Stirred vessel cultures of rat brain cells aggregates: Characterization of major metabolic pathways and cell population dynamics
Abstract
We report a study on neural metabolism of long-term three-dimensional cultures of rat embryonic brain cells in stirred vessels. Our experimental setup was optimized to keep viable aggregate cultures with neuronal maintenance for up to 44 days. Results show that aggregate size and shape could be hydrodynamically controlled depending on the impeller design, avoiding necrotic centers or significant losses in cell viability. Aggregates were composed mainly of neurons until day 16, whereas an effective growth of the glial population was observed after day 21. Cell metabolic status was evaluated by quantification of several metabolites in the culture medium; amino acid metabolism was used as a marker of metabolic interrelationships between neural cell types. Furthermore, 13C-NMR spectroscopy was used on day 31 to explore specific metabolic pathways: incubation with [1-13C]glucose for 45 hr produced an increase in label incorporation in extracellular alanine, lactate, and glutamine, reflecting mainly astrocytic metabolism. The contribution of anaplerotic vs. oxidative pathways for glutamine synthesis was determined: a 92% reduction in the pyruvate carboxylase flux during the first 41 hr of incubation suggested a decrease in the need for replacing tricarboxylic acid cycle intermediates. We believe that our data corroborate the aggregating cultures as an attractive system to analyze brain cell metabolism being a valuable tool to model metabolic fluxes for in vitro brain diseases. © 2007 Wiley-Liss, Inc.
The brain remains a challenging organ for study because of its tremendous cellular heterogeneity and network complexity. To bypass such complexity, simple model systems such as primary brain cell cultures have been widely used to decipher basic biochemical features of individual cell types. Despite monolayer cell cultures being well established as experimental models, their limitations in terms of restricted spatial environment have to be taken into account when interpreting results. It is known that the cellular microenvironment plays a vital role in numerous biological processes (Zhang et al., 2005). Different research applications of three-dimensional (3D) cell cultures, such as aggregates or spheroids, have been broadly exploited (for review see Mueller-Klieser, 1997; Kim, 2005). The major advantage of brain aggregate cultures is their well-organized cellular spatial arrangement, constituted by different cell types and interactions, with patterns of morphological differentiation resembling the ones present in intact brain tissue (Seeds and Vatter, 1971; Seeds, 1975). In addition, among the methodologies used to culture brain cells, the aggregates are suitable for growth and maintenance under well-defined and reproducible conditions, namely, in bioreactors. Therefore, these 3D structures constitute useful and attractive models to link more closely morphological/biochemical studies of brain cells within a structure that resembles the cerebral tissue in vivo.
Maintenance of a viable cell culture in suspension conditions requires optimization of several cultivation parameters. In particular, for aggregate cultures, it is mandatory to control their size hydrodynamically, avoiding the formation of necrotic centers inside the structure resulting from oxygen and nutrient transport limitations. Experimental parameters such as agitation rate and impeller design can be optimized to circumvent such drawbacks without the decrease in culture viability (Moreira et al., 1995a, b). To fully control the culture environment, one has to resort to bioreactors, with which it is possible to monitor and control important parameters such as pH, O2, and temperature while performing sampling over long time periods. A suitable small bioreactor apparatus for culturing primary brain astrocytes using microcarriers has been described (Sá Santos et al., 2005).
The brain is composed mainly of glial and neuronal compartments, glucose being its major energy substrate. The concept of metabolic compartmentation in the brain emerged with the discovery of specific metabolic pathways in astrocytes distinctly different from those of neurons (Hertz, 2004). The existence of specific enzymes such as glutamine synthetase (GS; EC 6.3.1.2) and glutamate decarboxylase (GAD; EC 4.1.1.15) provide unique synthesis capacities to astrocytes and to GABAergic neurons, respectively (Wilson et al., 1972; Martinez-Hernandez et al., 1977). In the brain, the anaplerotic enzyme pyruvate carboxylase (PC; EC 6.4.1.1) is present exclusively in astrocytes (Shank et al., 1985). This enzyme compensates for the loss of tricarboxylic acid (TCA) cycle intermediates in neurons by replenishing the extracellular glutamine, which will be accessible for neurotransmitter synthesis (Westergaard et al., 1995; Schousboe et al., 1997; Zwingmann and Leibfritz, 2003). Remarkable information about compartmentalized cerebral metabolism has been obtained by using 13C-label substrates combined wuith 13C-nuclear magnetic resonance (NMR) spectroscopy (Badar-Goffer et al., 1990; Bachelard, 1998; Gruetter, 2002).
To apply and validate the aggregating brain cell culture as an excellent model system for a wide range of experimental studies, it is indispensable to know the cellular composition during long-term cultures (Berglund et al., 2004) as well as to understand the dynamics of the overall metabolism throughout the time of culture. The present work attempts to characterize the cellular metabolism of long-term cultures of brain cell aggregates from rat embryonic cortex with stirred spinner flasks, providing useful knowledge to design experiments in a fully controlled setup, i.e., stirred vessel bioreactors.
MATERIALS AND METHODS
Materials
Basal medium Eagle's (BME), Dulbecco's modified Eagle's medium (DMEM), fetal bovine serum (FBS), glutamine, penicillin-streptomycin, and phosphate-buffered saline (PBS) were purchased from Invitrogen (Glasgow, United Kingdom). D-[1-13C]glucose (99% enriched) was from Omicron (South Bend, IN) and deuterium oxide (99.9% enriched) was from Sigma-Aldrich (Milwaukee, WI). The monoclonal antibodies used were specific to glial fibrilliary acidic protein (GFAP; Boehringer Mannheim Gmbh, Mannheim, Germany) and to neuron βIII-tubulin (Chemicon, Hampshire, United Kingdom). For immunofluorescence microscopy, the neurons were stained using the polyclonal antibody anti-neurofilament light (NFL; Chemicon). ProLong Gold antifade reagent with DAPI, Alexa Fluor 488 anti-mouse and Alexa Fluor 594 anti-rabbit were from Molecular Probes (Invitrogen). Horseradish peroxidase (HRP)-conjugated secondary antibodies were from Amersham Biosciences (Freiburg, Germany). Other chemicals were of the purest grade available from regular commercial sources.
Studies on stirred vessels were performed in glass spinner flasks with a maximum culture volume of 125 ml (Wheaton, Techne, NJ). The spinners were siliconized prior to experiments to avoid cellular adhesion to the inner surface of the spinner and the outer surface of the ball impeller.
Brain Aggregates Culture
Culture preparation and spinners inoculation
Primary brain cultures were prepared with cerebral hemispheres removed from 15–16-day-old Wistar rat embryos (Harlan Interfauna Iberica, Barcelona, Spain). The dissociation procedure was adapted from Yavin and Yavin (1974). Briefly, the tissue was pooled in ice-cold PBS, and an initial dissociation was carefully done by passing it through nylon filters (180-μm pore diameter) with the plunger of a syringe. Then, to guaranty a good mechanical disruption, gentle resuspension using a Pasteur pipette was performed. The cells were centrifuged at 300g for 15 min at 4°C; resuspended in ice-cold PBS, and centrifuged again. The cell pellet was resuspended in BME medium supplemented with 15% (vol/vol) fetal bovine serum (FBS), 2 mM glutamine, 100 U/ml penicillin-streptomycin and to a final concentration of 10 mM glucose. Single-cell suspensions (approximately 1.9 × 108 cells) were seeded in a 125 ml spinner flask (with ball impeller) containing 40–50 ml of the supplemented medium. The spinner flask was placed on a magnetic stirrer, agitated at 60 rpm, and kept at 37°C in a humidified atmosphere of 7% CO2 in air. After 2 hr in culture, to avoid the formation of large aggregates, the culture volume was increased to 80–100 ml [final concentration of 10% (vol/vol) FBS], and the agitation rate was increased to 80 rpm. After 24 hr, more culture medium was added, for a final culture volume of 125 ml.
Culture maintenance
Aggregating brain cell cultures were maintained in spinner flasks for 44 days. For maintenance of the aggregates, the operational mode applied was a 50% medium substitution (refeed mode) approximately twice per week in order to maintain nutrient availability and to decrease the accumulation of products of cellular metabolism that can be toxic to the cells. The replacement criterion was also that glucose concentration should never be lower than 2 mM. Medium substitutions were performed in a laminar flow cabinet with agitation stopped and gentle removal/refeeding of medium after sedimentation of aggregates; this manipulation was carefully controlled to avoid aggregate adhesion, cell lysis at the outer layers, and biomass loss during the refeeding procedure.
Incubation with [1-13C]glucose
At day 31 of cultivation, the medium was completely removed from the spinner and replaced by 125 ml DMEM without glutamine containing 5.5 mM [1-13C]glucose and 10% (vol/vol) FBS. The culture was then maintained for 45 hr, and 7.5 ml samples of the culture medium were collected at 6, 13, 25, 32, 41, and 45 hr, ensuring that no losses on the culture biomass occurred. Samples were lyophilized and afterward dissolved in 0.6 ml D2O containing 0.1% (vol/vol) dioxane while the pH was adjusted to 7 just before NMR analysis. All the experiments were performed in duplicate.
Analytical Methods
Biomass quantification and evaluation of cellular viability
Culture biomass was evaluated by quantification of total protein using the Pierce bicinchoninic acid (BCA) protein assay kit (No 23227; Pierce, Rockford, IL), after dissolving the cell pellet in 0.1 M NaOH at 37°C for 24 hr. The release of intracellular enzymes, namely, lactate dehydrogenase (LDH; EC 1.1.1.27), in the culture supernatant can be used to quantify the changes in culture viability. This approach assumes that higher rates of release of enzymatic activity correspond to increased cellular damage and thus a loss in culture viability (Racher, 1998). Hence, to assess cellular viability, supernatant samples were collected and the activity of LDH was measured by following the rate of pyruvate reduction to lactate. This reaction is coupled with the oxidation of NADH to NAD+, which can be measured spectrophotometrically at 340 nm.
Morphological analysis and determination of cell populations
Samples containing aggregates were taken daily for the first 2 weeks, and twice per week afterward. Aggregate morphology was monitored with an inverted microscope with phase contrast (DM IRB; Leica, Wetzlar, Germany), and their diameters were measured. An aggregate was considered as a group of cells with size of 40 μm or larger (Moreira et al., 1995a). An Olympus DP11 digital camera system (Olympus, Tokyo, Japan) was used for photomicrographs.
Paraffin sections of aggregates
The aggregates were fixed in 4% (vol/vol) paraformaldehyde in PBS for 20 min at room temperature. Afterward, they were rinsed in NaCl 0.85% (vol/vol), dehydrated through an ethanol range of 70–100% (vol/vol), washed with toluol, placed in paraffin, and stored at 4°C. Paraffin blocks were cut in slices of 10 μm thickness (micrometer: Leica RM 2135) and mounted on SuperFrost Plus microscope slides (Menzel GmbH, Braunschweig, Germany). For fluorescence/confocal microscopy, paraffin sections of aggregates were deparaffinazed in xylene, rehydrated [ethanol 70–100% (vol/vol)], and immunolabeled using standard techniques. The specific cellular markers GFAP and NFL (1:200) were combined with the secondary antibodies Alexa Fluor 488 (1:200) and Alexa Fluor 594 (1:500), respectively, for fluorescence and were visualized by confocal microscopy (TCS SP2 Leica microscope).
Western blotting procedure
Samples containing aggregates were collected at different time points during culture. Aggregates were washed with PBS, resuspended in lysis buffer [0.08 M Tris-HCl, pH 6.8, 2% (vol/vol) sodium dodecyl sulfate (SDS), 10% (vol/vol) glycerol] and resolved in a NuPAGE Novex 4–12% Bis-Tris Gel (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. For immunobloting assays, the lanes were loaded with equal amounts of protein (6 μg). Afterward, samples were transferred to nitrocellulose membranes (Hybond ECL; Amersham Biosciences, Buckinghamshire, United Kingdom) and then blocked with 5% (wt/vol) dry milk at 4°C overnight. Incubation with monoclonal antibodies GFAP (1:200) or βIII-tubulin (1:20) was followed by HRP-conjugated anti-mouse Ig (1:5,000). Signal detection was performed by ECL Plus Western blotting detection system and Hyperfilm ECL (Amersham Biosciences).
Evaluation of the metabolic status throughout the culture
Samples of the culture supernatant were taken on a daily basis. Glucose, glutamine, and lactate concentrations were analyzed by using an YSI 7100 Multiparameter Bioanalytical System (Dayton, OH). Ammonia was quantified enzymatically via UV assay (No 1112732035; Boehringer Manheim, R-Biopharm AG, Darmstadt, Germany).
Quantification of amino acids was determined in an Alliance HPLC System (Waters Corporation, Milford, MA). For sample hydrolysis and precolumn derivatization, a Waters Pico·Tag work station was used. Before analysis, norleucine (internal standard) was added, and samples were purified by solid-phase extraction (SPE) with Sep-Pak C-18 cartridges. Afterward, the amino acids were derivatized with phenylisothiocyanate (PITC) and separated by reverse-phase chromatography. The recovery of the SPE procedure differs slightly for the various amino acids, but it is always above 99.3% ± 0.5%.
Spectroscopy
Proton-decoupled 13C-NMR spectra were obtained on a Bruker DRX-500 spectrometer operating at a frequency of 125.77 MHz. Dioxane (67.19 ppm) was used as an internal standard for chemical shift calibration and also for metabolite quantification (by integration of peaks). Spectra were acquired at a temperature of 27°C using a 55° pulse angle, 31 kHz spectral width, and relaxation delay of 10 sec. The total number of scans was 1,024, and for data processing a 2-Hz line broadening was used.
RESULTS
Dynamics of Aggregate Formation
The first days in culture correspond to a period of cellular recovery from the shear force effects involved in tissue dissociation and adaptation to the culture system. Aggregation of single cells starts immediately after seeding and placing the spinner inside the incubator. Most of the aggregates were small 24 hr after inoculation, with an average diameter of less than 100 μm, and essentially spherical in shape. Within the following 7 days, the aggregates increased in both diameter and number, raising the biomass levels for the next 21 days. After 1month of culturing, the aggregates seem to go through a stable phase in which the biomass amount stays practically constant and with a well-defined spherical appearance. Only after almost 2 months in culture the biomass levels decline, with aggregates decreasing in size and number, with concomitant loss of spherical morphology and decrease in culture viability (data not shown). Similar results concerning size and shape were obtained in different aggregating cultures when aggregates were maintained under analogous experimental conditions.
Influence of Vessel Hydrodynamics on Aggregate Size and Shape
Initial conditions to establish viable cultures of brain aggregates were based on parameters optimized by our group for baby hamster kidney cell lines, i.e., ball impellers agitated at 60 rpm (Moreira et al., 1995a, b). As mentioned previously, the major drawback of aggregate cultivation is the formation of necrotic areas at the center of the aggregates because of size enlargement. The increase in aggregate size is related not only to cell division but also to fusion of small aggregates. Hence, whenever necessary, a step increase of the agitation rate of 5–10 rpm was done to control aggregate size. Culture viability was assessed before and after speed changes to evaluate eventual cell damage. No detectable cell lysis occurred within the agitation range studied, i.e., 60–110 rpm (data not shown). The maximum speed used could still be increased by 10–15 rpm before collision of the ball impeller with the spinner wall takes place. This strategy allowed the hydrodynamic control of aggregate size, with a final average diameter of 300/350 μm obtained at day 8 of cultivation. Aggregates of animal cells with such dimensions are known to be viable, without the risk of necrotic centers being formed (Moreira et al., 1995a).
In previous studies, it was observed that hydrodynamics is the controlling variable for aggregate size independently of vessel and impeller size and geometry (Moreira et al., 1995a). Also, comparative studies showed an increase in cell death for microcarrier cultures when high agitation rates were applied, i.e., 100 rpm or above for geometrically similar spinners (Moreira et al., 1994). This renders the use of natural aggregates an advantageous system for scale-up purposes; and, because the data obtained in small vessels could be used to predict the cellular performance in larger vessels, it is useful to have wide information about different hydrodynamic conditions. Therefore, after successfully cultivating brain aggregates with a ball impeller, testing the culture adaptation to a different impeller configuration, i.e., a paddle impeller, was necessary. The total culture volume of a spinner (ball impeller, 110 rpm) was split in two at day 22 of cultivation: one spinner was maintained with the ball impeller at 110 rpm (control), and the other was operated at 60 rpm with a paddle impeller; these conditions were kept for 6 days (days 22–28). The data obtained are shown in Figure 1, where the abbreviation B corresponds to the ball impeller and P to the paddle impeller.
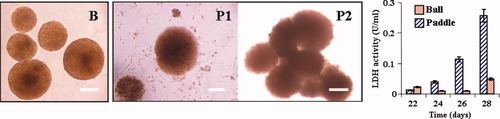
Effect of the impeller design (ball vs. paddle) on aggregates size, shape, and viability. B, ball impeller; P, paddle impeller. Left side images B, P1, P2: morphology under different hydrodynamically conditions. Ball impeller: 110 rpm, day 28 of culture (B); paddle impeller: 65 rpm, day 26 (P1), and 55 rpm, day 28 (P2). Right side graph: progression of culture viability (assessed by LDH activity quantification; see Materials and Methods). The experiments were carried out between day 22 and day 28 of cultivation. Scale bars = 150 μm.
Figure 1B represents the typical culture growth of aggregates (well-defined spherical structures) obtained with ball impellers at 110 rpm, ranging in size from 180 to 350 μm. The maintenance of these structures was possible for up to 44 days. During this period (days 22–28) no significant cell lysis occurred as assessed by quantification of LDH released to the culture supernatant (Fig. 1, graph).
In contrast, when a paddle impeller was used, there was significant cell lysis resulting from changes in aggregate morphology: size increase and structure disruption (Fig. 1P1, P2, and graph). At the higher agitation rate of 65 rpm a peel-off of cells from the outer layer of the aggregates was observed, and occasionally the larger structures were disrupted into smaller ones (Fig. 1P1). In fact, just by decreasing the agitation rate by 10 rpm (to 55 rpm), clusters of several aggregates were formed (Fig. 1P2) whose central zones could contain necrotic areas because of transport limitations, affecting the culture viability (Fig. 1, graph). Moreover, with the paddle impeller, a more heterogeneous size distribution with aggregate sizes ranging from 100 to 700 μm was observed.
Monitoring Cellular Composition in Aggregates
To assess the cellular population in culture, samples of aggregates were taken at different time points and analyzed by Western blot. A representative immunobloting of aggregates to assess neuronal and astrocytic content through a long-term culture is shown in Figure 2. After day 14, the expression of glial fibrilliary acidic protein (GFAP) could be detected. Subsequently, the amount of GFAP increased over time showing an effective growth of astrocytes, especially between days 21 and 36. The neuronal marker βIII-tubulin was detected in samples collected at day 7. Densitometry analysis showed an increase in βIII-tubulin expression from day 7 to day 21. At days 36 and 44 a less intense band was observed. To confirm the presence of neurons at the end of the culture, paraffin sections were performed with aggregates collected at day 44 and double-stained for neurofilament (NFL) and GFAP (Fig. 3). The immunopositive signal for anti-NFL, as observed in Figure 3, supports the existence of neurons until the end of the culture period. Neuronal distribution within the aggregates is highly organized (red signal), where the majority of neurons are arranged in specific areas with spherical shape and in close connection with the outer layers. On the other hand, the outer layers are characterized by a more intense signal for astrocytes (anti-GFAP, green signal). At the higher level of magnification (Fig. 3A), it is possible to see in more detail that astrocytes are more evenly distributed inside the aggregate, without the appearance of cellular density characteristic of the outer layers. No areas of necrosis were evident inside the aggregates (Fig. 3B).
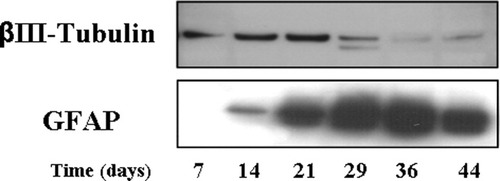
Representative immunobloting results of cellular dynamics through culture time. Increase of markers expression over time: substantial growth of astrocytic population after day 14 (GFAP-positive cells) and detection of neuronal population until the end of the culture time (βIII-tubulin-positive cells).
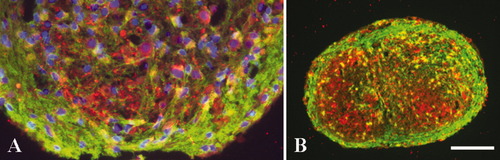
Immunoflurescence images of 10 μm paraffin sections of aggregate culture at day 44 obtained via confocal microscopy. Spatially, the sections are from the central region of the aggregates. Astrocytes were labeled using anti-GFAP/Alexa Fluor 488 (green) and neurons with anti-NFL/Alexa Fluor 594 (red). A: Nuclei labeled with DAPI (×400 amplification). B: Entire section (×100 amplification). Scale bar = 85 μm.
Aggregate Metabolism Through Different Culture Phases
Glucose and lactate metabolism
Glucose is metabolized via glycolysis and TCA cycle, and its carbon skeleton may be detected in lactate, alanine, glutamine, and glutamate (Sonnewald et al., 1997; Zwingmann and Leibfritz, 2003). Throughout the cultivation time the levels of glucose, lactate, and glutamine were monitored on a daily basis, and their specific rates of consumption or production (μmol · hr−1 per mg protein) are summarized in Table I. As mentioned previously, to assure a viable culture for a long-time it is necessary to replace the culture medium (refeed mode). Thus, the rates of consumption or production of the metabolites were calculated between refeedings. Usually, the culture medium was changed twice per week, and, for the sake of clarity, some time ranges were coupled with the criterion applied being the presence of similar ratios of lactate to glucose (Lac/Glc). As can be seen in Table I, ratios between 0.3 and 0.8 of Lac/Glc were obtained.
| Time range (days) | Rates (μmol · h−1 per mg protein) | Lac/Glc | ||
|---|---|---|---|---|
| Glucose consumption | Lactate production | Glutamine consumption | ||
| 0–2 | 0.39 ± 0.05 | 0.326 ± 0.001 | 0.087 ± 0.008 | 0.8 |
| 2–7 | 0.249 ± 0.009 | 0.176 ± 0.007 | 0.112 ± 0.004 | 0.7 |
| 7–16 | 0.32 ± 0.01 | 0.091 ± 0.006 | 0.031 ± 0.003 | 0.3 |
| 16–21 | 0.409 ± 0.006 | 0.20 ± 0.02 | NQ | 0.5 |
| 21–24 | 0.52 ± 0.03 | 0.37 ± 0.02 | 0.014 ± 0.003 | 0.7 |
| 24–31 | 0.47 ± 0.02 | 0.30 ± 0.05 | NQ | 0.6 |
| 33–42 | 0.25 ± 0.03 | 0.16 ± 0.04 | 0.017 ± 0.003 | 0.6 |
| 42–44 | 0.26 ± 0.05 | 0.20 ± 0.07 | −0.015 ± 0.0091 | 0.8 |
- * Data are mean ± SD of two independent experiments, with each sample (daily point) quantified in triplicate or more. Lac/Glc, ratio lactate to glucose; NQ, not quantifiable: values below the detection limit of the analytical method. Some time ranges are sets of two culture periods with the criteria applied for the coupling being the presence of similar lactate to glucose ratios (culture period is described by the time between refeeds).
- 1 Glutamine production between day 42 and day 44.
Until day 7 the ratio Lac/Glc did not change. This time range covers the period when aggregates are being formed with spatial reorganization of cells, coupled with a slight increase in the biomass levels.
Between day 7 and day 16, the rate of lactate release declines to such an extent that the ratio Lac/Glc was reduced by 57%; however, the uptake rate for glucose was 1.3 times higher than that in previous periods. It is worthwhile to mention that neurons are the major cell constituents at this time (Fig. 2) and also that they have a lower production of lactate than astrocytes; furthermore, the extracellular lactate can be taken up by neurons (Schousboe et al., 1997).
It is clearly shown that, after day 16, lactate production rates were increased by fourfold (0.37 ± 0.02 μmol · hr−1 per milligram protein, days 21–24). Afterward, although a reduction in the lactate production rate was obtained, no significant changes in glycolytic metabolism were observed until day 44, when the ratio Lac/Glc was approximately 0.7.
Glutamine metabolism
Glutamine was consumed during almost the entire culture time (with the exception of the last 2 days; Table I). Ammonia was produced during the first 7 days of the culture (when glutamine uptake was highest), achieving a maximal concentration of 1 mM on day 7. The ratio of ammonia production to glutamine consumption was 1.4 and 0.5 between days 0–2 and days 2–7, respectively. After day 7, no significant production of ammonia was detected, so its level was not higher than 0.3 mM.
Between day 7 and day 16 the glutamine uptake decreased by 72%, to a value of 0.031 ± 0.003 μmol · hr−1 per milligram protein. After 21 days in culture, the glutamine uptake rates ranged from 0.014 to 0.017 (±0.003) μmol · hr−1 per milligram protein.
Alanine, branched-chain amino acids, and glutamate metabolism
The amounts of alanine, branched-chain amino acids (BCAA: isoleucine, leucine, and valine), and glutamate were quantified during three different periods of the long-term cultivation selected as representative of different cellular compositions (Fig. 2): mostly neurons (days 5–7), appearance of astrocytes (days 13–16), and mostly astrocytes (days 29–31). It is worthwhile to mention that those amino acids were present in the medium from the beginning of the culture period and that their concentrations were partially restored after each 50% medium substitution (refeeding). The results of the amino acids consumption are shown in Figure 4.
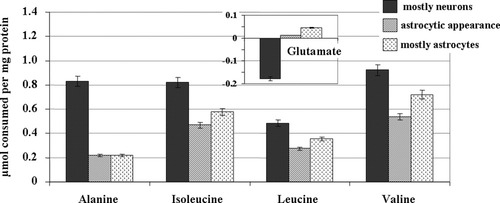
Alanine, branched-chain amino acids (BCAA: isoleucine, leucine, and valine) and glutamate consumed from medium culture during three different periods selected as representative of different cellular composition (Fig. 2): mostly neurons (days 5–7), astrocytic appearance (days 13–16), and mostly astrocytes (days 29–31). Results are expressed as mean ± SD (n = 3).
During the different time intervals evaluated (between refeeds), both alanine and BCAA were taken up by aggregates. Their uptake was higher during the first time range (days 5–7). The consumption of alanine was reduced by fourfold afterward, i.e., at days 13–16and days 29–31. With regard to BCAA metabolism, the uptake of both isoleucine and valine was always higher (1.7- and 1.9-fold, respectively) compared with the leucine uptake. The glutamate levels were almost tenfold lower compared with the other amino acids (Fig. 4). A release of glutamate into the medium was detected only when the neuronal compartment was dominant (days 5–7), corresponding to the period when more glutamine was consumed (Table I). Afterward, almost no changes were observed in the amounts of glutamate, with just a slightly consumption at days 29–31 (mostly astrocytes) taking place.
Studies on Carbon Metabolism Using [1-13C]Glucose
To gain more information about the metabolic pathways, such as the flux through a specific enzyme, a kinetic study at day 31 of cultivation was carried out with [1-13C]glucose, in the absence of glutamine as a medium supplement. The labeling from [1-13C]glucose in metabolites released to the culture medium was analyzed at different time points for 45 hr (Table II); whereas the biomass content was constant.
| Incubation time (hr) | ||||||
|---|---|---|---|---|---|---|
| 6 | 13 | 25 | 32 | 41 | 45 | |
| Lactate (C3) | 17.0; 17.2 | 22.8; 31.7 | 36.3; 39.4 | 36.7; 37.6 | 38.9; 41.4 | 41.3; 48.1 |
| Glutamine | ||||||
| C2 | 11.61 | 14.6; 17.7 | 10.2; 15.4 | 10.6; 15.6 | 10.7; 11.5 | 11.0; 19.0 |
| C3 | ND | ND | 4.6; 6.8 | 8.9; 9.3 | 9.3; 9.9 | 14.6; 17.0 |
| C4 | ND | 8.4; 10.0 | 4.9; 9.0 | 8.2; 11.3 | 9.3; 12.7 | 19.4; 22.2 |
| PC/PDH | NQ | 1.76 | 1.0 | 0.40 | 0.14 | — |
- * Data obtained from two independent experiments. PC/PDH, ratio pyruvate carboxylase to pyruvate dehydrogenase. ND, not detectable; NQ, not quantifiable.
- 1 Quantifiable in only one sample.
The labeling pattern from glucose metabolism into several metabolites is sketched in Figure 5. Through glycolysis, one molecule of [1-13C]glucose can be converted to one of [3-13C]pyruvate and another of unlabeled pyruvate, from which lactate can be formed ([3-13C]lactate and unlabeled lactate, respectively). The label may go through the TCA cycle via pyruvate dehydrogenase (PDH; EC 1.2.4.1) with [2-13C]acetyl-CoA formation or via pyruvate carboxylase (PC; EC 6.4.1.1), which catalyzes the label entrance in oxaloacetate C3. This latter reaction is an anaplerotic pathway specific to glial cells. [2-13C]Acetyl-CoA enters the TCA cycle, and glutamate/glutamine will be labeled in the C4 position (first cycle turn); this label will be scrambled to the C2 or C3 glutamate/glutamine or [2,4-13C]- or [3,4-13C] glutamate/glutamine according to whether another molecule of [2-13C]acetyl-CoA enters the TCA cycle (second cycle turn). On the other hand, through PC activity, the [3-13C]pyruvate will yield a C2 labeled glutamine (for more detailed information see Sonnewald et al., 1997; Zwingmann and Leibfritz, 2003).
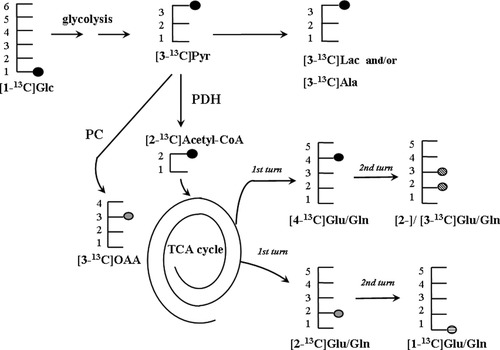
13C labeling patterns of metabolites derived from [1-13C]glucose via metabolism in the TCA cycle: 13C-label is shown as a solid circle for first turn and as a striped circle for the second turn. It should be noted that the label pattern will depend on whether the label is going to the TCA cycle via pyruvate dehydrogenase (PDH; black circle) or via pyruvate carboxylase (PC; gray circle), as described in Results. Ala, alanine; Glc, glucose; Gln, glutamine; Glu, glutamate; Lac, lactate; Pyr, pyruvate; OAA, oxaloacetate.
13C-NMR spectra of the medium during the incubation are shown in Figure 6. Lactate, glutamine, and alanine were released in the culture medium and an increase in 13C-incorporation through time was detected (Fig. 6, Table II). Glutamine was labeled on C2, C3, and C4, alanine and lactate on C3. Glucose was always present in the medium during the incubation period (45hr). Its concentration at the end of the experiment was 1.1 ± 0.1 mM. The percentage of 13C enrichment in each carbon was determined as described elsewhere (Badar-Goffer et al., 1990). Table II shows that, until 41 hr of incubation, glutamine C2 was much more enriched than C3. Moreover, the enrichment of C4 and C2 did not change during the 25–41 hr period. These 13C enrichments were used to determine the enzymatic contribution of PC relative to PDH for glutamine synthesis by calculating the ratio PC/PDH = (C2–C3)/C4 (Hassel et al., 1995). The flux through the anaplerotic pathway was 1.76-fold higher than the flux through PDH at 13 hr of incubation, followed by a sequential decrease and, therefore, achieving a minimum of 0.14 at 41 hr; that corresponds to a 92% reduction through PC in 28 hr (Table II). The amounts of 13C in glucose C1 and lactate C3 are useful for calculating the percentage of lactate that is coming from glycolysis. In the last 20 hr of incubation, this percentage was always higher than 95%, indicating that all the lactate produced derives from glucose. During the same period, a reduction in the medium levels of glutamate, isoleucine, leucine, cysteine, and glycine was also observed (data not shown).
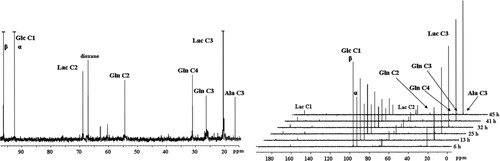
13C-NMR spectra of medium from aggregates incubated for 45 hr with [1-13C]glucose on day 31 of cultivation in a spinner flask. Medium was collected at 6, 13, 25, 32, 41, and 45 hr (spectra sequence). The left spectrum corresponds to the kinetic final (45 hr), expanded between 97 to 15 ppm. Relevant metabolites are identified concerning each carbon position. Dioxane was used as an internal standard. Ala, alanine; Glc, glucose; Gln, glutamine; Lac, lactate.
DISCUSSION
The major aim of this work was to depict the major metabolic pathways of brain cell aggregate cultures from rat embryonic cortex, combining the bioreaction technology to the knowledge available in the literature for culturing primary brain cells. The results presented here are crucial to validate this cell model, supporting it as an alternative to monolayer cultures; in its three-dimensionality and cell–cell interaction, the model resembles more closely the in vivo environment.
Operational Mode and Bioreactor Design
To fully characterize and develop an “essentially tissue-like culture” it is important to ensure a functional and dynamic model, and, therefore, the experimental conditions have to be optimized. Most of the research with aggregating brain cell cultures is performed in small Erlenmeyer flasks (orbital shakers). In these culture systems, the pH and O2 cannot be monitored and small quantities of media are used. The latter aspect can jeopardize the normal cellular behavior, because of starvation conditions or system disturbances resulting from the necessity of replenishing medium daily. Previous studies reported by our group showed that primary brain astrocytes can be grown and maintained under stirred vessel conditions in a bioreactor (Sá Santos et al., 2005). Beyond the fact of being a monotypic culture, the major different requirement of using primary astrocytes is the necessity to employ an extracellular matrix (microcarriers) for cell adherence and growth.
As mentioned earlier, for aggregate culture maintenance, a half media refeed strategy was considered. This operational mode was selected in order to maintain, at least partially, important signalling factors already secreted by cells and essential for the aggregate structures differentiation and maturation. Additionally, because a selective neuronal death has been reported when glucose concentration drops bellow 2 mM (Pardo and Honegger, 1999), a minimum glucose concentration of 2 mM was ensured through refeeding. This was also justified by its normal concentration in the brain (1–2 mM; Zwingmann and Leibfritz, 2003).
As addressed here, the choice of the most suitable impeller to grow embryonic brain aggregates is an important parameter affecting culture performance significantly. Results shown in Figure 1 reveal that, once aggregates were adapted to the ball impeller design, the cellular adjustment to the paddle configuration was impossible. The fact that paddle application caused cellular death was equally supported by the decline in cellular viability and the alterations in the morphology of structures. The latter was characterized by a “switch” between aggregates disruption (due to shear stress) and coadhesion (formation of necrotic centers). After day 28, the attempt to “recover” the damaged aggregates by replacing the paddle for the ball was unsuccessful, signifying that the cellular injury was too profound (data not shown). Therefore, the use of the ball impeller was adopted in aggregating cultures that were further prepared for the long-term metabolic characterization.
Characterization of Aggregate Cultures: Cellular Composition and Metabolism
To assess the dynamics of neural population in aggregating cultures, immunofluorescence and Western blot techniques were performed using specific antibodies for astrocytes (GFAP) and neurons (βIII-tubulin and NFL). Previous studies reported astrocytes and neurons as the most abundant cell types in brain aggregating cultures (Berglund et al., 2004).
An increase in the neuronal phenotype expression was observed from day 7 until day 21 due to differentiation and maturation of precursor cells, since it is known that neurons lack the capability to proliferate (Fig. 2). The results also showed an effective growth of astrocytes in culture after day 21. The astrocytic distribution inside the aggregates reveals a more compact localization at the outer layers (Fig. 3), which can be explained by considering that newly formed cells will be positioned in a shield-like way to protect neurons from shear stress that can occur, because neurons are more sensitive to culture environment than astrocytes. Detection of βIII-tubulin- and NFL- positive cells until the end of culture (Fig. 2, 3) indicates that our setup apparatus was adequate to maintain neurons for long periods of time.
Glucose and lactate metabolism
It is known that glucose is the major energy source for the brain, although a correlation between developmental brain stage and the preference of specific energy-yielding substrates has been reported; i.e., lactate is used as cerebral substrate in the early neonatal period (Cremer, 1982; Vicario and Medina, 1992). Additionally, astrocytes are capable to switch their metabolism to consume lactate under hypoglycemic conditions (Tildon et al., 1993; Sá Santos et al., 2005). In neuronal cultures, the oxidative metabolism of lactate is more intense than that of glucose, and lactate produced by astrocytes may to some extent serve as an energy substrate in neurons (Waagepetersen et al., 1998a, b; Pellerin, 2003).
To analyze the glucose metabolized via glycolysis, the ratio of lactate to glucose (Lac/Glc) was calculated for different time periods (Table I). If all glucose is converted to lactate, the ratio Lac/Glc would be 2. This value is never achieved under physiological conditions, since glucose has many roles to fulfill in cellular necessities, e.g., energy production via the TCA cycle and lipid and amino acid biosynthesis. In cases in which oxygen deprivation occurs, the stimulation of the glycolytic pathway in astrocytes, with higher lactate release, leads to an increased Lac/Glc ratio. Therefore, because oxygen was fully available in our system, it is not surprising that Lac/Glc results obtained are much lower than 2 (Table I). The obtained Lac/Glc ratios plus the cellular population dynamics (Fig. 2, 3) are in agreement with previous studies in cultures of monolayers (Schousboe et al., 1997); indeed, from day 7 to day 21, essentially neuronal metabolism took place, with Lac/Glc (0.3–0.5) similar to that of monotypic cultures of neurons; and, after day 21, the Lac/Glc (average of 0.7) resembles neurons and astrocytes cocultures.
In the present study, a preferential use of lactate by neurons seems to spare glutamine as a carbon substrate: this is suggested by the results presented in Table I (days 7–16), where the glutamine uptake was 24% lower than the reduction in lactate production. The consumption rates of glucose were higher, between day 16 and day 31 (Table I) with an increase in lactate release, as astrocytic population starts to proliferate and maturate (Fig. 2). This could be explained by the tremendous glycolytic capacity of astrocytes compared with neurons. In fact, under hypoxic stress conditions, the astrocytic response with up-regulated glycolysis might determine the degree of neuronal survival (Marrif and Juurlink, 1999). Interestingly, between day 33 and day 44, the glucose requirements decreased by 46% but the lactate to glucose ratio was maintained (average of 0.7; Table I). This may suggest the achievement of a steady-state period inside the aggregates, related to an advanced maturation stage, in which the proliferation does not appear to be appreciable (Fig. 2), so the glucose needs are reduced.
Metabolism of glutamine, glutamate, alanine, and BCAA
The glutamine-glutamate cycle plays a pivotal role in neuron–glia interactions, in that the glutamine released by astrocytes is used by neurons to form glutamate, and then to form GABA (in GABAergic neurons). Both cellular compartments can use glutamine as a carbon source, and astrocytes seem to be more susceptible to the effects of high extracellular amounts of glutamine (Rama Rao et al., 2005). Brain aggregate cultures required the consumption of glutamine, especially when neurons are the main cellular compartment (until day 16; Table I). Afterward, glutamine is still consumed, but such uptake is reduced with the increase in astrocytic population, confirming that astrocytes were functionally capable of producing glutamine for the neurons. This astrocyte-specific property has been reported to be clearly stimulated in the presence of neurons, i.e., neuronal induction for glutamine de novo synthesis (Mearow et al., 1990; Zwingmann et al., 2003). Our results are in agreement with this fact, in that, when glutamine was not supplied to the medium (incubation with [1-13C]glucose), the production of this amino acid was 3.6-fold higher than that in our monotypic cultures of astrocytes under similar conditions (unpublished data).
With regard to amino acid metabolism, changes in alanine, glutamate, and BCAA (isoleucine, leucine and valine) were also analyzed. These amino acids were chosen because of their importance for the interrelationships between neurons and astrocytes: glutamate for the glutamate-glutamine cycle, whereas alanine and BCAA might be involved in shuttles related to ammonia transfer (Yudkoff, 1997; Schousboe et al., 2003).
Glutamate can be released during neuronal activation, and its major uptake occurs in astrocytes, which is fundamental to avoid selective neuronal death by excitotoxic mechanisms (Sonnewald et al., 2002). From the results obtained (Fig. 4), the low levels of glutamate were observed not only when astrocytes were present (astrocytic uptake) but also at the beginning of the culture when the neuronal population was in the majority (days 5–7). The latter can be explained by the fact that neurons isolated from the immature cerebral cortex are predominantly GABAergic, and major release of glutamate does not appear to occur from these neurons (Schousboe et al., 1992).
The data obtained concerning alanine metabolism indicate a more evident consumption in the early stages (days 5–7) when astrocytic populations are not detected. This is in keeping with the large alanine uptake capacity by GABAergic neurons, whereas the astrocytes have a higher rate of alanine release (Schousboe et al., 2003). Ammonia metabolism is a concern, because glutamine/alanine degradation can induce an increase in ammonia levels, and the maintenance of a nitrogen balance must occur to support the normal brain activity. From this point of view, it is clear that intracellular metabolism of nitrogen was efficient in aggregates, since the ammonia levels achieved were always below 0.3 mM, in agreement with the normal brain values (Felipo and Butterworth, 2002).
It is well known that BCAA can also serve as amino donors for the glutamate-glutamine cycle, comprising a shuttle between neurons and astrocytes that consumes glutamate and provides a mechanism for “glutamate buffering” if levels become excessive (Yudkoff, 1997; Yudkoff et al., 2005). The “apparent higher consumption” of isoleucine and valine in the present study may be a culture “strategy” to ensure a more steady concentration of leucine. This can be explained based on the work of Hall et al. (1993) showing that BCAA aminotransferase (BCAT; EC 2.6.1.42.) has a higher affinity for leucine, which suggests that this amino acid might be preferentially used. In addition, the role of leucine and isoleucine catabolism in lipid build-up (via acetyl-CoA formation) should not be disregarded, relating their possible contribution to relevant features in cellular survival and differentiation, e.g., membrane structure and myelin production.
As mentioned above, glutamine is a good precursor for GABA via glutamate (Schousboe et al., 1992). During the entire culturing time of aggregates, GABA was not detected in the medium culture. This is due to the fact that this amino acid is stored in vesicles at nerve endings, and its vesicular release can be selectively induced by depolarization conditions (Waagepetersen et al., 1999) that were not applied in our system. Nevertheless, evidence in the literature supports that aggregates prepared from telencephalon of 16-day-old rat embryos and cultured under comparable conditions show a GABAergic neuronal phenotype (Honegger and Richelson, 1979; Honegger et al., 1998; Pardo and Honegger, 1999; Teunissen et al., 2000).
[1-13C]glucose metabolism
Carbon metabolism was also assessed by using [1-13C]glucose and NMR spectroscopy on day 31 of cultivation (Fig. 6, Table II). This time point was selected based on reports stating that brain cells adopted mature biochemical properties by this time in aggregate culture (Honegger and Richelson, 1976). An incubation time of 45 hr was chosen to maximize the incubation period while still ensuring that there was no limitation of glucose until the end of the experiment. The appearance of [3-13C]alanine, [3-13C]lactate, and 13C-label in C2,3,4 of glutamine was expected, because in this phase the aggregate metabolism reflects mainly the astrocytic compartment, as was mentioned above. Based on the glycolytic flux determination, the extracellular lactate was totally formed from glucose. Similar labeling patterns in alanine, glutamine, and lactate were reported in the literature for cocultures of astrocytes and neurons incubated with [1-13C]glucose for 48 hr (Sonnewald et al., 1991).
Interestingly, during the incubation with [1-13C]glucose, the total release of glutamine was twice the amount released between day 42 and day 44 (Table I). This suggests an increase of GS activity in astrocytes to compensate neurons for the absence of glutamine as a medium supplement. In fact, the extensive glial export of glutamine during this period originated a level of glutamine concentration (∼0.3 mM) similar to that in brain under physiological conditions (Zwingmann and Leibfritz, 2003). Glutamine synthesis is coupled to the PC activity, and the flux through PC depends on the necessity to fill up TCA intermediates (Hassel et al., 1995). As revealed by the ratio of PC to PDH (Table II), it seems that the metabolic requirements for TCA replacement decreased throughout the 45 hr incubation. This PC flux decline can be explained if amino acids are being channeled to TCA, compensating for the glutamine efflux. As mentioned previously, a decrease in the concentration of several amino acids in the culture medium was observed during the incubation time, namely, glutamate, isoleucine, leucine, cysteine, and glycine. This may suggest the breakdown of glutamate and isoleucine to guarantee the normal function of the TCA cycle. Furthermore, after 25 hr of incubation, more than 95% of the lactate was derived from the labeled glucose, indicating that the consumed amino acids did not contribute significantly to the production of lactate. In addition, the glutamate taken up into astrocytes could be directly converted to glutamine by GS; whereas the transamination of BCAA, particularly leucine, is the source of the amino group transferred to the TCA cycle intermediate α-ketoglutarate to yield glutamate for glutamine synthesis (Yudkoff, 1997; Yudkoff et al., 2005).
CONCLUSIONS
This study confirms that long-term cultivation of embryonic brain cell aggregates can be performed in stirred vessel systems, allowing establishment of viable cultures with neuronal maintenance up to 44 days. Furthermore, this setup proved to be very valuable for interpretation and confirmation of the detailed events of cellular metabolism through the different developmental stages of brain aggregate cultures. The data presented herein corroborate the aggregating culture model as an attractive system for depicting brain cell metabolism; it will also be a valuable tool for future research, namely, for the development of in vitro disease models with quantitative estimations of the metabolic fluxes involved.
Acknowledgements
The authors are grateful to C. Peixoto and R. Clemente for technical support and to P. Chicau for providing data from the Amino Acid Analysis Service at the Instituto de Tecnologia Química e Biológica, Universidade Nova de Lisboa, Oeiras, Portugal. Financial support from Merck, Sharp & Dohme, Portugal, is also gratefully acknowledged.




