Fyn kinase is involved in oligodendroglial cell differentiation induced by apotransferrin
Abstract
Mechanisms that regulate oligodendroglial cell (OLGc) differentiation are the focus of intensive research in the field of cellular and molecular neurobiology. We have previously shown that the addition of apotransferrin (aTf) to primary OLGc cultures accelerates their differentiation and induces an increase in the expression of different components of the myelin cytoskeleton (CSK) such as actin, tubulin, and some of the microtubule-associated proteins, particularly the stable tubulin only peptide (STOP). Fyn protein-tyrosine kinase (Fyn kinase), a member of the Src family, participates in signalling pathways that regulate OLGs/myelin cytoskeletal reorganization. It is essential for myelin development in the central nervous system (CNS), and its absence results in hypomyelination. In the present study, we used both primary cell and N19 cell line cultures to investigate further the mechanisms of action involved in the accelerated differentiation of OLGcs induced by aTf. In particular, we were interested in studying the participation of Fyn kinase in the different pathways involved in the reorganization of the OLGc/myelin cytoskeleton. In agreement with results already published, we found that in OLGcs, Fyn kinase is associated with Tau and tubulin. Using a dominant-negative of Tau in which the Fyn-Tau-microtubules (MTs) interaction is blocked, we found that aTf was unable to induce OLGc morphological differentiation. It was also observed that aTf decreases the activated RhoA content in coincidence with a redistribution of actin immunoreactivity. These results give support to our hypothesis that Fyn kinase plays a key role in the differentiation process of OLGcs promoted by aTf. © 2008 Wiley-Liss, Inc.
Myelination involves the recognition and ensheathment of axons and the final compaction of the wrapping lamellae to generate this unique membrane. It occurs when precursor cells proliferate, migrate, and differentiate into mature oligodendrocytes. The progression from progenitors to myelinating oligodendrocytes involves a sequence of events that includes cell cycle withdrawal, cytoskeletal changes, and synthesis of myelin components (Casaccia-Bonnefil and Liu, 2003).
The intracranial injection (ICI) of apotransferrin (aTf) at early stages of development induces oligodendroglial cell (OLGc) differentiation and early deposition of myelin (Escobar Cabrera et al., 1994, 1997; Marta et al., 2000; Paez et al., 2002). With a transgenic mouse overexpressing the human transferrin gene, Saleh et al. (2003) have shown changes in the expression of myelin cytoskeletal proteins. In these animals, there was a significant increase in the expression of myelin basic protein (MBP), tubulin, Tau, and stable tubulin only peptide (STOP; Marta et al., 2002), similarly to what was previously found in our laboratories in aTf-injected rats (Escobar Cabrera et al., 2000).
Activation of Fyn kinase is one of the earliest events triggered in oligodendrocyte precursor cells (OPCs) when they start to differentiate (Umemori et al., 1994). Fyn kinase regulates the extension of cell processes and myelin formation by OLGc (Osterhout et al., 1999), and work by Umemori et al. (1999) has suggested that it could participate in OLGc differentiation and in the first steps of myelination, through the phosphorylation of different transcription factors that activate the expression of the MBP gene.
The involvement of Fyn kinase in the mechanisms controlling the morphological differentiation of the OLGc appears to occur through the phosphorylation of p190RhoGAP (GTPase-activating protein) and the RhoGTPase signaling pathways (FpR; Wolf et al., 2001), which result in actin cytoskeletal reorganization. Finally, Fyn kinase also participates in OLGc differentiation, acting as a scaffolding protein between Tau and the microtubular (MT) network, leading to MT stabilization (Klein et al., 2002).
In this work we investigated whether the effects of aTf on OLGc morphological differentiation were mediated by the Fyn-Tau-microtubules (FTM) cascade. For this purpose, we used a Tau dominant-negative construct (+PXXP) in which this cascade is blocked. Klein et al. (2002) have shown in primary oligodendrocyte cultures expressing +PXXP Tau that cells exhibited a reduced number of processes and a reduction in process length, suggesting that the Fyn-Tau interaction is important for the generation and elaboration of cellular processes. Furthermore, and to evaluate the activation of RhoGTPase, we carried out pull-down experiments using a Rho-GST chimeric protein. The results show that, when the mutant Tau is overexpressed in OLGcs, neither the control cells nor the aTf-treated cells attains differentiation. Moreover, after aTf treatment, there was a decrease in activated RhoA content, followed by a redistribution of actin immunoreactivity. Taken together, these results show that aTf activates the Fyn kinase pathway, leading downstream targets of Fyn kinase to exert their action on the OLGc/myelin cytoskeleton and strongly suggest that Fyn kinase plays a key role in the differentiation process of OLGcs promoted by aTf.
MATERIALS AND METHODS
Human apotransferrin, paraformaldehyde, bovine serum albumin, and Triton X-100 were obtained from Sigma Chemical Co. (St. Louis, MO). DMEM/F12 was from Hyclone (Logan, UT). Fetal calf serum was from Natocor (Córdoba, Argentina). PP2 (4-amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo[3,4-d]pyrimidine) and PP3 (4-amino-7-phenylpyrazol[3,4-d]pyrimidine) were from Calbiochem (La Jolla, CA), and rhodamine-phalloidin was obtained from Molecular Probes (Eugene, OR). The following antibodies were used: anti-PrPC, anti-Fyn kinase (anti-total Fyn), and anti-Rho A (SC-179) were from Santa Cruz Biotechnology (Santa Cruz, CA); mouse monoclonal anti-active SFK (clone 28; anti-active Fyn) was from Biosource (Camarillo, CA); mouse anti-beta-tubulin monoclonal antibody was purchased from Chemicon (Temecula, CA); and anti-GAPDH was from Abcam (Cambridge, MA). Cy2 and Cy3 secondary antibodies were from Jackson Immunoresearch (West Grove, PA). All other chemicals were analytical grade reagents of the highest purity available.
OLGc Cultures
Primary OLGc cultures were carried out according to McCarthy and de Vellis (1980). Briefly, newborn Wistar rat cerebral hemispheres were mechanically dissociated and the cells collected in 10% fetal calf serum (FCS), 15 mM HEPES, DMEM/F12 1:1. Cells were seeded on 75-cm2 culture flasks previously coated with poly-L-lysine and incubated at 37°C in 5% CO2. After 12–14 days in culture, the subpopulation of OLGcs was obtained by using a differential cell adhesion protocol. Cells present in the supernatant after the shaking procedure were separated by centrifugation at 150 rpm for 10 min and resuspended in a glial defined medium (GDM) containing DMEM/F12 supplemented with 4.0 g/liter glucose, 2.4 g/liter NaCO3, 25 mg/liter insulin, 8 mg/liter putrescine, 20 nM progesterone, 8 μg/ml sodium selenite, 10 μg/liter biotin, and 9.8 μg/liter T3 (Casaccia-Bonnefil et al., 1996) and penicillin + streptomycin. aTF was omitted from the medium, except when otherwise indicated. For the different experiments, cells in suspension were plated either on poly-L-lysine-coated coverslips, which were placed in 18-mm-diameter multiwell dishes, or in 100-mm Petri dishes. Cultures were incubated for 24 hr with platelet-derived growth factor (PDGF)/basic fibroblast growth factor (bFGF; 10 ng/ml each) before aTf treatment. For inhibition studies, PP2 and PP3 were dissolved in dimethyl sulfoxide (DMSO) and added to the culture media at a 5 μM concentration during 48 hr. In some experiments, N19 cell line cultures were used. Cells were kindly provided by Dr. A.T. Campagnoni, UCLA. These cells were grown in DMEM-F12 with 10% FCS and 100 μg/ml G418 to 80% confluence at the permissive temperature (34°C) in Petri dishes (100 mm in diameter; 2–3 × 106 cells/dish). For the experiments, the cells were placed at 39°C (nonpermissive temperature) during 2 days and then treated with aTf for different times (12, 24, and 48 hr).
MTT Assay
The MTT survival assay was performed as described by Mosmann (1983). The sterile solution of MTT was added to the cells, and then the microplate was incubated at 37°C for 3 hr. The reaction was stopped by addition of SDS, and the product was quantified by spectrophotometry at 570 nm.
Immunocytochemical Studies
Multiwells containing cultured OLGcs were kept for 48–72 hr at 37°C in GDM or GDM containing aTf (100 μg/ml). They were then fixed with 4% paraformaldehyde, 0.12 M sucrose in PBS for 1 hr at room temperature. Fixed cells were permeabilized by incubation with 0.01% Triton X-100 in PBS for 5 min, blocked with 1% bovine serum albumin in PBS for 2 hr, and incubated overnight at 4°C with one of the following primary antibodies: anti-total Fyn (1/50) and anti-active Fyn (1 μg/ml). For double labeling against Fyn kinase and PrPC, cells were incubated with anti-PrPC (1:100) for 1.5 hr at room temperature after blocking. Then, cells were permeabilized, blocked, and incubated with antitotal Fyn (1:100) for 1.5 hr at room temperature. The coverslips were rinsed and incubated with the appropriate secondary antibodies conjugated to Cy2 (for active Fyn and PrPC) or Cy3 (for total Fyn). The coverslips were rinsed and incubated with the appropriate secondary antibody. The preparations were mounted in Fluorsave.
Cultures of the N19 cell line were fixed with 4% paraformaldehyde in PBS for 20 min at room temperature. Fixed cells were permeabilized by incubation with 0.1% Triton X-100 in PBS for 10 min, blocked with 1% bovine serum albumin in PBS for 2 hr, and incubated overnight at 4°C with anti-beta-tubulin (1/1,000) followed by incubation with the secondary antibody conjugated to Cy2 plus rhodamine-phalloidin (1/1,000) for 2 hr at room temperature.
Western Blot Analysis
After 12, 24, and 48 hr in culture, Petri dishes (5 × 106 cells/dish) were washed twice with PBS. The cells were collected by centrifugation (600g for 10 min at 4°C) and extracted with 20 mM Tris-HCl, pH 7.4, 150 mM NaCl, 10 mM EDTA, 0.5% Triton X-100 for 1 hr at 4°C. The insoluble material was cleared out by centrifugation (100,000g for 20 min at 4°C), and the resulting protein extracts were subjected to 12.5% SDS-PAGE. After transfer, samples were analyzed by Western blotting using an anti-total Fyn antibody (1/500), an anti-active Fyn antibody (0.1 μg/ml), or an anti-GAPDH (1/300). Antibody binding was visualized with horseradish peroxidase-conjugated secondary antibodies (1/20,000) and the ECL Plus substrate kit (Amersham Pharmacia Biotech UK Limited, Buckinghamshire, England).
Microscopic Examination and Image Processing
Stained preparations were examined by epifluorescence using either regular (Olympus B-50) or confocal (Olympus FV300) microscopy. Images were analyzed with the Image Pro Plus software (Media Cybernetics).
Fyn Kinase Auto Phosphorylation Assay
After 12, 24, and 48 hr in culture, Petri dishes (5 × 106 cells/dish) were washed twice with PBS. The cells were collected by centrifugation (600g for 10 min at 4°C) and extracted with 1 mM PMSF, 2% Nonidet P-40, 100 mM Na3VO4, and 10 mM NaF in PBS. They were precleared with protein A-Sepharose (PAS), incubated overnight in a head-over-tail rotator with anti-total Fyn antibody plus PAS, and washed three times under stringent conditions in RIPA buffer (100 mM Tris/HCl, pH 7.4, 2%Triton X-100, 0.2% SDS, 2% Na-deoxycholate, 1 mM ditiothreithol, 100 mM Na3VO4, 10 mM NaF) and once with PBS. Immunocomplexes were resuspended in 50 ml kinase buffer (40 mM HEPES pH 7.4, 10 mM Cl2Mg, 2 mM Cl2Mn, 100 mM Na3VO4) and incubated with 2 mCi [γ32P]ATP for 30 min at 37°C. The samples were then washed, resuspended in Laemmli sample buffer (62.5 mM Tris/HCl, pH 6.8, 2% SDS, β-mercaptoethanol, 10% glycerol, bromophenol blue) and subjected to 12.5% SDS-PAGE, and the gels were scanned in a STORM 840. The results of the densitometric evaluation of the bands were standardized by Western blot of the corresponding sample.
Cell Transfections
Two different plasmids were used in these studies: one dominant-negative construct, +PXXP, amino acid 1–227, which should disrupt the endogenous Tau-Fyn interaction when overexpressed in cells, and a control construct, –PXXP, amino acids 1–223, lacking the Fyn kinase binding motif, which should not interfere with Fyn-Tau interaction. Plasmids were a generous gift from Dr. J. Trotter, University of Heidelberg. Primary OLGc cultures were transfected by electroporation as described by Krueger and colleagues (1998), with slight modifications. The OLGc obtained after the shaking procedure described above were resuspended in DMEM:F12, 1% FCS. Equal numbers of cells (2–3 × 106) were mixed with 15 μg of a DNA construct consisting of the +PXXP Tau sequence driven by the CMV promoter plus 1 μg of the enhasnced green fluorescent protein (EGFP) sequence also driven by the CMV promoter (EGFP plasmid) in a final volume of 0.5 ml per sample. A set of cells was used as a transfection control with the –PXXP plasmid, plus the EGFP plasmid. The cells were electroporated at 300 V and 300 μF. After electroporation, the cells were allowed to rest undisturbed at room temperature for 10 min prior to plating onto poly-L-lysine-treated glass coverslips in GDM containing 1% FCS, 10 ng/ml PDGF, and 10 ng/ml bFGF. After 18–20 hr, the medium was replaced by the same medium without PDGF/bFGF in the presence or absence of aTf (100 μg/ml). Then, the cells were incubated at 37°C for different times (48–72 hr). Identification of the transfected cells was done by fluorescence microscopy for EGFP expression or by myc immunostaining.
Analysis of Morphological Complexity and Confocal Microscopy
Analysis of OLGc morphological complexity was done following the method of Sperber and McMorris (2001). Individual O4-positive cells were scored according to their morphological complexity. Five different categories of complexity were established based on the length and number of the processes, by whether they were radially distributed, and by the presence of secondary and/or tertiary processes.
Colocalization studies were done by confocal microscopy with Olympus FV300 equipment. The digital images were then analyzed using the Pearson's correlation coefficient (Rr) facility provided in the Image Pro Plus software (v4.5.01; Media Cybernetics). Rr is a well-defined and commonly accepted means for describing the extent of overlap between image pairs. It is a value ranging between –1 and 1, with –1 meaning no overlap between images whatsoever and 1 being perfect image registration. Pearson's correlation coefficient takes into account only the similarity of shapes between images and does not take into consideration image intensity (Manders et al., 1993).
Rho Pull-Down Assay
The plasmid pGEX2T-RBD coding for the glutathione S-transferase (GST)-Rho binding domain of the Rhotekin fusion protein was kindly provided by Dr. A. Fournier, McGill University. These studies were carried out in N19 cell line cultures. Cells were first rinsed and then lysed in RIPA buffer with 500 mM NaCl containing protease inhibitors. GTP-Rho was affinity precipitated from cell lysates using an immobilized GST fusion construct of the Rho-binding domain (RBD) of Rhotekin. Sedimented Rho was separated by SDS-PAGE, transferred to PVDF membranes, and blotted with an antibody against RhoA (1/200 dilution). For each pull-down assay, the level of RhoGTPase sedimented was normalized relative to the amount of the RhoGTPase in the whole-cell lysate.
Statistical Analysis
Statistical analysis was done in Graph Pad Prism 3.0 software (Graph Pad Software Inc., San Diego, CA). We used an F test (Fisher) to compare variances, followed by a two-tailed Student's t-test. Significance was calculated with P values: P > 0.05 = nonsignificant (n.s.), *P < 0.05, **P < 0.01, and ***P < 0.001. In the MTT assay, statistical significance was determined by one-way ANOVA, followed by the Dunnett test.
RESULTS
The immunocytochemical analysis showed that aTf treatment of OLGc cultures for 48 hr resulted in a 19% increase in Fyn kinase expression (Fig. 1A). Analysis of Fyn kinase by Western blot, showing 80% higher integrated optical density (IOD) values for the corresponding bands in the aTf-treated cells than in the control cells, agrees with the above-mentioned results (Fig. 1B).
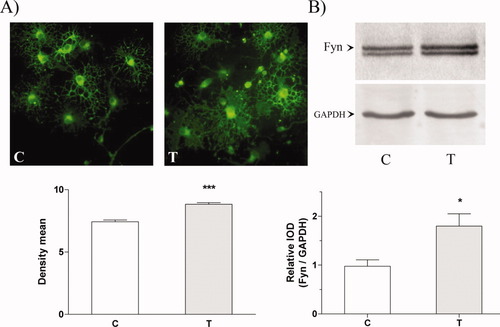
Apotransferrin increases Fyn kinase expression in OLGc primary cultures. A: Control (C) and aTf-treated cells (T) were kept in culture for 48 hr, and the expression of total Fyn was determined by immunocytochemistry. Density mean (DM) values show a significant difference between control and aTf-treated cells. Results are the mean ± SEM. The DM for control cells was 7.43 ± 0.14 (n = 168 cells) and for the aTf-treated cells 8.84 ± 0.12 (n = 282 cells; ☆☆☆P < 0.0001). B: Western blot analysis of total Fyn, which correlates with results of the inmunocytochemical study, shows that the expression of total Fyn in aTf-treated cells was about 80% higher than that in untreated controls. Data are expressed as mean ± SEM of the integrated optical density (IOD) of total Fyn relative to GAPDH. IOD for control cells was 0.98 ± 0.13 (n = 6) and for aTf-treated cells was 1.80 ± 0.25 (n = 6; ☆P < 0.05). [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Fyn kinase activation at different incubation times was measured by using an autophosphorylation assay. We found that, in the control cells after 20 min of incubation, there was a low level of auto phosphorylation activity. After 24 hr, the specific activity was almost three times higher; the highest specific activity being observed after 48 hr of incubation. In aTf-treated cells after 20 min of incubation, there was a low level of activity, similar to that observed in control cells. The peak in Fyn kinase-specific activity occurred much earlier (after 12 hr of incubation) and was higher than in controls, whereas, after 48 hr of incubation with aTf, Fyn kinase activity fell to almost undetectable levels (Fig. 2).
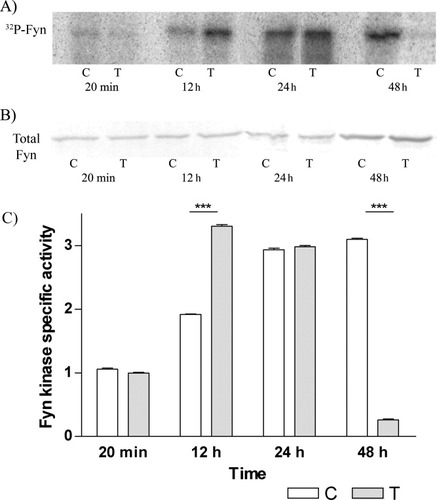
Autophosphorylation of Fyn kinase occurs earlier in OLGcs treated with aTf. OLGc primary cultures were used to determine Fyn kinase activation (autophosphorylation) as described in Materials and Methods. A: 32P incorporation by Fyn kinase. B: Western blot analysis of total Fyn. C, control; T, aTf-treated cells. C: Specific activity of Fyn kinase at different incubation times. Specific activity was calculated as the ratio between the IOD of phosphorylated Fyn kinase and the IOD of total Fyn. Results are the mean ± SEM of three different experiments. The specific activity of Fyn kinase in control cells shows a peak at 48 hr, whereas, in aTf-treated cells, the highest peak of specific activity occurs at 12–24 hr. ☆☆☆P < 0.0001.
Immunocytochemical analyses in control and aTf-treated OLGc were performed to investigate whether there were changes in Fyn kinase localization on the cell as a consequence of the increased activation induced by aTf treatment. Cells were doubly labeled with a polyclonal antibody that recognizes total Fyn and with a monoclonal antibody that recognizes only the active Fyn form (clone 28). The two Fyn kinase states displayed different distribution patterns: total-Fyn showed a nonuniform cell distribution, with a strong immunoreactivity in the tips of the process and as immunoreactive cumulus dispersed along the processes and the cell body (Fig. 3A). On the other hand, active Fyn immunoreactivity displayed a fibrillar distribution pattern in the cell processes and a structure that seemed to nucleate the immunoreactivity in the soma (Fig. 3B). The degree of colocalization of total Fyn and active Fyn immunoreactivities seemed to depend on the morphological complexity of the cells, the superimposition of the two labels being much higher in the more differentiated cells (see merged images at right in Fig. 3). Colocalization analysis of immunoreactivities using the Pearson's coefficient showed Rr values for total Fyn and active Fyn of 0.51 ± 0.01 (N = 40) in controls and 0.70 ± 0.01 (N = 53) in samples treated with aTf for 48 hr. Differences between means showed a significant increase of Rr in treated samples with reference to controls (P < 0.0001), indicating that aTf treatment induces changes in Fyn localization.
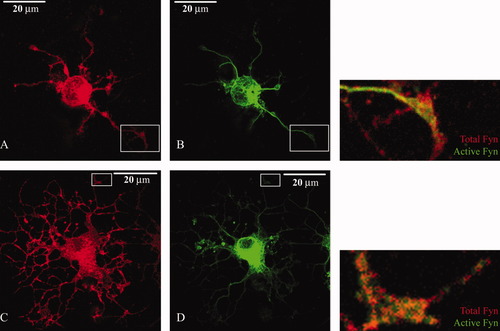
Total Fyn and active Fyn are differentially distributed in OLGcs. Control cells (A,B) and aTf-treated cells (C,D) were kept in culture for 48 hr, and the expression of total Fyn (red) and active Fyn (green) was determined by immunocytochemistry. Total Fyn antibody strongly labels the tip of the processes, and cumuli are also dispersed along the processes and the cell body (A,C). The active Fyn immunoreactivity displays a fibrillar pattern in the cell processes and a structure in the soma that seems to nucleate the fibrillar reactive pattern (B,D). Merged images of the areas enclosed within the rectangle are shown at higher magnification. Colocalization analysis of immunoreactivities using the Pearson's coefficient showed Rr values for total Fyn and active Fyn of 0.51 ± 0.01 (n = 40) in controls and 0.70 ± 0.01 (n = 53) in samples treated with aTf for 48 hr, indicating that there was an increase in the colocalization of the two labels in aTf-treated cells. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Fyn kinase localization in lipid rafts depends on its activation state. It was our interest to investigate the relationship between Fyn kinase and lipid rafts using an antibody against PrPc, the prion cellular protein attached to the plasma membrane by a glycosyl-phosphatidyl-inositol anchor that is localized in raft domains (Taylor and Hooper, 2006). No changes in the distribution of PrPc were observed after treatment with aTf. In control, untreated cultures, in which the cells are less well differentiated than in those treated with aTf, PrPc immunoreactivity closely colocalized with total Fyn kinase immunoreactivity, and the same colocalization pattern was shown with active Fyn (Fig. 4A–Ci–iv). At the tip of the processes, there were areas in which a high colocalization of the immunoreactivity of total and active Fyn with PrPC was detected, indicating the presence of Fyn activity in rafts (Fig. 4i–iv). After aTf treatment, it was found that PrPc immunoreactivity remained localized on the plasma membrane whereas the superimposed reactivity of total Fyn kinase and PrPc decreased significantly. Also, colocalization studies of the three immunoreactivities showed absence of active Fyn expression in the lipid rafts identified by PrPc immunoreactivity (Fig. 4v–viii).
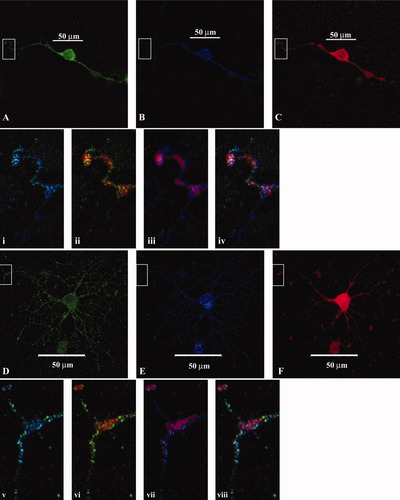
aTF decreases Fyn kinase colocalization with a lipid raft marker. Cells cultured for 48 hr in a medium with or without aTf were used for immunocytochemistry to detect PrPc (green), total Fyn (blue), and active Fyn (red). A–C: Control cells. D–F: Cells incubated in the presence of aTf. PrPc and total Fyn immunoreactivities are in close apposition at the leading edge of the OLGc processes. In aTf-treated cells, there is a decrease in the colocalization of total Fyn and PrPc, with a concomitant increase in the superimposition of total Fyn and active Fyn. Merged images of the areas enclosed within the rectangle are shown at higher magnification. Upper row (controls): PrPc and total Fyn (i), PrPc and active Fyn (ii), total Fyn and active Fyn (iii). Merge of the three images is shown in iv. Lower row (aTf-treated): PrPc and total Fyn (v), PrPc and active Fyn (vi), total Fyn and active Fyn (vii). Merge of the three images is shown in viii.
The distribution of active Fyn was studied in OLGcs incubated for 48 hr with or without aTf, followed by treatment with 5 μM PP2 (Hanke et al., 1996), an inhibitor of the Src family of proteins. At the concentration used, PP2 did not affect survival rate and was found to inhibit Fyn kinase specifically. Cell viability under the diffferent experimental conditions was determined by using the MTT assay. The results (mean ± SD of four experiments performed in triplicate) expressed as percentage of control values were 101.2 ± 3.9 for aTf-treated cells, 90.8 ± 4.3 for controls + PP2, 91.8 ± 6.9 for aTf-treated cells + PP2, 100.1 ± 7.1 for controls + PP3, and 102.1 ± 7.4 for aTf + PP3.
Cell cultures were pretreated with PP2 for 1 hr before the addition of aTf and evaluated 2 days after. In these experiments, OLGc similarly treated with PP3, an inactive chemical analog of PP2, were used as controls. Morphological evaluation of cell cultures treated with PP2 showed a clear disorganization of the processes, and eventually the processes became almost undetectable (Fig. 5). The morphological changes observed in OLGcs cultures in the presence of aTf and PP2 were similar to those detected in controls (Fig. 5). In PP3-treated cultures, the morphological appearance of the cells did not change compared with normal, untreated cultures in the presence or absence of aTf.
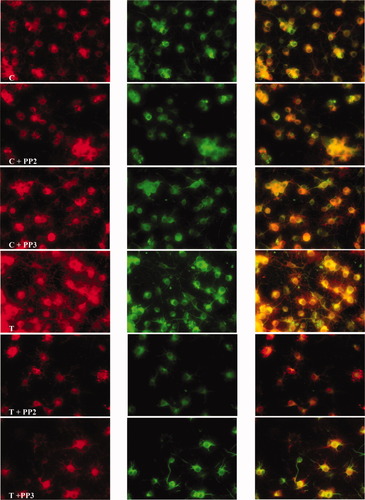
The Src inhibitor PP2 changes the distribution of active Fyn and promotes process retraction in control and aTf-treated cells. Control cells (C) and aTf-treated cells (T) were cultured for 48 hr in the absence or presence of PP2 (C + PP2 and T + PP2) or PP3 (C + PP3 and T + PP3) and used for immunocytochemistry. Left column: total Fyn (red). Middle column: active Fyn (green). Right column: merge. A clear disorganization of the fibrillar pattern and a retraction of the processes promoted by the Src inhibitor are observed in C + PP2 cells. Addition of aTf (T + PP2) did not change the morphology. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
The participation of Fyn acting as a scaffolding protein in aTf-treated OLGc was analyzed with a +PXXP Tau dominant-negative construct. Control transfections were performed with a –PXXP construct. The Tau mutant construct was cotransfected with the pCMV-EGFP plasmid to visualize the positive cells. The cells were immunostained with an anti-myc antibody or with an O4 antibody. All the EGFP-positive cells were found to be myc positive, and more than 98% of the EGFP-positive cells were also O4 positive (data not shown). Morphological examination of the control cells that overexpress the –PXXP protein showed that, after 48 hr in the presence of aTf, the OLGc population of low complexity diminished significantly (9%, P < 0.001) in comparison with that observed in aTf-untreated cells. The other populations showed an increase of 2% each, but these changes were not statistically significant (Fig. 6A). In exploring the effect of aTf treatment of the cells for 72 hr, it was found that, in the control –PXXP transfected cells, there was a drop in the cell population of low complexity from 26% to 12% (P < 0.001) concomitant with an increase in the cell population of medium complexity (+7.47%, P < 0.001) and in the medium-high-complexity cell group (+3.47%, P < 0.001). A statistically nonsignificant increase in low-medium- and high-complexity cell populations was also observed (Fig. 6C). On the other hand, morphological complexity studies with cells transfected with the dominant-negative + PXXP construct showed no differences in the number of cells after 48 hr of incubation in the presence or absence of aTf (Fig. 6B). Similarly to what occurs after 48 hr of incubation, the cells overexpressing + PXXP protein, incubated with or without aTf for 72 hr, showed no changes in the percentage of differentiated cell populations. However, aTf treatment induced an increase in the low-medium-complexity group (+6.5%, P < 0.0001), with a decrease in the low-complexity population (–7.57%, P < 0.001; Fig 6D). The sum of the two populations reached 85–90% of all the cells.
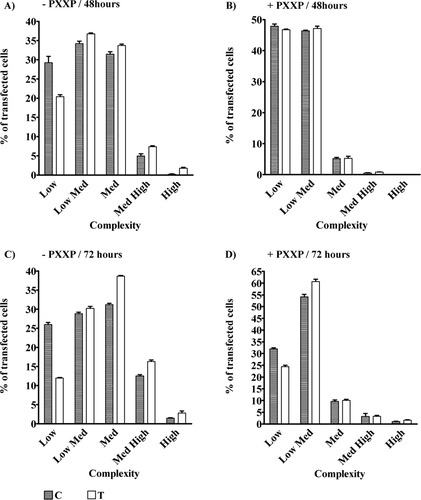
Tau deletion protein +PXXP seems to block the morphological differentiation effect of aTf. OLGc in primary cultures were transfected with the Tau deletion constructs –PXXP or +PXXP. After 48 and 72 hr in culture in the presence or absence of aTf, their morphological complexity was analyzed by O4 immunostaining. A: In cells transfected with –PXXP and incubated for 48 hr in the presence of aTf, there was a decrease in the number of bipolar cells (low-complexity population; –9%, P < 0.001) compared with control cells, whereas the other populations showed an increase of 2% each (nonsignificant). B: Morphological analysis of the cells transfected with +PXXP and incubated for 48 hr in the presence of aTf showed no indication of changes in differentiation. Note that, in –PXXP cells, all the differentiation stages are present, whereas, in the +PXXP cells, the more differentiated cell populations are absent. C: Experiments in this group were similar to those described for A, but cells were incubated for 72 hr. In this case, the low-complexity cell population after aTf treatment decreases from 26% to 12% (P < 0.001). The medium-complexity cell population increases by 8% (P < 0.001), and the medium-high-complexity group increases 4% (P < 0.001), with no changes in the low-medium- and high-complexity populations. D: Same as B, but after 72 hr in culture. Cells transfected with +PXXP treated or not with aTf showed no indication of increased differentiation in the medium-, medium-high-, and high-complexity groups. However, aTf treatment induces an increase in the low-medium-complexity group (+7%, P < 0.001) and a decrease in the low-complexity population (–8%, P < 0.001).
To determine whether aTf could inactivate RhoA to promote actin cytoskeletal reorganization, a Rho pull-down assay was carried out. Treatment of N19 cells with aTf has been shown to promote effects similar to those in OLGc primary cultures (Paez et al., 2004), providing us with the high amounts of protein needed for the assays. An early activation of Fyn kinase (i.e., at 12–24 hr) was found after aTf treatment of N19 cells, whereas, in untreated control cells, this activation occurred much later (at 24–48 hr; results not shown). For N19 cells, we found that, after 24 hr of incubation in the presence of aTf, there was a significant decrease in active RhoA. Quantitative analysis of RhoA by Western blot with whole-cell lysates showed that the levels of total RhoA in aTf-treated cells did not change at 12 hr or at 24 hr (Fig. 7A). Furthermore, when actin distribution was analyzed after 48 hr of incubation, it was found that, although the immunoreactivity in control cells was present in the cell soma, in aTf-treated cells, the immunolabeling was found in the cell processes, particularly at their branching points (Fig. 7B).
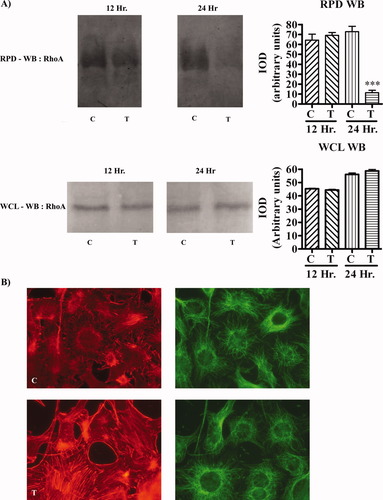
Activated RhoA and actin immunoreactivity in control and aTf-treated N19 cells. A: Cell lysates of the N19 cell line were used to evaluate activation of RhoA by Western blot of Rho pull-down (RPD-WB). C, control; T, aTf-treated. The means ± SEM of IOD at 12 hr were 64.28 ± 5.94 for control cells and 69.10 ± 2.77 for treated cells (n = 3, n.s.). At 24 hr, the IOD values for control and aTf-treated cells were 72.83 ± 5.36 and 11.42 ± 2.35, respectively (n = 3, P = 0.005). Whole-cell lysates (WCL-WB) of the same samples that were used for the Rho pull-down assay are shown in the Western blot. The IOD values at 12 hr were 45.27 ± 0.42 for control cells and 44.34 ± 0.51 for aTf-treated cells (n = 3). At 24 hr, the IOD for control cells was 56.12 ± 1.05 and 58.73 ± 1.14 (n = 3) for aTf-treated cells. There were no significant differences between control and aTf-treated cells at the time points analyzed. B: Immunocytochemistry of actin (red) and beta-tubulin (green) in N19 control (C) and aTf-treated (T) cells cultured for 48 hr. In controls, the immunoreactivity of actin was located mainly in the soma of the cells, whereas in aTf-treated cells it appeared to be redistributed to the cell processes after 48 hr of treatment. ☆☆☆P < 0.0001. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
DISCUSSION
The mechanisms by which OLGc extend branched processes have not been elucidated as yet, but morphological differentiation of these cells is known to require the coordination of rearrangements in the cell cytoskeleton. Song et al. (2001) showed that actin drives the formation of new processes at the leading edges of the OLGc processes. There are many signals involved in the reorganization, formation, and stabilization of the microfilament and MT networks in this cell during its maturation process, and multiple functions have been assigned to Fyn kinase in OLGc during their morphological differentiation (Kramer et al., 1999; Umemori et al., 1999; Seiwa et al., 2000, 2007; Nakahara et al., 2001; Wolf et al., 2001; Klein et al., 2002; Mi et al., 2005). Osterhout et al. (1999) have demonstrated that, as soon as OLGcs become mature, there is an increase in the levels of Fyn expression. The expression of full-length Fyn containing a point mutation that prevents ATP binding showed a myelin content reduced by 66%, very similar to the decrease observed in the complete absence of Fyn kinase, indicating that the kinase activity is necessary for the myelination process (Sperber et al., 2001).
In a previous study, we hypothesized that aTf added to OLGc in culture interacts with its receptor on the soma, activating signals that could be conveyed to the leading edge of the processes, where the signals would be transduced to modify the cytoskeletal arrangement of the cell (Ortiz et al., 2005). Fyn kinase could be one of the candidates to be activated by this mechanism at the tip of the cell processes. Fyn kinase activation and accelerated OLGc maturation seem to occur in parallel. In our case, in control cells in culture, the specific activity of this kinase peaked at 48 hr, whereas the mere presence of aTf in the culture medium induced Fyn kinase activity in OLGcs much earlier than in cells cultured in a medium containing no aTf, indicating a parallelism between increased changes in morphology and Fyn activation. Our results are in agreement with those of Osterhout et al. (1999), who showed that, in OLGc primary cultures, Fyn is activated after 24 hr of incubation in a prodifferentiating media that induces OLGc differentiation.
At variance with what has been suggested by Sperber et al. (2001), our results indicate that Fyn kinase seems to participate in the rearrangement of the OLGc cytoskeleton, in the outgrowth of cell processes, and in the wrapping of axons, insofar as inhibition of Fyn kinase activity in cultured OLGc showed that process outgrowth and cell differentiation were completely blocked. It has been reported that PP2 has toxic effects on OLGcs (Sperber and McMorris, 2001), but a number of other investigators who have used PP2 did not observe such cell deleterious effects. In our case, survival experiments carried out in cultures in the presence of PP2 showed that cells exhibited 90% viability compared with controls.
With the appearance of myelinating oligodendrocytes, detergent-insoluble glycosphingolipid-rich microdomains (or rafts) are up-regulated, and, as a consequence, F3/contactin and Fyn kinase will be enclosed in these microdomains (Kramer et al., 1999). The ligation of F3/contactin (and probably N-CAM) by an axonal ligand activates Fyn kinase in the OLGc, increasing its autophosphorylation and consequently inducing myelination. Results obtained in this study with an anti-PrPc antibody to study colocalization of a marker of microdomains with different activation states of Fyn kinase appear to indicate that, when Fyn is activated after treatment with aTf, colocalization of Fyn kinase and PrPc immunoreactivities decrease. According to our results, a higher activation of Fyn kinase occurs in cells treated with aTf at 12 hr. However, after 12 hr in culture, even in cells treated with aTf, the morphological studies indicate that the number of bipolar OPCs seems to be higher than at 48 hr, when aTf-treated cells have been able to differentiate much more than control cells. Insofar as the formation of cell processes is a mechanism dependent on Fyn kinase activation, it seems reasonable to find at 12 hr of culture a high degree of colocalization of active Fyn with a raft marker, which disappears when the OLGcs mature.
Lee et al. (1998) have demonstrated that the last of the seven PXXP Tau motifs is necessary and functional for the interaction with the Fyn SH3 domain. For this reason, we used a construct (+PXXP) containing the amino acids 1–227 of the rat Tau sequence. This Tau amino acid sequence contains the PXXP motif, which interacts with SH3 of Fyn but lacks the MT interactive sequence. When the +PXXP construct is overexpressed in OLGcs, it binds to Fyn SH3 but cannot bind to MTs, disrupting the FTM endogenous interaction (Klein et al., 2002). Using this dominant-negative of Tau, we found that aTf was unable to induce an accelerated OLGc differentiation. The morphological analysis of control OLGcs showed that, after 48 hr, most of the –PXXP transfected cells fell within the low, low-medium, and medium stages of morphological complexity. Treatment with aTf induced a slight switch toward higher complexity values. After 72 hr of aTf treatment, the switch toward the more differentiated OLGc was more evident, in agreement with the results described by Paez et al. (2006). As a result of the FTM cascade impairment, the +PXXP transfected cells were unable to differentiate beyond the low-medium stage of complexity (∼90% of the cells fell within the two first differentiation stages), and aTf treatment could not bypass the block, even after 72 hr of treatment, indicating that aTf is acting in this site of action of Fyn kinase.
The Rho family of GTPases regulates the polymerization of actin and plays an essential role in the control of cell morphology. Wolf et al. (2001) demonstrated that the overexpression of a dominant-negative RhoA results in hyperextension of the OLGc processes, and Liang et al. (2004) described that RhoA expression and activation were found to be down-regulated during OLGc differentiation. The primary function of p190 RhoGAP appears to be activation of the GTPase activity of Rho, promoting the formation of Rho-GDP and inducing Rho inactivation. In agreement with these authors, we found that, after 24 hr of treatment with aTf, there was a decrease in RhoA activity, with no changes in the levels of total RhoA. It was also demonstrated that, when OLGcs were transfected with p190RhoGAP and after 48 hr of incubation, the levels of expression of RhoA did not change, indicating that, as long as the cells need the active protein, they will maintain its expression at adequate levels and that, when the activity decays, the cells trigger a negative feedback on the expression of the protein (Liang et al., 2004). In comparison with controls, aTf promotes a premature Fyn activation, so we assumed that p190RhoGAP is more active in aTf-treated cells than in control cells and, as a consequence, the levels of RhoA GTP decrease. As in OLGc primary cultures, treatment of the N19 OLGc line with aTf induces an early activation of Fyn tyrosine kinase. The increase in Fyn activation is followed by a decrease in activated RhoA and by a redistribution of actin. These results allow us to conclude that, for the rearrangement of the cytoskeleton that leads to a more mature OLGc, aTf appears to be acting through both the FTM cascade and the Fyn-p190-RhoGTPase pathway. Fyn is an essential signaling component for OLGc morphological differentiation, and Umemori et al. (1999) demonstrated that signals acting through Fyn stimulate transcription of the MBP gene. We are at present studying the participation of Fyn in the stimulation of MBP expression mediated by aTf.
Acknowledgements
This paper is dedicated to the distinguished scientist Steve PFeiffer, a beloved friend that we will never foget. The authors thank Dr Gabriel Corfas for critical reading of the manuscript.




