Kinetic properties of the redox switch/redox coupling mechanism as determined in primary cultures of cortical neurons and astrocytes from rat brain
Abstract
We investigate the mechanisms underlying the redox switch/redox coupling hypothesis by characterizing the competitive consumption of glucose or lactate and the kinetics of pyruvate production in primary cultures of cortical neurons and astrocytes from rat brain. Glucose consumption was determined in neuronal cultures incubated in Krebs ringer bicarbonate buffer (KRB) containing 0.25–5 mM glucose, in the presence and absence of 5 mM lactate as an alternative substrate. Lactate consumption was measured in neuronal cultures incubated with 1–15 mM lactate, in the presence and absence of 1 mM glucose. In both cases, the alternative substrate increased the Km (mM) values for glucose consumption (from 2.2 ± 0.2 to 3.6 ± 0.1) or lactate consumption (from 7.8 ± 0.1 to 8.5 ± 0.1) without significant changes on the corresponding Vmax. This is consistent with a competitive inhibition between the simultaneous consumption of glucose and lactate. When cultures of neurons or astrocytes were incubated with increasing lactate concentrations 1–20 mM, pyruvate production was observed with Km (mM) and Vmax (nmol/mg/h) values of 1.0 ± 0.1 and 109 ± 4 in neurons, or 0.28 ± 0.1 and 342 ± 54 in astrocytes. Thus, astrocytes or neurons are able to return to the incubation medium as pyruvate, a significant part of the lactate consumed. Present results support the reversible exchange of reducing equivalents between neurons and astrocytes in the form of lactate or pyruvate. Monocarboxylate exchange is envisioned to operate under near equilibrium, with the transcellular flux directed thermodynamically toward the more oxidized intracellular redox environment. © 2007 Wiley-Liss, Inc.
The oxidation of carbon skeletons and reducing equivalents from plasma glucose to CO2 and H2O provides, in the adult mammalian brain, the energy required to support cerebral functions (Sokoloff, 1989; Hertz and Dienel, 2002). Both metabolic processes are known to follow different routes. Whereas the carbon skeletons are degraded through glycolysis and oxidized in the tricarboxylic acid cycle to CO2 and H2O, the reducing equivalents are coupled first to nicotinamide or flavin nucleotides, and finally transferred to oxygen and water through the operation of the mitochondrial respiratory chain (Rodrigues and Cerdan, 2007). In the past decades much attention has been given to the flow of carbon skeletons from glucose to CO2 and their relationship to cerebral energetics and neurotransmission (Cruz and Cerdan, 1999; Rothman et al., 2003). The pathways for the transfer of reducing equivalents to O2 and H2O remained much less explored. In particular, the highly heterogeneous cellular composition of the cerebral tissue led to considerable uncertainties concerning the roles, relative contributions, subcellular compartmentation, and coordination of the metabolisms of carbon skeletons and reducing equivalents, in neurons and glial cells.
The flow of carbon skeletons from glucose to CO2, in neurons and astrocytes during cerebral activation is currently understood to proceed as outlined in the astrocyte to neuron lactate shuttle (ANLS) hypothesis (Pellerin and Magistretti, 1994; Tsacopoulos and Magistretti, 1996; Magistretti et al., 1999). According to this proposal, glutamatergic stimulation induces glutamate and Na+ influx into the astrocyte, resulting in partial depolarization and a reactive increase of plasma glucose consumption and lactate production. Extracellular lactate is then thought to be consumed selectively by the neurons and oxidized to CO2 in the tricarboxylic acid cycle. Glucose derived from plasma is, however, also available in the extracellular fluid and can be transported and glycolytically degraded in the neurons. Consequently, both lactate and glucose are available as substrates to support the energetic demands of neuronal activation, but the mechanisms underlying the selection between the consumption of these two substrates and their relative contributions to neuronal or glial metabolism remain insufficiently understood.
Although the intracellular exchange of reducing equivalents between cytosol and mitochondria of neurons and astrocytes is receiving increasing attention (Arco and Satrustegui, 2005; McKenna et al., 2006), their transcellular exchange between these cells has not been, to our knowledge, explored previously. In the original ANLS hypothesis, a flux of lactate was proposed to operate from astrocyte to neuron. The reversibility of the lactate dehydrogenase isozymes and the monocarboxylate transporters of neurons and astrocytes allows these neural cells to produce and consume lactate or pyruvate, resulting in recycling of carbon and reducing equivalents through the corresponding plasma membranes (Rodrigues and Cerdan, 2005b; Rodrigues et al., 2005). Moreover, because both lactate and pyruvate can exchange reversibly between the cytosol of each cell and the extracellular space, it follows that these metabolites can also be exchanged transcellularly between both cells in a lactate/pyruvate shuttle, an aspect previously not envisioned. The competitive metabolism of glucose or lactate and the transcellular exchange of the monocarboxylates lactate and pyruvate between neurons and astrocytes, provided the basis for the redox switch/redox coupling hypothesis (Cerdan et al., 2006b). However, despite a number of studies on the consumption of pyruvate by neurons and astrocytes (Matsumoto et al., 1994; Desagher et al., 1997; Cruz et al., 2001; Zwingmann and Leibfritz, 2003), no evidence had been presented to our knowledge on the crucial aspect of pyruvate production by these cells.
We explore the kinetics of the redox switch/redox coupling mechanisms by investigating the consumption of glucose and lactate in primary cultures of cortical neurons and show that pyruvate can be produced from lactate, both by neurons and astrocytes. Our results show that lactate and glucose are consumed competitively and simultaneously, the dominant substrate being determined by their relative extracellular concentrations, the specific kinetic constants for substrate consumption, and the cytosolic redox state. In addition, we show that pyruvate is produced from lactate in primary cultures of neurons and glial cells, establishing a crucial link for the operation of the redox coupling mechanism. A preliminary account of this work has been presented (Cerdan et al., 2006a).
MATERIALS AND METHODS
Materials
Dulbecco's modified Eagle's medium (DMEM), fetal bovine serum (FBS), horse serum (HS), and phosphate-buffered saline (PBS) were purchased from GIBCO (Glasgow, UK). Kits for analytical determinations of glucose, lactate, and pyruvate were from Roche (Mannheim, Germany). Sodium (3-13C)-L-lactate (99% 13C) was purchased from Cambridge Isotope Laboratories (Andover, MA). 2H2O (99.9% 2H) was purchased from Apollo Scientific (Bradbury, Stockport, UK). All other chemicals were of the purest grade available from commercial sources.
Preparation and Characterization of Primary Cultures From Neural Cells and Incubation Conditions
Primary cultures of cortical neurons were prepared from embryos of Wistar rats with 17 days of gestation, essentially as described previously (Cruz et al., 2001). Briefly, the brains were isolated from the embryos, the meninges removed, and the cortical areas collected in a PBS solution with 6 mM glucose and 1% BSA. The cerebral tissue was minced and resuspended with a pipette. The suspension was then passed through an 80-μm filter and the cells seeded at 1.3 × 106 cells/ml in poly-lysine-coated 8-cm plates with 10 ml of culture medium (DMEM with 15 mM HEPES, 2.5 μg/ml of fungizone, 100 U/ml of penicillin/streptomycin, 5 ng/ml Na2SeO4, 5 μg/ml insulin, 5 μg/ml transferrin, 6.3 ng/ml progesterone, and 100 μM putrescine) with 5% FBS, 5% HS. Plates were maintained in a humidified incubator chamber at 37°C with 5% CO2. After 24 hr, the cells were transferred to culture medium without serum and maintained for 6 more days in culture. Forty-eight hours before the experiments, 10 μM cytosine β-D-arabinofuranoside was added to the culture medium.
On the day of the experiment, the DMEM medium was removed and substituted by Krebs ringer bicarbonate buffer (KRB) containing glucose concentrations of 0.25, 0.5, 1, 2.5, and 5 mM in the absence and presence of 5 mM lactate, or lactate concentrations of 1, 2.5, 5, 10, and 15 mM in the absence and presence of 1 mM glucose. Two aliquots from the incubation medium (0.5 ml each) were withdrawn after 3 and 6 hr of incubation to determine glucose or lactate consumption and investigate the kinetic constants of these processes. An independent set of experiments was carried out to evaluate neuronal pyruvate production from lactate. Primary cultures of neurons, prepared as described above, were incubated in KRB containing increasing lactate concentrations in the same range. In some experiments, the neuronal cultures were incubated with (3-13C) lactate, to monitor (3-13C) pyruvate production by 13C NMR. Two aliquots (0.5 ml) from the incubation medium were withdrawn at 3 and 6 hr and the pyruvate concentration determined spectrophotometrically. The incubation was continued up to 24 hr, the medium collected and concentrated for high resolution 13C NMR analysis. At least three primary cultures from neurons prepared from fetuses derived from different animals were used for every experimental condition.
Primary cultures of cortical astrocytes were prepared from newborn (up to 2-day-old) Wistar rats. Briefly, the cortices were removed and cleaned from the meninges. Cerebral tissue was then minced, resuspended with a pipette and vortexed for 1 min. The resulting suspension was passed through an 80-μm filter and the cells seeded in poly-lysine-coated 8-cm plate with 10 ml DMEM with 10% FBS, 10% HS, 10 mM HEPES, 2.5 μg/ml fungizone, and 100 U/ml penicillin/streptomycin. Plates were maintained in a humidified incubator chamber at 37°C with 5% CO2. After 1 week in culture, cells were transferred to DMEM with 5% FBS, 5% HS, 10 mM HEPES, 2.5 μg/ml fungizone, and 100 U/ml penicillin/streptomycin. Confluent cultures were obtained and used for experiments after 2 weeks in culture. In the day of the experiment, the DMEM medium was removed and substituted by KRB containing increasing concentrations of lactate in the range 1–15 mM in the absence and presence of 1 mM glucose. In some experiments, astrocyte cultures were incubated with (3-13C) lactate, to evaluate (3-13C) pyruvate production under similar conditions to those described above for neurons. At least three primary astrocyte cultures prepared from different animals were used for every experimental condition.
Cell culture purity was assessed using standard immunolabeling techniques. Cells were washed three times with PBS for 10 min, fixed in 4% (w/v) paraformaldehyde in PBS for 5min, and permeabilized with 0.2% (v/v) Triton in PBS. Neurons were visualized (negative staining) using rabbit anti-glial fibrillary acidic protein (GFAP, 1:200) and (positive staining) rat anti-β-III-tubulin (1:200) as primary antibodies and biotin conjugated anti-rat immuno gamma globulin (IgG,1:200), streptavidin Alexa Fluor 488 (1:2,000), and Alexa Fluor 594 goat anti-rabbit IgG (1:2,000) as secondary antibodies. Astrocytes were visualized with rabbit anti-GFAP (1:200) and Alexa Fluor 594 goat anti-rabbit IgG (1:2,000). Cellular nuclei were labeled with 4′-6-diamidino-2-phenylindol (DAPI). The purities of neuron or astrocyte cultures determined in this way were >95%.
Analytical Determinations
Glucose, lactate, or pyruvate in the incubation medium of neurons or astrocytes, were determined spectrophotometrically (340 nm, 37°C) using a vertical spectrophotometer (Spectramax TM 340 PC; Molecular Devices, Sunnyvale, CA) in 96-well polypropylene plates. To this end, we adapted conventional enzymatic end point methods coupled to NAD(P)H production or consumption (Bergemeyer, 1983). Briefly, glucose was determined through the NADPH production by hexokinase and glucose-6-phosphate dehydrogenase, lactate, and pyruvate through NADH production or consumption by lactate dehydrogenase. Total protein was determined in cell pellets obtained after the incubation using the Bradford assay (Bradford, 1976).
Determination of Kinetic Constants
The apparent Km and Vmax values for glucose consumption in the absence and presence of lactate, lactate consumption in the presence and absence of glucose, and pyruvate production, were determined by non-linear least squares fit of the measured rates to the Michaelis-Menten expression v = Vmax × [S]/Km + [S] where v or [S] represent the initial rates of substrate consumption or the initial concentrations of substrate and Km and Vmax, the corresponding apparent kinetic constants. The apparent inhibition constant Ki was determined using the expression for competitive inhibition KmI = Km (1 + [I]/Ki) where KmI and Km represent the Km values in the presence and absence of the inhibitor and [I] the inhibitor concentration. Non-linear fits were carried out using the Mathematica v4.0 software (Wolfram Research, Champaign, IL) on an Intel Centrino Pentium M 1.5 GHz platform.
13C NMR
High resolution 13C NMR spectra (4°C, pH = 7.2) from aliquots of the incubation medium after incubation with (3-13C) lactate were acquired in a Bruker AVANCE 500 WB NMR spectrometer using a commercial 5-mm high resolution triple probe (13C,1H,2H). Conditions were: π/3 pulses, 1.09 sec acquisition time, 7.0 sec recycle time, 30 kHz sweep width, 64,000 points zero-filled to 256,000 points before Fourier transformation and 20,000 scans. Broadband proton decoupling was gated on only during acquisition. Chemical shifts were measured with respect to the resonance of a 10% dioxane solution in water at 67.4 ppm, placed in a coaxial capillary. Assignments were carried out using literature values and confirmed through the addition of the authentic compounds (Cruz and Cerdan, 1999; Rodrigues and Cerdan, 2005a).
Statistics
Kinetic constants obtained under different incubation conditions were compared using Student's t-test, as implemented in the SAS package (Statistical Analysis System, Cary, NC) running on an Intel Centrino Pentium M 1.5 GHz Platform. Results with P < 0.05 were considered statistically significant.
RESULTS
Competitive Consumption of Glucose and Lactate
Table I summarizes the kinetic constants for glucose consumption in the absence and presence of 5 mM lactate or those for lactate consumption in the presence and absence of 1 mM glucose. Aliquots withdrawn at 3 and 6 hr of incubation showed a linear decrease in the initial glucose or lactate concentrations allowing the determination of the initial rates. Glucose or lactate consumption rates depicted a clear hyperbolic pattern, consistent with Michaelis-Menten kinetics. These experiments were reproduced with neuronal cultures from fetuses of three different litters and the combined results fitted to the Michaelis-Menten expression to determine the apparent Km and Vmax values. The Km for glucose consumption increased from 2.2 ± 0.2 mM in the absence of lactate, to 3.6 ± 0.1 mM (P < 0.001) in the presence of 5 mM lactate, with no significant effects on the Vmax value. This is consistent with a competitive inhibition mechanism, with a Ki value of 3.6 mM for the inhibition of glucose consumption by extracellular lactate. The Km and Vmax values for lactate consumption increased from 7.8 ± 0.1 mM and 440 ± 3 nmol/mg/hr in the absence of glucose to 8.5 ± 0.1 and 451 ± 3 nmol/mg/hr (P < 0.05) in the presence of 1 mM glucose, also consistent with a competitive inhibition mechanism with a Ki value of 11.1 mM.
| Process | Incubation condition | Km (mM)a | Vmax (nmol/mg/hr)a | Ki (mM)b |
|---|---|---|---|---|
| Glucose consumption | Glucose 0.25–5 mM | 2.2 ± 0.2 | 600 ± 65 | na |
| Glucose consumption in the presence of lactate | Glucose 0.25–5 mM and 5 mM lactate | 3.6 ± 0.1 | 674 ± 54 | 3.6 |
| Lactate consumption | Lactate 1–15 mM | 7.8 ± 0.1 | 440 ± 3 | na |
| Lactate consumption in the presence of glucose | Lactate 1–15 mM and 1 mM glucose | 8.5 ± 0.1 | 451 ± 3 | 11.1 |
- a Apparent kinetic constants were obtained from non linear fits of substrate consumption curves to the Michaelis-Menten equation as described in Materials and Methods.
- b Apparent Ki values were obtained as described in Materials and Methods from the expression Km′ = Km (1 + [I]/Ki) where Km′ is the Km in the presence concentration of inhibitor [I] and Km the value obtained in the absence of inhibitor. na, not applicable.
To evaluate the effects of the competing substrates over the full range of substrate and inhibitor concentrations (0.25–5 mM glucose and 1–15 mM lactate) we generated 3D plots of the glucose (Fig. 1A) or lactate (Fig. 1B) consumption rates, as a function of the variations in the concentration of the competing substrate. To this end, we used the expression for hyperbolic competitive inhibition v = Vmax [S]/((Km (1+[I]/Ki) + [S]) for the consumption of glucose or lactate in the presence and absence of one competitive inhibitor. Our results showed that at resting glucose and lactate concentrations of around 1 mM in the extracellular fluid, primary cultures of neurons consumed 81% glucose and 19% lactate. When the extracellular glucose concentration was maintained at 1 mM and the extracellular lactate concentration increased to 2 mM and 5 mM, however, the relative lactate consumption rate increased to 30% and 60%, respectively.
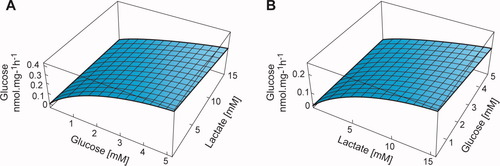
3D simulations of (A) glucose consumption (nmol/mg/hr) for the range 0.25–5 mM glucose and 1–15 mM lactate and (B) lactate consumption (nmol/mg/hr) for the range 1–15 mM lactate and 0.25–5 mM glucose. 3D Simulations were carried out with the Mathematica v4.0 software package, using the kinetic constants of Table I.
Pyruvate Production
We continued by investigating the reversible exchange of monocarboxylate reducing equivalents between astrocytes and neurons. This process involves necessarily not only the reversible transfer of reduced substrates such as lactate between neurons and astrocytes, but the transfer of oxidized equivalents in the form of pyruvate, in the opposite direction. Figure 2 illustrates the kinetics of pyruvate production in the medium of primary cultures of astrocytes (Fig. 2A) or neurons (Fig. 2B) incubated with increasing concentrations of lactate in the range 1–20 mM. Pyruvate production showed hyperbolic kinetics in both cell types. Neurons were able to release pyruvate with Km and Vmax values of 1.0 ± 0.2 mM and 109 ± 4.4 nmol/mg/hr, whereas astrocytes incubated under similar conditions released pyruvate to the medium with Km and Vmax values of 0.3 ± 0.1 mM and 341 ± 53.7 nmol/mg/hr, respectively.
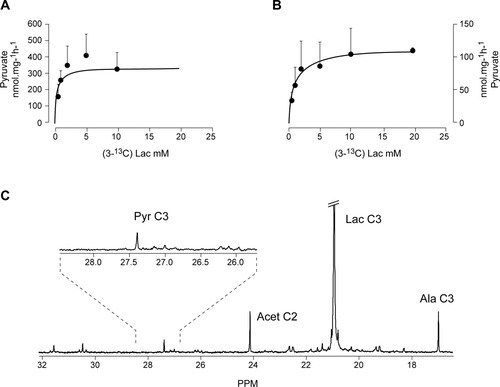
Kinetics of pyruvate production in primary cultures of cortical astrocytes (A) and neurons (B). Initial rates of pyruvate consumption were determined spectrophotometrically in the incubation media of neuronal and glial cultures containing lactate concentrations in the range 1–20 mM. Results are expressed as the mean ± SD. 13C NMR spectra of the media of cortical astrocytes incubated with 5 mM (3-13C) lactate for 24 hr (C).
Finally, we investigated if the pyruvate produced by primary cultures of neurons or astrocytes during incubation with (3-13C) lactate could be detected by 13C NMR in the incubation medium. Figure 2C shows a representative 13C NMR spectrum from the incubation medium of astrocytes incubated for 24 hr with 5 mM (3-13C) lactate. After extensive concentration of the 8 ml medium to dryness and resuspension in 0.5 ml of 2H2O, it was possible to observe the resonance of (3-13C) pyruvate at 27.4 ppm, together with other additional resonances derived from the (3-13C) lactate (20.9 ppm) used as substrate or the (4-13C) glutamine (27.7 ppm) produced. The lower capacity of pyruvate production by neurons made it not possible to detect the corresponding (3-13C) pyruvate resonance in their incubation media after similar incubation conditions and concentration steps (not shown).
DISCUSSION
Simultaneous and Competitive Consumption of Glucose and Lactate
The competition between glucose and lactate as oxidative substrates for the neurons has been addressed a number of times previously, both in vitro and in vivo (Waagepetersen et al., 1998; Dienel and Hertz, 2001; Bouzier-Sore et al., 2003, 2006). In vitro studies used primary cultures of neurons or astrocytes and combinations of 13C labeled glucose or lactate in the presence of the unlabeled competitor whereas in vivo studies infused different combinations of labeled and unlabeled glucose and lactate (Bouzier-Sore et al., 2003, 2006). The in vitro results showed a preferential oxidation of lactate by neurons as shown by increased 13C labeling of the amino acids glutamate and glutamine. Similarly, the in vivo data were consistent with a preferential oxidation of lactate in a compartment devoid of pyruvate carboxylase activity, presumably the neuronal environment (Bouzier et al., 2000).
In contrast, the inhibitory effects of glucose or lactate on the consumption of each other received less attention and no kinetic studies are available to our knowledge. Primary cultures of cortical neurons incubated with glucose or lactate only, consumed preferentially glucose because of its more favorable kinetic constants Km and Vmax. Lactate increased the Km for glucose consumption without a significant effect on Vmax and glucose increased the Km for lactate consumption, without apparent effects on Vmax. These results showed that glucose and lactate are consumed competitively and simultaneously, rather than alternatively as thought previously. Moreover, the kinetics for glucose and lactate consumption in the presence of each other are consistent with a one-site inhibition by the competing substrate in both cases. We hypothesize that the common inhibition site occurs at the glyceraldehyde 3-phosphate dehydrogenase step, the inhibition mechanism relying in the competition for cytosolic NAD+ between glycolysis and lactate oxidation (Cruz et al., 2001; Garcia-Espinosa et al., 2004). The apparent Ki values indicate that relatively high lactate concentrations (∼3.6 mM) are required to inhibit by 50% glucose consumption. This concentration is in the range of the high Km value of the dominant lactate dehydrogenase activity of synaptosol (O'Brien et al., 2007), suggesting that this isoform is the main participant in the regulation of the redox switch.
The relevance of the present results to the in vivo situation in the adult brain remains difficult to evaluate at present. Lactate concentrations in the in vivo brain have been measured previously by 1H NMR spectroscopy and in vivo microdialysis methods, with values in the range of 0.5 μmol/g or 0.4 mM, respectively (Gruetter et al., 1998; Darbin et al., 2006). Moderate increases ranging 50–150% have been reported on cerebral activation (Giove et al., 2003). These concentrations seem to be too small to elicit a significant inhibition of glucose consumption or an important relative rate of lactate consumption, in our cultures. Under these in vivo conditions, present results indicate that neurons would consume preferentially glucose. This conclusion agrees with the in vivo accumulation of fluorescently labeled deoxyglucose observed in neurons from the hippocampus or cerebellar Purkinje cells (Itoh et al., 2004). It is also possible, however, that the actual concentrations of lactate in the highly constrained space of the synaptic cleft are significantly higher than the average concentrations measured by NMR over large voxel volumes or determined by microdialysis in the extracellular space. Under these conditions, synaptic lactate could operate effectively the redox switch, provided that its concentration approaches or exceeds significantly the Ki value. Extracellular lactate can also approach a complete switch off of glycolysis under hypoxic or ischemic conditions, when lactate concentrations reach the 10–15 mM range. This confirms previous proposals of lactate becoming a predominant substrate under ischemic conditions (Schurr et al., 1988; Schurr, 2006).
In addition to these considerations, it should be noted that cerebral activation can induce changes in the rates of lactate production and consumption by the neuronal and glial compartments in vivo (Serres et al., 2003, 2004, 2005). Increased glutamate concentrations obtained during activation have been shown to increase the uptake of fluorescently labeled glucose by astrocytes and reduce it in neurons (Loaiza et al., 2003; Porras et al., 2004). Together, this evidence indicates that the cerebral activation process in vivo could involve changes in the kinetic parameters investigated in this study under resting conditions. In summary, our results suggest that glucose and lactate can be consumed simultaneously, the consumption of one substituting partially the need for the other. This is a useful bioenergetic circumstance because both substrates seem to be equally effective energizing activity dependent synaptic vesicle turnover (Morgenthaler et al., 2006).
It should be noted that the kinetics of relative glucose or lactate consumption reported here are different from the relative glucose or lactate oxidation rates described previously (Bouzier-Sore et al., 2003, 2006). For the combination of 1 mM glucose and 1 mM lactate, it was calculated that lactate contributed 75% of the carbons oxidized in the neuronal cycle, whereas the lactate consumption values reported in the present study would indicate that lactate consumption represents only 20% of the glucose consumed. These results indicate that even though lactate consumption may be smaller than glucose consumption, the lactate consumed may be preferentially oxidized. This situation is consistent with our previous findings of two kinetically different pyruvate pools in neurons, one derived from glucose (the Pyrg pool) and the other from monocarboxylates (Pyrp) pool, able to be oxidized preferentially under different substrate and redox conditions (Cruz et al., 2001; Garcia-Espinosa et al., 2004). Thus, glucose or lactate consumption rates may not be linearly related to the respective oxidation rates.
Transcellular Monocarboxylate Redox Shuttle
A number of studies have reported previously lactate and pyruvate consumption by primary cultures of neural cells (Cruz et al., 2001; Dienel and Hertz, 2001; Zwingmann and Leibfritz, 2003; Bouzier-Sore et al., 2006). In addition, the presence of pyruvate in the extracellular fluid has been classically reported in a variety of microdialysis experiments (Ronne-Engstrom et al., 1995; Valtysson et al., 1998). The origin of the cerebral pyruvate present in the extracellular fluid and consumed by neural cells has not, however, been addressed sufficiently. The present study provides an advance in this direction by showing pyruvate production and its kinetics in primary cultures of neurons and glial cells. Interestingly, our results indicate that both neural cells are able to produce pyruvate from lactate but with different kinetics. For the same extracellular lactate concentration, astrocytes present a larger capacity of pyruvate production than neurons. This occurs, most probably, because of the different kinetic properties of the lactate dehydrogenase isozymes present in both cells (O'Brien et al., 2007). Although both neurons and astrocytes have been shown recently to contain the five isoforms of LDH, the muscle isoenzyme LDH5 seems to be predominant in astrocytes whereas the heart isoenzyme LDH1 is predominant in neurons (Bittar et al., 1996; Pellerin et al., 1998). The kinetic constants of LDH5 favor lactate production whereas those of LDH1 favor pyruvate formation. The fact that from the same amount of lactate neurons produce less pyruvate than astrocytes indicates that the pyruvate produced by neurons may be, as expected, preferentially oxidized rather than exported to the extracellular medium. It should be noted that the capacity for pyruvate production in neurons may account for a very large proportion of the lactate consumed. Considering the kinetic constants of lactate consumption (Table I) and pyruvate production for a 1 mM extracellular lactate concentration, it can be calculated that both lactate and pyruvate can be consumed and produced virtually at the same rate of 50 nmol/mg/hr. This represents an important observation because many previous studies considered that all lactate consumed by neural cells was completely oxidized. On this basis, our results suggest that the rates of lactate oxidation reported previously, based on metabolic balances not considering pyruvate production, could be overestimated.
The demonstration of pyruvate production by neurons and astrocytes fulfills an essential requirement for the operation of the redox shuttle proposed recently (Cerdan et al., 2006b). Figure 3 describes schematically its operation between both cells, considering monocarboxylate compartmentation (Zwingmann et al., 2000; Cruz et al., 2001). Astrocytes are able to transfer lactate to neurons after the stimulation of glycolysis by glutamate or K+ ions. As counterpart, neurons are able to transfer back to the astrocytes part of the lactate received in the form of pyruvate, closing the exchange of reducing equivalents between both cells. The exchange of reducing equivalents in the form of monocarboxylates is reversible, because of the reversibility of the lactate dehydrogenase isoenzymes and monocarboxylate transporters. It is also thought to operate close to equilibrium in both cells, because the large activity of lactate dehydrogenase and monocarboxylate transport (Stubbs et al., 1972; Veech, 1991; Halestrap and Price, 1999; Hertz and Dienel, 2005; O'Brien et al., 2007). Under these conditions, the direction of the transfer of reducing equivalents is determined thermodynamically, depending on the relative redox states of the initial and final steps, neurons or astrocytes, respectively. Because of the larger oxidative capacity of neurons, the redox state in these cells is conceived to be more oxidized than in astrocytes. Thus, the regular operation of the shuttle would be to transfer reducing equivalents in the form of lactate from astrocytes to neurons, and return pyruvate from neurons to astrocytes. Indeed, our results show that most of the neuronal lactate uptake may be returned to the astrocytes in the form of pyruvate. The inverse situation with a neuronal lactate transfer to the astrocytes may become operative, whenever the redox state of astrocytes becomes relatively more oxidized than that of the neurons. Thus, the operation of this transcellular redox coupling mechanism seems to relay ultimately in the regulation of the intracellular redox states of neurons and astrocytes and, consequently, is determined by the activity of the intracellular redox shuttles through the inner mitochondrial membrane (Arco and Satrustegui, 2005; McKenna et al., 2006) and the bioenergetic requirements of both cells. Under these strong redox coupling conditions, neurons and glial cells behave effectively as a single neural cell, containing one anaerobic (astrocytic or cytosolic) and one aerobic (neuronal or mitochondrial) compartment, exchanging NADH reducing equivalents through very potent transcellular and intracellular shuttle mechanisms.
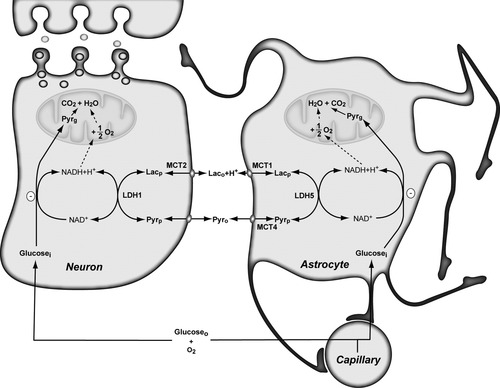
The transcellular monocarboxylate redox shuttle between neurons and astrocytes. Reducing equivalents are exchanged transcellularly, under reversible, and close to equilibrium conditions, in the form of the monocarboxylates lactate and pyruvate. The net direction of the flow occurs toward the more oxidized cytosolic redox environment, as determined mainly by the local oxygen availability, the oxidative capacity of the corresponding respiratory chain and the activity of the mitochondrial redox shuttles (dotted line) coupling cytosolic and mitochondrial redox environments. The exchange of monocarboxylates involves primarily the fast turnover pool of cytosolic monocarboxylates (Lacp and Pyrp), eventually inhibiting glycolytic pyruvate production (Pyrg pool) by NAD+ competition. Lacg, slow turnover glycolytic lactate pool; Laco, extracellular lactate; Lacp, fast turnover intracellular lactate pool derived from extracellular lactate; LDH, lactate dehydrogenase; Glucosei, intracellular glucose; Glucoseo, extracellular glucose; MCT, monocarboxylate transporters; Pyrg, slow turnover glycolytic pyruvate pool; Pyro, extracellular pyruvate pool; Pyrp, fast turnover intracellular pyruvate pool derived from extracellular pyruvate.
Transcellular exchange of reducing equivalents underlies most probably the biphasic changes in NADH fluorescence observed in hippocampal slice preparations (Kasischke et al., 2004) or the extracellular lactate dynamics calculated during activation (Aubert and Costalat, 2005; Aubert et al., 2005). After stimulation, the dynamics of NADH fluorescence and extracellular lactate concentration follow a biphasic time course with an initial dip followed by a pronounced overshoot. Dip and overshoot kinetics have been interpreted to reflect the initial neuronal oxidation of lactate followed by an increase in its glycolytic delivery from the astrocytes. Our results suggest that the decrease in neuronal NADH fluorescence, or in the extracellular lactate concentration, may not necessarily reflect neuronal lactate oxidation. In fact, both processes could well be due to an increase in the transcellular transfer of neuronal pyruvate to the astrocyte, a process also resulting in neuronal NADH oxidation and reduced extracellular lactate levels. Similarly, the overshoot in NADH fluorescence in the astrocytes or the increase in extracellular lactate concentrations may not simply reflect lactate production and its stoichiometric oxidation in the neurons. These processes must show the net balance between lactate and pyruvate produced and oxidized in the astrocyte, lactate, and pyruvate transferred to the neuron and the balance between lactate oxidized in the neurons and returned to the astrocyte as pyruvate in the transcellular redox shuttle.
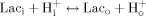 (1)
(1)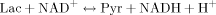 (2)
(2)Consequently, the transcellular redox shuttle seems to be intimately related to intra- and extracellular pH regulation and, because of the proton involvement in many monovalent and divalent cation transport processes, linked to the overall ionic homeostasis.
In this context, the transcellular monocarboxylate shuttle investigated in this study is envisioned to play a central role in the integration of redox processes, pH regulation, and ion homeostasis in the central nervous system. Redox shuttles based on monocarboxylate exchange may present a more general biologic significance, coupling hypoxic and oxidative environments in many other heterogeneous tissues including muscle and tumors.
Acknowledgements
The authors are deeply indebted to the reviewers of our previous contributions who expressed the importance of addressing pyruvate production in neural cells. In addition, they wish to express their gratitude to S. Arroyo for expert technical assistance and the drafting shop of the Institute of Biomedical Research Alberto Sols for professional preparation of the illustrations. B.G.R. was a postdoctoral fellow of C.S.I.C. and I.R.V. received an Erasmus fellowship from the University of Coimbra. M.M.C.A.C. was on a sabbatical visit from the University of Coimbra (Portugal). T.B.R. and L.L.F. received predoctoral fellowships from the Portuguese FCT. M.L.G.M. holds a tenure track “Ramón y Cajal” contract from CSIC. S.C. receives a grant from Institute of Health Carlos III, as does M.L.G.M. Portuguese Fundação para a Ciência e a Tecnologia provides funding for T.B.R. and L.L.F.




