MT79-treated culture, whereas many elongated cells with strong nestin signals are observed in the IgG-treated culture. Moderately nestin-positive cells are flat, similar to the differentiated astrocytes. F: Number of differentiated cells that were determined by flat cell morphology and weaker nestin signal is significantly (*P < 0.05) increased compared with the IgG-treated culture. G,H: Morphological differences of GFAP-positive cells were observed in IgG-treated culture (radial glia-like shape) and in MT79-treated (squamous shape). Almost all of GFAP-positive cells are radial glia-like shape in the IgG-treated culture. In contrast, GFAP-positive cells with squamous shape appears in the MT79-treated culture. I: There is significant (*P < 0.05) difference in the number of cell with squamous shape. Statistical analysis is performed by Mann-Whitney U-test. Data are mean ± SE. Scale bar = 100 μm.
We also found distinct morphological changes in cells of the astrocyte lineage in the cultures. Nestin is expressed in neuronal stem cells and astrocytes, and in both immature and reactive ones. In control treated cultures, nestin-positive cells had an elongated and radial glia–like shape (Fig. 8E). Their shapes might reflect their immature properties because radial glia have some characteristics of neuronal stem cells (Miyata et al., 2001). In contrast, in the MT79-treated cultures, nestin signals decreased in the entire explant; moreover, it was moderately positive in flat-shaped cells observed at the edge of explants (Fig. 8D,F). This morphology was very similar to that of mature astrocytes, and so this finding suggests that MT79 treatment facilitates generation of mature astrocytes. Additionally, most of the glial fibrillary acid protein (GFAP)-positive cells were radial glia–like in shape in the IgG-treated culture, whereas many GFAP-positive cells with squamous shape migrated out of the explants in the MT79-treated culture (Fig. 8G–I).
DISCUSSION
Precursor cells form clusters and sustain certain neurogenic ability both in vitro (neurosphere) and in the adult neurogenic regions in vivo (chain migration in the SVZ and RMS). In both situations, microenvironments surrounding these cells are controlled by adhesive interactions and soluble signals set to support neurogenic ability and to slow the progression to fully differentiated neurons and glial cells. In the present study, we demonstrate that homophilic cell–cell interactions mediated by N-cadherin may be one of the elements that sustains neurogenic microenvironments both in vitro and in vivo.
Comparison of stem cell niches in several systems has revealed common features: cell–cell interactions and diffusible signals are key elements for controlling stem cell activation and differentiation. Within a niche, stem cells are frequently anchored to a basal lamina or stromal cells that can provide a substrate for oriented cell division. In turn, this determines segregation of key determinants into one or both daughter cells. In the Drosophila ovary, germline stem cells are anchored to cap cells by DE-cadherin-mediated adhesion junctions (Song et al., 2002). Cap cells regulate self-renewing divisions of germinal stem cells via secreted or transmembrane proteins, whose biological activities may only be effective in the short range; therefore, target cells need to be in close proximity. Disruption of DE-cadherin results in stem cell loss, underscoring the importance of adhesive interactions in niche function.
In the current study, we demonstrated that N-cadherin is highly expressed in neurospheres prepared from these regions in vitro and precursor cells in the SVZ and RMS in vivo. N-cadherin expression is down-regulated when differentiation of neurospheres is induced in vitro. Furthermore, function blocking of N-cadherin-mediated interactions disrupts neurosphere formation and enhances glial cell differentiation. These results suggest that homophilic cell–cell interactions between precursor cells mediated by N-cadherin are crucial for retaining precursor cells in these brain regions and for modulating their differentiation. Possible biological implications for N-cadherin-mediated homophilic cell interactions between precursor cells are 1) anchoring precursor cells in the SVZ, 2) regulating cell positions by a sorting-out mechanism, 3) modulating signaling between precursor cells, and 4) supporting cell migration in the SVZ and RMS.
Cells that express the same cadherin adhere to each other and segregate out other cells that express different cadherins through their homophilic binding abilities (Nakagawa and Takeichi, 1995, 1998). This cell-recognition system is important in tissue morphogenesis. In the present study, we show that N-cadherin is expressed in the SVZ and RMS. PSA-NCAM-positive cells are abundant in these regions and express N-cadherin abundantly. PSA-NCAM is also expressed in neuroblasts and in a part of precursor cells (Ben-Hur et al., 1998), which express IQGAP1 (Balenci et al., 2007). Precursor cells expressing both PSA-NCAM and IQGAP1 can form neurospheres and produce neurons and glia in vitro. Down-regulation of N-cadherin function in PSA-NCAM-positive precursor cells may depress neurogenic ability and enhance glial cell differentiation. In the adult SVZ and RMS, loss of N-cadherin expression might induce dissociation of cells from the neurogenic microenvironments, N-cadherin-rich region, through in situ cell sorting mechanisms. These cells, in turn, released from adhesion to their neighbors, might switch on their differentiation program and migrate away from these brain regions. On the other hand, expression of N-cadherin on neurogenic cells will retain these cells in the neurogenic region, maintaining their neurogenic ability. It has been shown that in songbird brains, lifelong neurogenesis takes place. Interestingly, Barami et al. (1994) reported that N-cadherin levels were high in precursor cells in the SVZ of the adult songbird, but down-regulated in the newly born neurons migrating out into the striatal parenchyma. They also demonstrated that down-regulation of N-cadherin was required for these cells to leave the SVZ.
It has been reported that Notch1 is expressed in the adult SVZ and RMS (Irvin et al., 2001). Lateral inhibition by Notch signaling was found to be required to maintain neuronal stem cells (Hitoshi et al., 2002) and regulated oligodendrocyte differentiation (Wang et al., 1998). For Notch signaling to be activated, cells have to be in contact with each other, and therefore, the N-cadherin-mediated adhesion system may cooperate with the Notch-signaling pathway. Another possible mechanism to regulate cell differentiation is through β-catenin signaling. β-Catenin binds to the N-cadherin cytoplasmic region and links to actin filaments. It also functions as a signaling molecule in the cells and works as a nuclear transcriptional factor. N-cadherin down-regulation might increase free β-catenin and influence this signaling pathway. Because glial differentiation is likely to be a default process (Doetsch, 2003), activation and maintenance of neurogenic signals are necessary to keep neurogenesis ongoing. Therefore, N-cadherin-mediated cell–cell interactions might be required for these processes, and perturbation of N-cadherin function might reduce neurogenic signals, resulting in enhancement of glial differentiation. Other signaling molecules that have been reported as key molecules to regulate cell fate in the neurogenic microenvironments include bone morphogenic proteins (BMPs) (Lim et al., 2000) and sonic hedgehog (Machold et al., 2003; Palma et al., 2005). N-cadherin-mediated cell adhesion may bring and retain precursor cells in the close proximity of these signaling molecules whose activities may be short range. Losing N-cadherin-mediated cell adhesion may free cells that may escape the effects of these signals. In sum, we propose a model in which N-cadherin, highly expressed in precursor cells, is involved in maintaining the clustering of these cells, providing the neurogenic microenvironments. In this situation, differentiation to the glial cell lineage is actively inhibited. Either by losing N-cadherin expression or by being removed from neurogenic signals, precursor cells differentiate further to glial cell lineage.
In mammalian brain, it has been shown that PSA-NCAM plays a role in chain migration as a mildly adhesive cell adhesion molecule (Tomasiewicz et al., 1993; Cremer et al., 1994). Inhibition of PSA-NCAM results in moderate disruption of cell migration and enhancement of glial cell differentiation. However, in PSA-NCAM-deficient mice, there are still junctional structures between chain migrating cells, suggesting that other cell adhesion molecules are involved in the cell–cell interactions. In fact, when NCAM and cadherins are coexpressed on the same cell surface, intercellular adhesion is mediated by the cadherins present, with NCAM occupying the lateral cell surfaces as apposed to the adhesive surfaces (Mege et al., 1988). It has also been reported that integrins play an important role in chain formation in the adult RMS (Jacques et al., 1998; Murase and Horwitz, 2002; Belvindrah et al., 2007). Integrins consist of α and β subunits and bind to extracellular matrix. β1 integrins are expressed in chain-migrating cells in the RMS and chain formation is disrupted in β1-subunit deficit mice (Belvindrah et al., 2007). Laminin, an extracellular matrix molecule, is a binding partner of integrins and also is expressed in chain-migrating cells in the RMS. Chain formation also is disrupted in laminin α2/α4 deficit mice. These results suggest that β1 integrins may mediate cell cluster formation through the extracellular matrix in the neurogenic region. As we report here, N-cadherin is also involved in the interactions between the chain-migrating cells, and we assume that N-cadherin might be involved in chain migration of precursor cells in these brain areas.
Acknowledgements
We thank members of Colman lab for helpful discussions. RIP antibody was obtained from the Developmental Studies Hybridoma Bank maintained by the University of Iowa.




