Multiple functions of the paranodal junction of myelinated nerve fibers†
This paper is dedicated to the memory and the legacy of Steve Pfeiffer.
Abstract
Myelin sheaths include an extraordinary structure, the “paranodal axoglial junction” (PNJ), which attaches the sheath to the axon at each end of each myelin segment. Its size is enormous and its structure unique. Here we review past and current studies showing that this junction can serve multiple functions in maintaining reliable saltatory conduction. The present evidence points to three functions in particular. 1) It seals the myelin sheath to the axon to prevent major shunting of nodal action currents beneath the myelin sheath while still leaving a narrow channel interconnecting the internodal periaxonal space with the perinodal space. This pathway represents a potential route through which juxtaparanodal and internodal channels can influence nodal activity and through which nutrients, such as glucose, and other metabolites can diffuse to and from the internodal periaxonal space. 2) It serves as a mechanism for maintaining discrete, differentiated axolemmal domains at and around the node of Ranvier by acting as a barrier to the lateral movement of ion channel complexes within the axolemma, thus concentrating voltage-gated sodium channels at the node and segregating fast voltage-gated potassium channels to the juxtaparanode under the myelin sheath. 3) It attaches the myelin sheath to the axon on either side of the node and can thus maintain nodal dimensions in the face of mechanical stresses associated with stretch or other local factors that might cause disjunction. It is therefore the likely means for maintaining constancy of nodal surface area and electrical parameters essential for consistency in conduction. © 2009 Wiley-Liss, Inc.
Among the intercellular junctions known, one stands out by virtue of its mammoth size and unique structure. The paranodal axoglial junction (PNJ; Robertson, 1959), by far the largest intercellular junction known (see Fig. 1), is situated at each end of each myelin segment in every myelinated nerve fiber of the vertebrate central and peripheral nervous systems. A single such junction may occupy as much as 150 μm2, depending on the diameter of the fiber and the thickness of the myelin sheath. A long fiber might have ∼1,000 myelin segments, with an aggregate junctional surface area of ∼300,000 μm2 along a single axon. Both the parent neuron of the axon ensheathed and the cells that form each segment of myelin contribute specific proteins to this junction. The nerve cell body must therefore make a considerable expenditure in generating the requisite components, at least one of which, the axonal protein Caspr (Einheber et al., 1997), or paranodin (Menegoz et al., 1997), has no known function in nerve cells except at the PNJ.
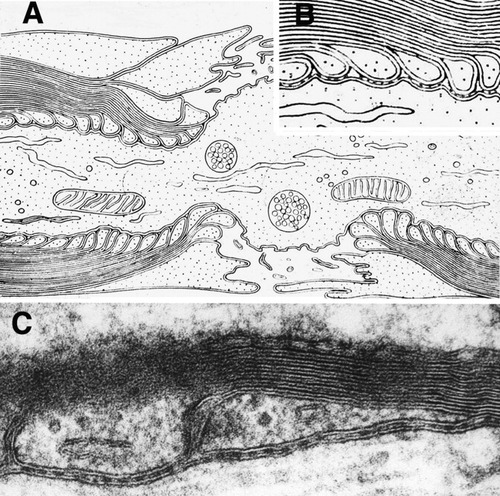
A: Robertson's original diagram showing overlapping “terminal loops” indenting the paranodal axolemma and perinodal microvilli originating from the outermost layer of the Schwann cell (from Robertson, 1959). B: Detail of Robertson's diagram modified to show a junctional gap containing “transverse bands.” C: TEM image showing two terminal loops abutting an axon (bottom) forming the paranodal junction comprising the axonal and glial “unit membranes” separated by a gap containing transverse bands (modified from Rosenbluth, 1995).
Surely the investment in forming this extraordinary junction must reflect important functional roles that cannot be met by any simpler structure. Much has been published in recent years about the architecture and molecular constituents of the nodal, paranodal, and juxtaparanodal domains of myelinated neve fibers. This article focuses on the functions of the PNJ and considers why it evolved to the size and conformation it has.
RESTRICTING SHORT-CIRCUITING
Saltatory conduction has the virtue that it requires axonal activity only at the interruptions between myelin segments, the nodes of Ranvier, occupying only ∼0.1–0.3% of the axonal surface. The exchange of ions involved in generating action potentials is therefore markedly restricted in amount compared with that in equivalent nonmyelinated axons that use continuous conduction. Thus, the overall entry of sodium into a myelinated axon during activity is reduced, and correspondingly less energy is required to pump it out. The quantity of potassium ions released into the general extracellular space is similarly restricted, thus facilitating maintenance of a constant extracellular ionic milieu and reducing the possibility of extracellular potassium accumulation, especially in the narrow intercellular spaces of the CNS, that could lead to spontaneous activity and seizures.
Nodes are separated by a considerable distance, ranging from hundreds to thousands of microns, and, for saltatory conduction to work, it is essential that activity at one node lead reliably to activity at neighboring nodes. The current generated at a node must therefore be sufficient to depolarize adjacent nodes passively to a threshold level. Accordingly, the current circuit set up at one active node, at which the resting potential is reversed, must be completed through neighboring nodes, where the passive current must generate a depolarization adequate to initiate a new action potential there. Whether it does or not depends on how much current is generated by the first node, what fraction of that current in fact reaches the adjacent nodes, and what the size and structure of the adjacent nodes are.
If the edges of the myelin sheath flanking each node were not closely applied to the axon, a fraction of the current generated by the active node would follow a local short-circuit pathway beneath the myelin sheath, and correspondingly less current would then reach adjacent nodes. The myelin sheath must therefore be relatively well sealed to the axon immediately adjacent to the electrically active nodal membrane.
One might expect that this need would be most simply met by a tight junction between the paranodal myelin sheath and the axon. Tight junctions provide very high resistance to longitudinal current flow between apposed plasma membranes and are capable of maintaining the separation between very different extracellular environments. Tight junctions certainly occur between myelin lamellae near the node, but, as shown in Figures 1B,C and 2, the junction between the paranodal loops of myelin and the axon is quite different from a tight junction. It is characterized, rather, by three elements: 1) indentation of the axolemma; 2) a very narrow intercellular gap (Elfvin, 1961); and 3) ridge-like spacers, the “transverse bands,” bridging that gap (Bargmann and Lindner, 1964; Andres, 1965). Freeze-fracture replicas (Fig. 3B) show that the transverse bands are oblique in orientation (Rosenbluth, 1976), and the narrow channels between them therefore pass obliquely across the entire junction between the perinodal space and the juxtaparanodal periaxonal space.
Thus, the paranodal seal between the myelin sheath and the axon membrane is far from tight. It includes a thin but apparently patent space, accessible to tracers (Hirano and Dembitzer, 1969; Mierzwa and Rosenbluth, 2006; Rosenbluth et al., 2006), and thereby provides a route for short-circuiting of nodal action currents beneath the myelin sheath. Nevertheless, because the gap is so narrow and the junction so long, the resistance of this pathway is high relative to that of the extracellular pathway outside the myelin sheath, thus imposing a significant restriction to short-circuiting. In brief, although a patent pathway beneath the myelin sheath is present, its dimensions are such that relatively little current is likely to follow this route.
So why is there a gap? What advantage does this enormous, cumbersome, incomplete seal provide over a simple tight junction? Two possibilities come to mind.
First, a small fraction of the nodal action current does follow the short-circuit pathway beneath the myelin sheath and may activate internodal channels, including the voltage-gated potassium channels segregated to the juxtaparanodal region of the axon. Earlier studies found that 4-AP, a Kv1 channel blocker, does not affect action potentials either at PNS nodes (Chiu and Ritchie, 1980; Sherratt et al., 1980) or at CNS nodes (Kocsis and Waxman, 1980), implying that these juxtaparanodal Kv1 channels are normally isolated and do not play a role in action potential propagation.
Later studies concluded, however, that potassium channels do play a role in the optic nerve (Gordon et al., 1989; Rasband et al., 1999a), in the PNS during development (Vabnick et al., 1999), and probably also in adult PNS fibers, in view of the effects of potassium channel blockade on afterpotentials (David et al., 1993), the backfiring seen after Kv1 channel knockout (Chiu et al., 1999), and the neuromyotonia caused by potassium channel antibodies (Vincent, 2004; Kleopa et al., 2006). Some of this disparity may reflect the effects of the abnormal conditions inherent in any in vitro study. However, it is also possible that short-circuit pathways through the paranode and the Schmidt-Lanterman (SL) clefts of normal fibers are able to undergo plastic changes in patency or caliber in response to physiological or minor pathological stresses in vivo so that juxtaparanodal and internodal events may play variable roles in nerve conduction under different conditions (cf. Moran and Mateu, 1983; Stys and Waxman, 1994).
Second, a pathway under the myelin sheath would not only allow the flow of some current but also constitute a pathway for slow diffusion between the perinodal extracellular space and the internodal periaxonal space. Such a pathway may be essential to provide the axon with access to glucose and other water-soluble metabolites that could not pass across the myelin sheath (Mierzwa and Rosenbluth, 2006).
In the case of large fibers in the PNS, patent extracellular pathways are also present in the SL clefts (Robertson, 1958), providing another means for slow exchange between the extracellular space outside the axon and the internodal periaxonal space. In the SL clefts, too, the spiral extracellular path is very long and narrow and therefore has high resistance with respect to current flow but is nevertheless open to slow diffusion of metabolites. SL clefts are largely absent from the CNS, however, and there the paranodal junction is the only extracellular pathway to and from the internodal periaxonal space.
In short, the paranodal junction appears to have evolved to meet two competing needs: 1) it must be sufficiently long, narrow, and therefore resistive, to restrict short-circuiting of nodal action currents, thus permitting saltatory conduction, and 2) it must be sufficiently open to permit slow diffusion of metabolites to and from the internodal axon. It also constitutes a potential pathway for activation of internodal channels, including the juxtaparanodal potassium channels.
DEFINING AND MAINTAINING AXOLEMMAL DOMAINS
Numerous reviews, most recently that by Salzer et al. (2008), have gone over the identity and roles of the ever-increasing number of molecules associated with nodal sodium and juxtparanodal potassium channels. It would be redundant to rereview this topic, so I will briefly sketch the evolution of the subject, referring to just a few relevant recent studies.
Structural nonuniformity of the axolemma first became apparent in freeze-fracture studies of myelinated axons, which demonstrated regional specialization of the axolemma, resulting in the formation of discrete domains at and around the node of Ranvier (Figs. 2, 3). The axolemma was found to have a paracrystalline structure in the paranodal region (Livingston et al., 1973; Schnapp and Mugnaini, 1975) and high concentrations of outer leaflet particles, representing membrane proteins, in both nodal and juxtaparanodal regions (Rosenbluth, 1976). No such regional differentiation was found in unmyelinated axons.
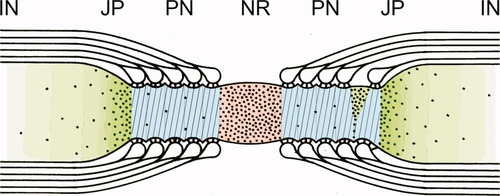
Diagram of a normal CNS node of Ranvier showing the domain organization of the axolemma based on freeze-fracture particle distribution (black dots) and superimposed immunofluorescence stains. Node of Ranvier (NR) is shown in red (pan Na channels), paranode (PN) in blue (Caspr), juxtaparanode (JP) in dark green (Kv1.1 and -1.2 channels), and internode (IN) in light green. Transverse band impressions on the paranodal axon are shown as oblique lines (cf. Fig. 3B).
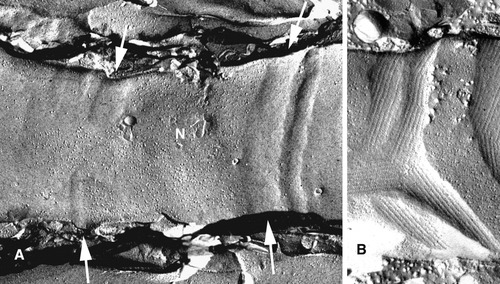
Freeze-fracture views of paranodal axolemmal outer leaflet (from inside the axon looking outward). A: Developing PNJ from normal mouse PNS. The axon runs horizontally across the field, with a particle-rich immature node (N) in the middle. It is sharply delimited on the right by a vertical, strip-like paranodal loop indentation (right arrows), which is free of particles. On the left side of the node, particle-free indentations are not yet complete (left arrows) and do not extend completely across the axolemma. Where the indentations are incomplete, particles stream through the defect toward the internode at left. At this early time in development, transverse bands have not yet formed, so the indentations do not show the embossed paracrystalline pattern visible in mature junctions, as in B (modified from Tao-Cheng and Rosenbluth, 1982). B: Dysmyelinated axon from mature shiverer mouse CNS. In contrast to the regularly arrayed paranodal junctional indentations seen normally (Fig. 2), the shiverer paranode is strikingly irregular. Indentations are particle free and show a paracrystalline pattern embossed on the axolemma by oblique, ridge-like transverse bands. Node-like particles interspersed among indentations are sharply delimited by them, but the domains interdigitate and are highly irregular (cf. Rasband and Shrager, 2000).
It was proposed that the accumulations of nodal particles represent the voltage-gated sodium channels (“ionophores”) involved in saltatory conduction (Rosenbluth, 1976). Indeed, the nodal particle density later proved to correspond closely to nodal sodium channel density estimated from gating current measurements (Chiu, 1980). Subsequent immunoflurorescence studies demonstrated specific labeling of sodium channels at the node (Dugandzija-Novakovic et al., 1995) and of fast voltage-gated potassium channels in the juxtaparanodal axolemma and abnodal end of the paranodal axolemma (Wang et al., 1993), the distribution of the immunofluorescence corresponding exactly to that of the membrane particles. As indicated above, both channel types have now been shown to form complexes with adhesion and cytoskeletal molecules, implying that the membrane particles probably correspond to sodium and potassium channel complexes.
What is the value of segregating potassium channels to the juxtaparanodal region beneath the myelin sheath? One likely consequence is that the amount of potassium released during saltatory conduction by myelinated fibers is significantly lower than that released during continuous conduction by unmyelinated or demyelinated fibers. Maintaining a consistent potassium concentration in the narrow extracellular spaces of the CNS is therefore simplified, and the amount of energy expended on clearing excess extracellular potassium is accordingly reduced. The likelihood of potassium-induced depolarization and spontaneous activity, such as that leading to seizures in myelin-deficient rats (Young et al., 1989), is also reduced.
How the differentiated structure of the axolemma forms and is maintained has been a matter of controversy. It was originally proposed that the nodal particle accumulation “is restricted by the paranodal junction,” which serves “to retain intramembranous particles at the node.” Attachment of channels to the cytoskeleton was proposed as well (Rosenbluth, 1976). In support of the paranodal barrier hypothesis, subsequent work showed clearly that incomplete formation of the PNJ during development (Tao-Cheng and Rosenbluth, 1982; Fig. 3A) or abnormal formation of the PNJ in dysmyelinating mutants (Rosenbluth, 1995; Fig. 3B) resulted in complementary abnormalities in the nodal domain.
Later studies showed that the nodal complex is initiated by “pioneer” molecules in the presumptive nodal axolemma, including NrCAM (Custer et al., 2003) and neurofascin (NF) 186, which binds specifically to gliomedin, in Schwann cell microvilli (Eshed et al., 2005), followed by binding of other molecules at those sites, including ankyrinG and sodium channels. Gliomedin is absent from the CNS, and there brevican binds NF186 (Ogawa et al., 2008). However, evidence from the CNS shows that nodal sodium channel clustering depends on the presence of paranodal junctions (Rasband et al., 1999b) and not on the presence of nodal NF186 (Zonta et al., 2008), consistent with the barrier hypothesis.
In the PNS, evidence from Sherman et al. (2005) indicates that knockout of NF186 results in diminution of nodal sodium channel complexes but not their elimination. The quantitative data in that study show that the fluorescence intensity of NF–/– nodes (∼4.4 after subtracting out the internodal background level) is only ∼3.5× lower than that of wild-type nodes (∼15 over background). Assuming that fluorescence intensity is approximately proportional to channel density, this would mean a decrease in nodal sodium channel density from the normal level of ∼1,200/μm2 to ∼350/μm2 in the NF–/– nodes. This figure is markedly less than normal and probably insufficient to support saltatory conduction; nevertheless, 350 channels/μm2 is a concentration ∼15× higher than the internodal level of ∼20–25 channels/μm2 (Shrager, 1989), implying that significant nodal sodium channel accumulation can occur at PNS nodes in the absence of NF186. Reconstituting paranodal NF155 did not change the result, implying that paranodal junctions with or without NF155, a major constituent of the transverse bands, can cause significant nodal sodium channel accumulation, albeit not to the normal level, in PNS myelinated axons lacking NF186.
These findings are consistent with the image in Figure 3A showing a PNS fiber at an early stage of paranode development. Even before transverse bands have formed, as indicated by the lack of a diagonal pattern in the junctional axolemma, the characteristic paranodal indentations exclude nodal particles and constitute barriers to them. In the CGT knockout as well, domains are defined by such indentations in the absence of transverse bands (Rosenbluth et al., 2003). Thus, current evidence supports a dual mechanism for forming and maintaining axolemmal domains at and around PNS nodes of Ranvier, an intramembranous diffusion barrier formed by paranodal junctions, and local attachment to adhesion and cytoskeletal elements.
Developmental studies have shown also that nodes originate as widely separated heminodes, which advance toward one another and eventually fuse, indicating that sodium channels can move within the plane of the axolemma, although not into the paranode (Dugandzija-Novakovic et al., 1995). Ultimately, the specific shape of the nodal domain is determined by the flanking paranodal junctions, since defects in those junctions result in distinct changes in the shape and extent of the nodal domain (Rasband et al., 2003; Rios et al., 2003; Rosenbluth et al., 2003). The juxtaparanodal location of fast, voltage-gated potassium channels as well depends on a barrier mechanism that excludes them from the paranode. Defects in the PNJ result in relocation of potassium channels into the paranode adjacent to the nodal domain (Dupree et al., 1998; Bhat et al., 2001; Boyle et al., 2001).
Loss of transverse bands seen in some dysmyelinating mutants (e.g., CGT−/− and CST−/−) appear to underlie gradual deterioration of axolemmal domain boundaries with lengthening of the nodal domain, widening of the paranodal junctional cleft (Boyle et al., 2001), and disjunction of paranodal loops with shortening of the PNJ, all leading to progressive neurological impairment and premature death (Rosenbluth et al., 2008).
In short, both paranodal barriers and linkage to the cytoskeleton and extracellular structures appear to contribute to the formation and maintenance of the axolemmal ion channel domains and the differentiated state of the axolemma at and around the node of Ranvier. In both cases, the sites at which nodes form are dictated by the myelin-forming cells, not the axon. Moreover, in dysmyelinating mutants, loss of transverse bands is directly correlated with gradual deterioration of paranodal structure and axolemmal domain organization, nodal elongation, and progressive neurological impairment.
KEEPING THE NODAL GAP CONSTANT
Perturbations of paranodal structure, as in the Caspr, CGT, and contactin knockouts, all result in significant slowing of conduction, despite preservation of nodal sodium channels and internodal myelin. This could be the result of 1) increased leakage of nodal action currents beneath the myelin sheath resulting from loss of transverse bands, widening of the junctional gap, and shortening of the PNJ caused by detachment of some paranodal loops. These changes would result in diminution in resistance along the path through the paranodal junction interconnecting the internodal periaxonal space and the perinodal space, thus increasing short-circuiting under the myelin sheath and diminishing passive current through adjacent nodes; 2) relocation of juxtaparanodal potassium channels to the paranode, where the myelin thins progressively and passive depolarization of the axolemma correspondingly rises, thus increasing the likelihood that the displaced Kv1 potassium channels will be activated. Potassium channel activity just adjacent to the node could significantly diminish nodal action currents; or 3) lengthening of the node resulting in increase in its surface area.
The relative importance of these factors is unknown. The overall time required for saltatory conduction depends largely on the cumulative delays at the nodes because of the time required for discharging nodal capacitance (Huxley and Staempfli, 1949). Computer simulations suggest that increased nodal surface area could be largely counterbalanced by a proportionate increase in the number of nodal sodium channels (Moore et al., 1978), as might occur in chronically lengthened nodes.
On the other hand, if nodes were lengthened acutely by withdrawal of adnodal paranodal loops, e.g., as a result of mechanical stress, nodal capacitance would increase; nodal membrane resistance would decrease; passive current density across the lengthened nodal membrane would diminish, as would the magnitude of passive depolarization; and time to reach threshold would increase. There would be no opportunity for insertion of additional sodium channels into the newly exposed membrane unless an intracellular pool were rapidly available within the axoplasm. The safety factor, resulting from the additional current generated beyond that needed to regenerate conduction, might avoid conduction block in the face of some nodal enlargement, but conduction velocity would still be reduced. More important, even if nodes lengthened gradually, channel insertion might not keep up with the increase in nodal surface area. As a result, the enlarged nodal domain might not become fully occupied by sodium channels.
Widening of the intercellular gap at PNJs that have defective transverse bands would increase shunting of nodal action currents under the myelin sheath in proportion to the decreased resistance of that pathway and would in effect increase the nodal surface area involved in passive depolarization, thus also contributing to slowing of conduction.
In brief, reliable saltatory conduction requires that nodal membrane surface area remain approximately constant and that the flanking PNJs remain reasonably tight in the face of acute stresses and nerve length changes associated with limb movements in the case of PNS fibers and flexion and extension of the spine in the case of CNS fibers. What is the mechanism for keeping the nodal area constant? Clearly, the flanking PNJs at which the glial terminal loops and the axolemma are bound together are in an ideal position to attach the myelin sheath to the axon immediately alongside the node securely enough to provide that constancy. Indeed, normal nodal length, ∼1 μm, varies within a very narrow range.
The attachment of paranodal myelin loops to the axon is mediated via interconnections with cytoskeletal elements in the cells forming the PNJ and by the transverse bands bridging the junctional gap (Garcia-Fresco et al., 2006; Ogawa et al., 2006). Glial NF155 and axonal Caspr/contactin are thought to make up the transverse bands, but recent evidence indicates that axonal netrin and its glial binding partner, DCC, are also constituents (Jarjour et al., 2008). Additional junctional components may yet be identified from among the myriad proteins known to be present in myelin (Taylor et al., 2004). Abnormalities of any of these constituents could result in absent or defective transverse bands and compromised adhesion at the paranodal axoglial junction, leading to disjunction and changes in nodal geometry and resulting in increased nodal surface and attendant conduction defects.
A common finding in dysmyelinating mutants that have transverse band defects, e.g., the md rat (Rosenbluth, 1987), CGT–/– mouse (Dupree et al., 1998), Caspr–/– mouse (Bhat et al., 2001), contactin–/– mouse (Boyle et al., 2001), and CST–/– mouse (Honke et al., 2002), is “eversion” of paranodal loops immediately adjacent to the nodal domain, as if these loops have become disconnected from the axon and retracted away. If this happened acutely, the effect would be an increase in length of the nodal domain corresponding to the axial length of the detached loops. Thus, eversion of five adnodal loops (0.1–0.2 μm each) on both sides of a node would lengthen the nodal domain by 1–2 μm over its normal length of ∼1 μm. This mechanism could account for the progressive lengthening of nodes and corresponding shortening of paranodes in these mutants, all of which are grossly deficient in transverse bands.
The consequences of nodal lengthening for conduction would depend partially on whether sodium channel complexes in the lengthened nodes came to be distributed uniformly at the same density found at normal nodes or uniformly but at lower density or nonuniformly in the form of islands surrounded by bare areas of axolemma virtually devoid of sodium channels. In the case of CGT–/– mice, noncircular accumulations at which the fluorescence intensity of the sodium channels is high around part of the nodal circumference but below the detection threshold around the remainder (Fig. 4) appear as early as 30 days (Rosenbluth et al., 2003). Thus, the nodal axolemma in these abnormal fibers appears to contain a subdomain of high sodium channel density adjacent to a bare, channel-deficient area. At 90 days, the nodal channel domain may appear as a noncircumferential streak as long as 15 μm. Progression of these changes would be expected to result in gradually increasing nodal capacitance and decreasing nodal transmembrane resistance, resulting in diminished passive depolarization, gradual diminution in the proportion of nodal membrane occupied by sodium channels, and eventually block when the proportion of the nodal domain occupied by sodium channels and passive depolarization are sufficiently reduced. This sequence of events could account for progressive disability in CGT−/− mice as well as other mutants with defective PNJs.

Confocal image of nodal Na channels in dysmyelinated CGT−/− CNS at 30 days demonstrated by immunofluorescence. The confocal image stack has been rotated to provide an end-on, axial view of the nerve fibers. Arrows indicate two nodes in which sodium channels do not encircle the axon completely, in contrast to the example visible at the lower left in which the Na channel fluorescence is annular (from Rosenbluth et al., 2003).
For similar reasons, the occasional node-like patches that occur in the amyelinated axons of dystrophic mice (Rosenbluth, 1979; Bray et al., 1980; Deerinck et al., 1997) and myelin-deficient rats (Rosenbluth, 1987; Arroyo et al., 2002) as well as those induced in vitro by a soluble factor released from astrocytes (Kaplan et al., 1997) or by soluble gliomedin (Eshed et al., 2007) may not be effective in impulse propagation, because all are surrounded by nonspecialized axolemma rather than circumferential PNJs that restrict current flow along the periaxonal space.
Enlargement of nodes can also result from intrusion of cellular processes into the PNJ from the adnodal or abnodal end. This is seen in genetic abnormalities, e.g., Caspr-null mice (Bhat et al., 2001), and can also arise as a result of acquired disease. Proteases released during inflammation, e.g., could lyse junctional components, resulting in stripping of the myelin sheath from the axon by invading cells (Griffin et al., 1996) or slippage of paranodal attachment sites (Yu and Bunge, 1975; Rosenbluth, 1984). Such a mechanism could cause significant conduction defects even without frank demyelination in diseases such as multiple sclerosis.
Apart from the changes in nerve fiber length associated with movement, more extensive and more rapid length changes can occur in association with trauma or can be generated artificially in experimental animals. In studies of CNS fibers, artificial stretch has been shown to result in selective damage to nodes characterized by the appearance of nodal membrane patches lacking the typical cytoskeletal “undercoating” and of bleb-like structures, lacking intramembranous particles and, presumably, sodium channels. Disruption of axoplasmic transport follows and ultimately, axonal interruption. The final pathological changes are thought to be mediated by increased influx of calcium through the stretch-induced nodal defects (Maxwell et al., 1999). In the PNS as well, slow, artificial stretch results in nodal elongation, leading to conduction block (Ikeda et al., 2000). Thus, mechanical stresses out of the physiologic range can have devastating effects on the geometry, integrity, and function of normal nodes.
In summary, in order that myelinated nerve fiber conduction be secure and consistent, nodal axonal surface area, and therefore nodal capacitance and transmembrane resistance, must be held reasonably constant. Attachment of the myelin sheath to the axon in the paranodal region is the probable mechanism for keeping nodal surface area constant as well as restricting current flow along the paranodal periaxonal space. Defective adhesion between paranodal terminal loops and the underlying axon, related in particular to defective transverse bands, as seen in some mutants, can lead to progressive abnormalities in the location of PNJs, resulting in increased nodal surface area, attendant defects in conduction velocity and security, and progressive neurological impairment.
SUMMARY AND CONCLUSIONS
The unique structure and size of the PNJ reflect its multiple, and to some extent competing, functions, three of which are of paramount importance in saltatory conduction.
The first is electrical isolation. The narrow, highly elongated paranodal periaxonal space meets this need by restricting current flow beneath the myelin sheath while not blocking it entirely. Thus, the junctional gap is of sufficiently high resistance to prevent significant short-circuiting of nodal action currents, while still being able to carry some current flow, thereby providing a route through which juxtaparanodal and internodal channel activity can affect nodal behavior under some conditions. At the same time, this path is also able to serve as a route for diffusion of aqueous materials between the perinodal extracellular space and the internodal periaxonal space. Thus, the axon has slow access to water-soluble molecules that could not diffuse across the multiple lipid bilayers of the myelin sheath. Traffic of aqueous materials presumably also moves in the reverse direction, carrying metabolites and allowing for gradual equilibration of the periaxonal and perinodal spaces.
The sceond is domain organization. The axolemma of the paranodal junction blocks lateral movement of nodal sodium channel complexes and juxtaparanodal potassium channel complexes within the plane of the membrane. Thus, acting as a barrier, it forms discrete boundaries that define and limit the domains, confine the respective ion channel complexes to those domains, and also contribute to the accumulation of the ion channel complexes. Channels are also held in place by associated molecules linked to the axonal cytoskeleton and to extracellular structural elements. The third is maintenance of the nodal gap. The PNJ is responsible for adhesion of the myelin sheath to the axon at specific sites and is thus in a position to maintain the length of the nodal domain in the face of local stresses. It probably also serves to keep extraneous cell processes from intruding beneath the myelin sheath and cleaving it away from the axon. Gradually developing disability in some mutants with abnormal PNJs, especially those with defective transverse bands, probably results from gradual shifts in pararanodal attachment sites, leading to expansion of the nodal axolemmal domain, increase in its capacitance, decrease in its resistance, and diminution in its sodium channel density, all contributing to progressive functional deficits in saltatory conduction.




