Peroxiredoxin 2 overexpression protects cortical neuronal cultures from ischemic and oxidative injury but not glutamate excitotoxicity, whereas Cu/Zn superoxide dismutase 1 overexpression protects only against oxidative injury
Abstract
We previously reported that peroxiredoxin 2 (PRDX2) and Cu/Zn superoxide dismutase 1 (SOD1) proteins are up-regulated in rat primary neuronal cultures following erythropoietin (EPO) preconditioning. In the present study, we have demonstrated that adenovirally mediated overexpression of PRDX2 in cortical neuronal cultures can protect neurons from in vitro ischemia (oxygen-glucose deprivation) and an oxidative insult (cumene hydroperoxide) but not glutamate excitotoxicity. We have also demonstrated that adenovirally mediated overexpression of SOD1 in cortical neuronal cultures protected neurons only against the oxidative insult. Interestingly, we did not detect up-regulation of PRDX2 or SOD1 protein in the rat hippocampus following exposure to either 3 min or 8 min of global cerebral ischemia. Further characterization of PRDX2's neuroprotective mechanisms may aid in the development of a neuroprotective therapy. © 2007 Wiley-Liss, Inc.
A clinically effective treatment that directly inhibits the neuronal death following cerebral ischemia and stroke remains elusive. However, in a process termed “preconditioning,” neurons can become temporarily resistant to normally lethal levels of ischemia (Kitagawa et al., 1990; Pringle et al., 1999; Meloni et al., 2002; Dirnagl et al., 2003). Because preconditioning is reliant on new protein synthesis, identification of the proteins involved may provide therapeutic targets for the development of drugs to inhibit ischemic neuronal death. To this end, we reported that Cu/Zn superoxide dismutase (SOD1) and peroxiredoxin 2 (PRDX2) are both up-regulated in cortical neuronal cultures following preconditioning with erythropoietin (EPO; Meloni et al., 2006). However, although the neuroprotective effects of SOD1 overexpression in primary neuronal culture injury models have been investigated (Ying et al., 2000; Borg and London, 2002), the effects of PRDX2 overexpression have not.
Peroxiredoxins are a family of intracellular antioxidant enzymes found in the cytosol, mitochondria, peroxisome, endoplasmic reticulum, and plasma membrane (Wood et al., 2003). Six distinct mammalian isozymes (PRDX 1–6) have been identified that share an N-terminal catalytic cysteine residue responsible for peroxidase activity (Fujii and Ikeda, 2002; Wood et al., 2003; Schroder et al., 2003). Along with SOD, catalase, and glutathione peroxidase, the peroxiredoxins form an integral component of the cellular antioxidant defense system. In conjunction with thiol-specific reducing agents such as thioredoxin and/or glutathione, peroxiredoxins are able to scavenge hydrogen peroxide (Fujii and Ikeda, 2002), peroxynitrite (Bryk et al., 2000), and organic hydroperoxides (Wood et al., 2003). Peroxiredoxins are also implicated in cellular differentiation (Choi et al., 2005), proliferation (Prosperi et al., 1998), modulation of H2O2-mediated signalling (Kang et al., 1998, 2004), and, more recently, protein chaperoning (Jang et al., 2004; Moon et al., 2005; Rand and Grant, 2006).
Peroxiredoxin 2, originally named “thiol-specific antioxidant” (TSA), is also called “thioredoxin peroxidase 1,” “thioredoxin-dependent peroxide reductase 1,” “thiol-specific antioxidant protein,” “RSA,” “PRP,” “porcine natural killer cell-enhancing factor B,” and “calpromotin” (Schroder et al., 1998; Kristensen et al., 1999). Peroxiredoxin 2 is highly expressed in the brain (Moore et al., 1991; Matsumoto et al., 1999) and is the predominant cytosolic neuronal isozyme (Sarafian et al., 1999; Jin et al., 2005). Recent evidence suggests that PRDX2 might also play an important role in the defense against neurodegenerative diseases. For example, in amyotrophic lateral sclerosis (ALS), in which oxidative stress is implicated in the selective death of motor neurons, PRDX2 protein levels are increased in surviving neurons, presumably as a compensatory response (Kato et al., 2005). However, at the terminal stage of disease, which is associated with accelerated neuronal degeneration and neuronal death, PRDX2 overexpression is lost (Kato et al., 2005). Similarly, increased PRDX2 levels have also been observed in post-mortem brain tissue from individuals with Down's syndrome, Alzheimer's Parkinson's, and Pick's disease (Krapfenbauer et al., 2003; Basso et al., 2004).
Overexpression of PRDX2 has been shown to inhibit or delay apoptosis induced by ceramide, etoposide, and cisplatin exposure (Zhang et al., 1997; Kim et al., 2000; Chung et al., 2001). In addition, PRDX2 overexpression can protect PC12 cells from nerve growth factor (NGF) and serum withdrawal and from the effects of oxidative stress induced by t-butylhydroperoxide (Ichimiya et al., 1997; Simzar et al., 2000). Although these studies have provided evidence that PRDX2 has cytoprotective properties in several injury models, no previous study has examined the protective role of this protein in primary neuronal cultures. Thus, given that PRDX2 is an important component of the cellular antioxidant defense system, and given our recent findings showing PRDX2 up-regulation in neuronal preconditioning, we sought to determine whether PRDX2 overexpression could protect cultured neurons against in vitro ischemia (oxygen glucose deprivation), oxidative stress (cumene hydroperoxide), and excitotoxic (glutamate) injury. In conjunction with PRDX2 examination, we also assessed the neuroprotective property of SOD1 overexpression in the same injury models. Finally, we also investigated whether a 3-min nondamaging or an 8-min neurodamaging period of global ischemia could increase PRDX2 and SOD1 protein levels in the rat hippocampus.
MATERIALS AND METHODS
Preparation of Recombinant Adenovirus
The rat cDNA sequence for PRDX2 was derived using standard cloning techniques. Briefly, total rat brain RNA was purified from a male Sprague-Dawley rat, reverse transcribed, and amplified by PCR using a forward primer (5′-GGT ACCCCACCATGGCCTCCGGCAACGCGCAC-3′) containing a KpnI restriction site (italicized) and Kozak sequence and a reverse primer (5′-CTCGAGTCAGTTGTGT TTGG AGAAGTATTCCTTGCTG-3′) containing a XhoI restriction site (italicized). The resulting PCR product was purified by agarose gel electrophoresis and cloned into pGEM-TEasy (Promega, Madison, WI). A pCMV-shuttle plasmid encoding the cDNA sequence of human SOD1 was kindly provided by Dr. Amerigo Carello and sequence verified. The cDNA for PRDX2 and SOD1 were released by digestion with KpnI and XhoI and directionally subcloned into the expression vector pRSV-WPRE/CMV-EGFP described previously (Boulos et al., 2006).
Recombinant adenoviruses were prepared according to the method of He et al. (1998), with some modifications. Briefly, pShuttle plasmid DNA was linearised by PmeI digestion and introduced into Escherichia coli strain BJ5183 carrying pAdeasy (Zeng et al., 2001) by electroporation (Gene Pulser II; Bio-Rad, Hercules, CA). Recombinants were selected on media containing 50 μg/ml kanamycin, and their plasmid DNA was checked by PacI digestion. HEK293 cells grown to 90% confluence in 25-cm2 flasks were transfected with 3 μg PacI linearized recombinant plasmid DNA using Lipofectamine2000 (Invitrogen, La Jolla, CA). Viral plaques appeared within 5–10 days, and viral material was used for subsequent amplification of the virus in HEK293 cells before purification and concentration using the Adeno-X virus purification kit (BD Biosciences, San Jose, CA). Viral titers were determined by end-point dilution assay, as indicated by enhanced green fluorescent protein (EGFP) reporter expression.
Preparation of Cortical Neuronal Cultures
All animal procedures were approved by the University of Western Australia Animal Ethics Committee. Establishment of cortical cultures was as previously described (Meloni et al., 2001); briefly, cortical tissue from E18–E19 Sprague-Dawley rats was dissociated in Dulbelcco's modified Eagle medium (DMEM; Invitrogen) supplemented with 1.3 mM L-cysteine, 0.9 mM NaHCO3, 10 U/ml papain (Sigma, St. Louis, MO), and 50 U/ml DNase (Sigma) and washed in cold DMEM/10% horse serum. Neurons were resuspended in Neurobasal (NB; Invitrogen) containing 2% B27 supplement (B27; Invitrogen). Before seeding, culture vessels, consisting of either 96-well plastic or glass dishes (6 mm diameter; Alltech, Australia) were coated with poly-D-lysine (50 μg/ml; 70,000–150,000; Sigma) and incubated overnight at room temperature. The poly-D-lysine was removed and replaced with NB (containing 2% B27, 4% fetal bovine serum, 1% horse serum, 62.5 μM glutamate, 25 μM 2-mercaptoethanol, 30 μg/ml streptomycin, and 30 μg/ml penicillin). Neurons were plated to obtain approximately 10,000 viable neurons per well on day in vitro (DIV) 9. Neuronal cultures were maintained in a CO2 incubator (5% CO2, 95% air balance, 98% humidity) at 37°C. On DIV 4, one-third of the culture medium was removed and replaced with fresh NB/2% B27 containing the mitotic inhibitor cytosine arabinofuranoside (Sigma) at 1 μM, and, on DIV 8, one-half of the culture medium was replaced with NB/2% B27. Cultures were used on DIV 12, when between 0.5–2% of cells stain positively for glial fibrillary acidic protein (GFAP; Meloni et al., 2001).
Adenoviral Transduction
On DIV 9, the conditioned medium was removed from cortical neuronal cultures and purified virus, diluted in 50 μl of NB/2% B27, at the required multiplicity of infection (moi: 75), was added to each well and incubated for 3 hr at 37°C. The virus-containing media were removed and replaced by an equal mix of conditioned media and fresh NB/2% B27. Unless otherwise indicated, transduced neuronal cultures were used on DIV 12. Exposure of cortical neuronal cultures to an moi of 75 results in neuronal transduction levels of ∼60% (Boulos et al., 2006; unpublished findings).
Western Blotting
For protein extraction, brain tissue and cultured cells were lysed in buffer [50 mM Tris-HCl, pH 7.5, 100 mM NaCl, 20 mM EDTA, 0.1% sodium dodecyl sulfate (SDS), 0.2% deoxycholic acid, containing Complete protein inhibitor (Roche, Indianapois, IN)], vortexed briefly, and clarified by centrifugation at 4°C. Protein concentrations were determined by the Bradford assay (Bio-Rad). Equivalent amounts of protein (5–10 μg per lane) were loaded and separated on 4–12% gradient SDS-polyacrylamide Bis-Tris minigels, (NuPAGE; Invitrogen) and transferred to a PVDF membrane. Membranes were blocked in phosphate-buffered saline/0.5% Tween 20 (PBS/T) containing ovalbumin (1 mg/ml) for 1 hr at room temperature before washing in PBS/T and PBS. Membranes were incubated at 4°C overnight in blocking solution containing primary antibody, washed, and incubated in blocking solution containing horseradish peroxidase (HRP)-conjugated secondary antibody for 1 hr at room temperature. Protein bands were detected using ECL Plus (Amersham, Amersham, United Kingdom), visualized by exposure to X-ray film (Hyperfilm; Amersham), and scanned and quantified in NIH Image. As required, membranes were incubated for 10 min at room temperature in stripping solution (Re-Blot Western Recycling kit; Chemicon, Temecula, CA) prior to immunodetection of control proteins. Primary antibodies used were rabbit polyclonal anti-PRDX2 (1:5,000; LabFrontier, Korea) antibody, anti-SOD1 antibody (0.2 μg/ml; Stressgen Bioreagents), mouse monoclonal anti-β-tubulin (0.5 μg/ml; Pharmingen, San Diego, CA). Secondary antibodies were donkey anti-rabbit IgG (1:25,000–1:50,000; Amersham), sheep anti-mouse IgG (1:10,000–1:20,000; Amersham), and rabbit anti-goat IgG (1:20,000; Zymed, South San Fransisco, CA).
Cell Death Assays
Exposure to In Vitro Ischemia
The media from glass wells were removed, and wells washed in 315 μl balanced salt solution (BSS; in mM: 116 NaCl, 5.4 KCl, 1.8 CaCl2, 0.8 MgSO4, 1 NaH2PO4, pH 7.0), then resupplied with 50 μl of BSS alone or BSS containing glutamate receptor antagonists [1 μM MK801/10 μM 6-cyano-7-nitroquinoxaline (CNQX; Tocris, Ellisville, MO)]. Cultures were placed into an anaerobic chamber (Don Whitely Scientific, England) with an atmosphere of 5% CO2, 10% H2, and 85% argon, 98% humidity at 37°C for 50 min. After anerobic incubation, an equal volume of DMEM containing 2% N2 supplement (Invitrogen) was added to each well before placing the wells into a CO2 incubator at 37°C for 24 hr. Control cultures received the same BSS wash procedures and media additions as ischemic cultures but were maintained in a CO2 incubator.
Exposure to Cumene Hydroperoxide (Cumene).
The media from cultures grown in plastic wells was removed and replaced with 100 μl DMEM/1% N2 containing freshly prepared cumene (25 μM; Sigma) alone or cumene containing glutamate receptor antagonists. Cultures were incubated in CO2 at 37°C for 24 hr
Exposure to Glutamate
The media from cultures grown in plastic wells was removed and replaced with 100 μl of a 50:50 mixture of conditioned media and fresh NB2/2% B27 containing glutamate (100 μM; Sigma) alone or with glutamate receptor antagonists. After a 5-min incubation at 37°C, the medium was replaced with 100 μl DMEM/1% N2 medium, and culture wells placed into a CO2 incubator at 37°C for 24 hr.
Cell Viability, Light Microscopy, and Statistical Analysis
Cell viability was assessed 24 hr after in vitro ischemia, cumene and glutamate treatment using the MTS assay (Promega). Although we did not distinguish between apoptotic and necrotic cell death following in vitro ischemia, as reported previously (Meloni et al., 2001; Arthur et al., 2004), based on light microscopy and nuclear staining, this model results in predominantly apoptotic-like neuronal death. Cumene exposure induces extensive cell rounding, indicative of apoptosis, which is supported by our findings that overexpression of the antiapoptotic protein Bcl-XL provides high-level neuronal protection in this injury model (Boulos et al., 2006). Similarly, although our glutamate model of excitotoxic injury exhibits elements of necrosis such as calpain activation and cell swelling, it also exhibits apoptotic features such as positive annexin V staining, and neuronal protection by Bcl-XL overexpression (recent unpublished findings). Image acquisition was performed in an Olympus IX70 under software control (DP controller; Olympus). Viability data are presented as mean ± SEM. Differences between groups were determined by ANOVA, followed by post-hoc Fischer's PLSD test. P < 0.05 was considered statistically significant. Unless otherwise stated, all experiments were conducted at least three times. All scanned and digitized images were uniformly resized in Adobe Photoshop, without any further alteration.
Transient Global Cerebral Ischemia Model
This study was approved by the Animal Ethics Committee of the University of Western Australia. The two-vessel occlusion with hypotension model of global cerebral ischemia was performed on 8–10-week-old adult male Sprague-Dawley rats as previously described (Zhu et al., 2004). During the procedure, both cranial and rectal temperature were measured via a thermocouple (Physitemp) and were maintained at 37°C ± 0.2°C with a heating fan and pad. Rats were anesthetized with 3% halothane/27% O2/balanced NO2 and ventilated before, during, and for at least 15 min after global cerebral ischemia. Cerebral ischemia was recorded from the time when the EEG became isoelectric and was maintained for a duration of 3 min or 8 min. Ten minutes before and fifteen minutes after the ischemic insult, PaO2, PaCO2 and pH were measured with a pH/blood gas analyzer (ABL5 Radiometer, Copenhagen, Denmark).
Experimental Groups
Animals were sacrificed and hippocampal tissue was immediately removed and stored at –80°C, for subsequent protein extraction, which was performed as described above. For Western blot analysis, experimental groups consisted of sham-operated or ischemic animals.
RESULTS
Construction of Recombinant Adenovirus To Overexpress PRDX2 and SOD1 in Cortical Neuronal Cultures
The expression cassettes of our control vector (AdRSV:Empty) and viral vectors used to overexpress PRDX2 and SOD1 (AdRSV:PRDX2 and AdRSV: SOD1) are presented schematically in Figure 1A. Visualization of EGFP reporter expression confirmed that neuronal cultures were successfully transduced and that transduction levels between each recombinant adenovirus were comparable (data not shown). Western blot analysis confirmed that transduction of cortical neuronal cultures with the PRDX2 and SOD1 viral vectors increased specific protein levels (Fig. 1B,C).
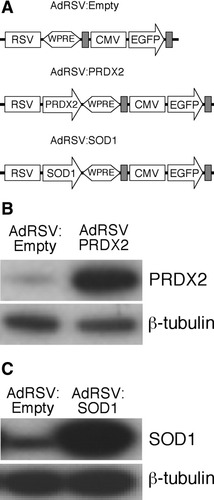
A: Schematic of recombinant adenoviral vector constructs AdRSV:Empty (control), AdRSV:PRDX2, and AdRSV:SOD1, showing the transgene and reporter expression cassettes. The DNA elements are: rous sarcoma virus (RSV) promoter, woodchuck posttranscriptional regulatory element (WPRE), cytomegalovirus (CMV) promoter, enhanced green fluorescent protein (EGFP); the gray rectangles denote the SV40 polyadenylation signal sequence. B: Western blot analysis of cortical neuronal cultures transduced with AdRSV: Empty (moi of 75) and AdRSV:PRDX2 (moi of 75) and probed with anti-PRDX2 antibody. C: Western blot analysis of cortical neuronal cultures transduced with AdRSV:Empty (moi of 75) and AdRSV:SOD1 (moi of 75) and probed with anti-SOD1 antibody.
Effect of PRDX2 and SOD1 Overexpression on Cell Viability Following Cumene Exposure
Virally mediated PRDX2 and SOD1 overexpression significantly increased neuronal survival following cumene exposure from 33% to 66% and 50%, respectively (Fig. 2A). Photomicrographs of cumene-treated cultures transduced with the control and PRDX2 and SOD1 viral vectors are provided in Figure 2B. Glutamate receptor antagonists significantly increased the viability of cultures following cumene exposure from 33% to 70%.
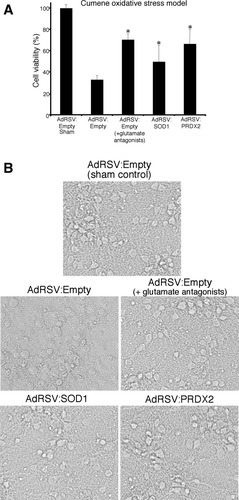
Cell viability of neuronal cultures transduced with adenoviral constructs AdRSV:Empty, AdRSV:PRDX2, and AdRSV:SOD1 at 24 hr following exposure to cumene. A: Cortical neuronal cultures were transduced with recombinant adenovirus (moi of 75) and exposed to either cumene, with or without glutamate antagonists, or were sham treated (n = 6). Cell viability in sham cultures was treated as 100%. Asterisks denote a statistically significant difference between that treatment group and the control group. B: Brightfield images of sham neuronal cultures and neuronal cultures 24 hr following cumene treatment.
Effect of PRDX2 and SOD1 Overexpression on Cell Viability Following In Vitro Ischemia
PRDX2 overexpression significantly increased the viability of cultures following in vitro ischemia from 17% to 36% (Fig. 3). By contrast, adenovirally mediated SOD1 overexpression had no effect on cell viability (Fig. 3). Glutamate receptor antagonists increased the viability of cultures following in vitro ischemia from 17% to 31%.
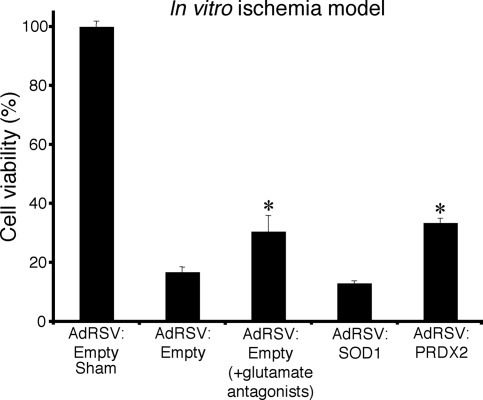
Cell viability of neuronal cultures transduced with adenoviral constructs AdRSV:Empty, AdRSV:PRDX2, and AdRSV:SOD1 at 24 hr following in vitro ischemia. Cortical neuronal cultures were transduced with recombinant adenovirus (moi of 75) and exposed to in vitro ischemia, with or without glutamate antagonists, or were sham treated (n = 3). Cell viability in sham cultures was treated as 100%. Asterisks denote a statistically significant difference between that treatment group and the control group.
Effect of PRDX2 and SOD1 Overexpression on Cell Viability Following Glutamate Exposure
PRDX2 and SOD1 overexpression did not protect neurons against glutamate exposure (Fig. 4). Extensive neuronal degeneration (cell membrane swelling and nuclear shrinkage) was evident at 3 hr following glutamate exposure (data not shown). Glutamate receptor antagonists significantly increased the viability of cultures following glutamate exposure from 11% to 94%.
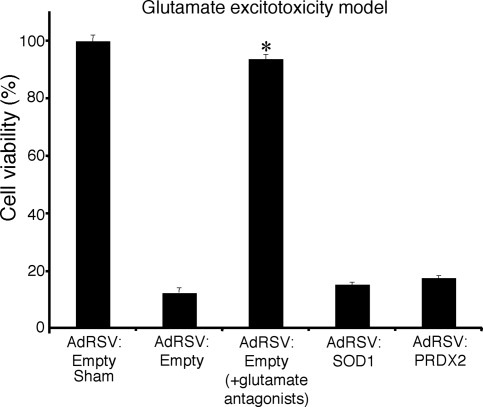
Cell viability of neuronal cultures transduced with adenoviral constructs AdRSV:Empty, AdRSV:PRDX2, and AdRSV:SOD1 at 24 hr following glutamate exposure. Cortical neuronal cultures were transduced with recombinant adenovirus (moi of 75) and exposed to either glutamate, with or without glutamate antagonists, or were sham treated (n = 3). Cell viability in sham cultures was treated as 100%. Asterisks denote a statistically significant difference between that treatment group and the control group.
PRDX2 and SOD1 Protein Levels in the Rat Hippocampus Following 3- or 8-Min Global Cerebral Ischemia
We initially investigated whether PRDX2 and/or SOD1 protein levels increased in the rat hippocampus 24 hr after 3 min of global ischemia and found no change in either protein compared with a 24-hr sham control (Fig 5A). To determine whether PRDX2 or SOD1 protein is increased at any other time points, we analyzed hippocampal protein levels at 6, 12, 18, and 36 hr after 3 min and 8 min of global cerebral ischemia. PRDX2 and SOD1 protein levels were not significantly different from control levels for any time point or duration of global cerebral ischemia (Fig. 5Ba–d).
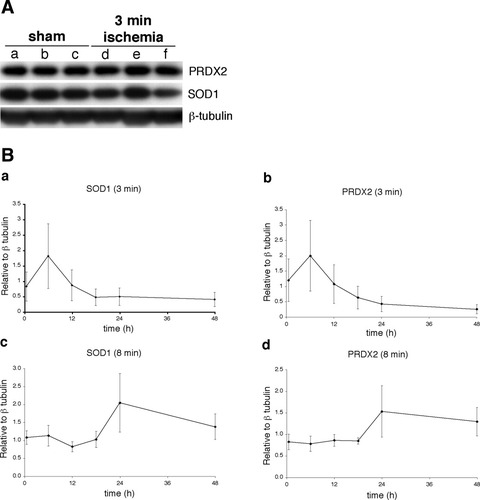
Detection of PRDX2 and SOD1 protein in the rat hippocampus following 3 or 8 min of global cerebral ischemia. A: Western blot analysis of protein lysates probed with anti-PRDX2, anti-SOD1 antibody, and anti-β-tubulin antibody (for loading control) from sham-operated animals (lanes a–c) and animals exposed to 3 min of ischemia (lanes d–f; n = 3). B: Time course of PRDX2 and SOD1 protein expression following 3 min or 8 min of ischemia. Western blot analysis of protein lysates probed with anti-PRDX2 antibody, anti-SOD1 antibody, and anti-β-tubulin antibody (for loading control). Each data point corresponds to the mean (n = 3) protein value (relative to β-tubulin) ± SEM. Control nonischemic animals (to determine baseline levels) are represented by the 0.5-hr time point.
DISCUSSION
In the present study, we used three in vitro neuronal injury models to assess the neuroprotective activity of SOD1 and PRDX2 overexpression. We used glutamate receptor antagonists as a positive control, and these increased neuronal viability following in vitro ischemia and glutamate exposure, as previously reported (Michaels and Rothman, 1990; Koh and Choi, 1991; Kaku et al., 1993). Similarly, glutamate receptor antagonists increased cell viability in the cumene-mediated oxidative stress model, indicating that cell death in this model also has an excitotoxic component. This finding is consistent with other studies, which have demonstrated an association among cumene, oxidative stress, and increased glutamate release (Tretter and Adam-Vizi, 1996; Matsumoto et al., 1996; Tretter et al., 2002).
We have also shown that adenovirally mediated SOD1 overexpression protected cultured cortical neurons against a cumene oxidative insult. This finding complements other studies reporting SOD1 overexpression protecting against oxidative stressors, such as 6-hydroxydopamine and 1-methyl-4-phenylpyridinium (Barkats et al., 2002, 2006). Cumene, which is a lipophilic organic hydroperoxide, localizes to the plasma membrane, causing malondialdehyde (MDA) generation, which contributes to oxidative stress and lipid peroxidation (Gavino et al., 1981; Koster et al., 1983; Persoon-Rothert et al., 1992; Vroegop et al., 1995; Tsai et al., 1997; O'Neil et al., 1999; Halliwell and Gutteridge, 1999). Exactly how SOD1 improves neuronal survival following cumene exposure is unclear, but presumably detoxifying superoxide ions is beneficial in this oxidative stress model. Nevetheless, the cell type and oxidative stressor are probably contributing factors with respect to cytoprotection provided by SOD1, insofar as overexpression of SOD1 in SH-SY5Y cells increases sensitivity to hydrogen peroxide (Sánchez-Font et al., 2003).
In contrast to the cumene model, SOD1 overexpression did not improve neuronal survival in the in vitro ischemic and glutamate injury models. These findings are at variance with reports showing that SOD1 overexpression can reduce neuronal cell death caused by both in vitro ischemic (oxygen glucose deprivation) and glutamate insults (Chan et al., 1990; Barkats et al., 1996; Borg and London, 2002). Superoxide production has been shown to occur following ischemic and glutamate insults in neuronal cultures, so we cannot explain the absence of SOD1-mediated protection in our models, but it is likely that differences in type and severity of injury models, neuronal culture system and method, and level of SOD1 overexpression are responsible for these discrepancies.
With respect to PRDX2, we have demonstrated for the first time that overexpression of this protein can protect cortical neuronal cultures from cumene-mediated oxidative stress and in vitro ischemia. In the oxidative stress model, a likely protective mechanism of PRDX2 overexpression may involve peroxidase-mediated inactivation of cumene by conversion to its less harmful corresponding alcohol. Another neuroprotective mechanism that could be operating in these models involves PRDX2 scavenging reactive oxygen and reactive nitrogen species (ROS/RNS) directly. The latter mechanism is in line with a previous study showing that PRDX2 overexpression reduces ROS generation in PC12 cells exposed to menadione, a superoxide generator; sodium nitropusside, a nitric oxide generator; and L-nitroarginine-D-methylester, an inhibitor of nitric oxide synthetase (Simzair et al., 2000). In addition, the PRDX2 protein is a homologue of alkylhydroperoxide reductase subunit C (AhpC) from Samonella typhimurium (Chae et al., 1994), which has been shown to protect HEK293 cells by eliminating peroxynitrite (Bryk et al., 2000).
Other recently identified functions of PRDX2 might also contribute to the neuroprotection observed in our injury models. For example, oxidative stress induces PRDX2 oligomerization, conferring a strong chaperone function (Moon et al., 2005), which may serve to attenuate the neurotoxicity caused by the misfolded and aggregated proteins known to form following ischemic and oxidative injury (Lee et al., 1999; Hu et al., 2000; Matsumoto et al., 2005). Indeed, Moon et al. (2005) demonstrated that the chaperone function of PRDX2 prevented not only H2O2-mediated cell death of HeLa cells but also the denaturation and aggregation of α-synuclein, a key component of Lewy bodies in Parkinson's and Alzheimer's diseases. Furthermore, it is possible that PRDX2 overexpression is suppressing neuronal cell death signalling pathways. For example, activation of c-Jun NH2-terminal kinase (JNK) and p38 is decreased in HeLa cells overexpressing PRDX2 (Kang et al., 1998, 2004). Finally, PRDX2 interacts with cyclophilin A (Jaschke et al., 1998; Lee et al., 2001), another abundant neuronal cytosolic protein (Goldner and Patrick, 1996) that we have shown is also neuroprotective in both the ischemic and the oxidative stress models used in the present study (Boulos et al., 2007).
As with SOD1 overexpression, we found that adenovirally mediated overexpression of PRDX2 was unable to prevent the neuronal death caused by acute glutamate excitotoxicity. Because of the severity of the excitotoxic insult in our glutamate model, it is more likely that the damaging action of calcium overload and protease activation is the dominant mechanism of cell death, rather than oxidative stress. However, in an in vivo study, adenovirally mediated overexpression of the mitochondrial PRDX3 isozyme was shown to inhibit protein nitration, reduce gliosis, and protect neurons in the rat hippocampus following treatment with the excitotoxic agent ibotenic acid (Hattori et al., 2003). It is possible that the mitochondrial location of the PRDX3 isozyme was an important factor in minimizing the detrimental effects of ibotenic acid neuronal toxicity in this model and/or that ibotenic acid-induced excitotoxicity does not cause the same type of acute injury observed in our in vitro glutamate model.
With respect to in vivo brain expression, we did not detect PRDX2 and SOD1 protein up-regulation in the rat hippocampus following either a nondamaging 3-min or an 8-min CA1-damaging period of global ischemia. Although a damaging period of global ischemia is known to inhibit protein expression, we were surprised that the 3-min global ischemia, which is likely to represent a preconditioning dose, did not up-regulate either protein at the time points examined. Nonetheless, it is possible that PRDX2 and SOD1 protein expression is up-regulated at a different time point(s) and/or within a subset of cells beyond detectable limits with Western blot analysis. Alternatively, chronic long-term exposure to oxidative stress, which is associated with neurodegenerative disorders, may be required to stimulate PRDX2 overexpression.
In summary, we have provided evidence for a neuroprotective function for both PRDX2 and SOD1. Our results suggest that PRDX2 is a more potent neuroprotectant than SOD1, and, unlike the case for SOD1, we have shown protection against in vitro ischemia. Furthermore, the level of protection we obtained for PRDX2 (and SOD1) is likely to be an underestimate because of the incomplete nature of adenoviral transduction of neurons (∼60%) in culture. Taken together, our data suggest that PRDX2 is a potential therapeutic target for the development of a treatment to prevent neuronal death in ischemic and neurodegenerative diseases.
Acknowledgements
The authors thank Ms. Kate Thomas for her technical support.




