Voluntary exercise-induced neurogenesis in the postischemic dentate gyrus is associated with spatial memory recovery from stroke
Abstract
Spatial cognitive impairment is common after stroke insults. Voluntary exercise could improve the impaired spatial memory. Newly generated neurons in the dentate gyrus are necessary for the acquisition of new hippocampus-dependent memories. However, it is not well known whether voluntary exercise after stroke promotes neurogenesis in the adult dentate gyrus, thereby promoting spatial memory recovery. Here, we examined in mice subjected to focal cerebral ischemia the effect of voluntary or forced exercise on neurogenesis in the ischemic dentate gyrus and spatial memory. Exposure to voluntary wheel running after stroke enhanced newborn cell survival and up-regulated the phosphorylation of cAMP response element binding protein (CREB) in the dentate gyrus and reversed ischemia-induced spatial memory impairment. However, the enhanced newborn cell survival and CREB phosphorylation in the dentate gyrus and improved spatial memory were not observed in the mice exposed to forced swimming. Moreover, there was a significant correlation between the total number of surviving newborn cells in the dentate gyrus and the ability of mice to locate the platform in the Morris water maze. These results suggest that, in the adult mice, exposure to voluntary exercise after ischemic stroke may promote newborn cells survival in the dentate gyrus by up-regulating CREB phosphorylation and consequently restore impaired hippocampus-dependent memory. © 2007 Wiley-Liss, Inc.
Stroke is a major leading cause of disability, and spatial cognitive impairment is common after stroke insults. Significant functional improvement occurs in most stroke survivors during the initial months after the stroke insult (Kotila et al., 1984). Postischemic environmental enrichment (Ohlsson and Johansson, 1995; Dahlqvist et al., 2004) or voluntary exercise (Stummer et al., 1994) could enhance the functional recovery, including spatial learning and memory. However, the mechanisms underlying these beneficial effects remain enigmatic. Recent studies have demonstrated that the spatial memory improvement induced by environmental enrichment and voluntary exercise is not the result of reduced infarction volume or attenuated thalamic atrophy (Johansson and Ohlsson, 1996).
Spatial memory is largely dependent on the hippocampal formation (Morris et al., 1982). It is well established that progenitor cells in the adult brain can proliferate and differentiate into mature neurons (Eriksson et al., 1998; Gould et al., 1999). Although many of the newly generated neurons undergo physiological cell death (Gould et al., 1999), it has been suggested that some of these new neurons are functionally recruited in the dentate gyrus circuitry, and form appropriate synapses with already existing neurons (van Praag et al., 2002; Liu et al., 2003). The newly generated neurons in the dentate gyrus are necessary for the acquisition of new hippocampus-dependent memories (Shors et al., 2001). Moreover, recent advances in the study of neural regeneration suggest that neurogenesis may be a critical element in brain repair. Our studies as well as those of others indicate that focal cerebral ischemia increases the number of newly generated neurons that migrate from the subgranular cell zone (SGZ) into the granule cell layer (GCL) of the dentate gyrus in adult rats (Jin et al., 2001; Zhu et al., 2003). A more recent study extended these findings to demonstrate that activation of endogenous progenitors after transient forebrain ischemia leads to massive regeneration of pyramidal neurons in the CA1 area of hippocampus (Nakatomi et al., 2002). These results have been interpreted as evidence for the direct migration of neuronal precursors toward injured areas, possibly to trigger brain repair and contribute to amelioration of the neurological deficits (Nakatomi et al., 2002). However, the ischemia-induced neurogenesis declines rapidly and returns to the basal level in approximate 3–4 weeks (Yagita et al., 2001; Zhu et al., 2003). Moreover, the survival rate of newborn cells is low (Takasawa et al., 2002; Sun et al., 2003), so the therapeutic potential of neurogenesis stimulated by cerebral ischemia itself is very limited. Previous investigations have demonstrated that voluntary wheel running (van Praag et al., 1999a) could enhance neurogenesis in the intact adult animals. However, it is not well known whether voluntary exercise after stroke promotes neurogenesis in the adult dentate gyrus and spatial memory rebuilding. We show here that voluntary wheel running after transient focal cerebral ischemia may promote newborn cells survival in the dentate gyrus by up-regulating cAMP response element binding protein (CREB) phosphorylation and consequently restore impaired hippocampus-dependent memory.
MATERIALS AND METHODS
Animals and Experimental Protocol
All protocols conformed to the NIH Guide for the care and use of laboratory animals and were approved by the Institutional Animal Care and Use Committee of Nanjing Medical University. Adult male C57/BL/6 mice (25–30 g) were used. The design of the experiments is illustrated in Figure 1. One week prior to surgery, mice were housed in standard laboratory cages (30 × 18 × 20 cm, four or five mice per cage) at temperatures of 20–21°C, with lights on 06:00–18:00 hr and with free access to food and water. On day 0, mice were subjected to middle cerebral artery occlusion (MCAO), then returned to standard cages after 24 hr of recovery in individual cages. One week after surgery, the mice with hemiparesis (Bederson et al., 1986) were randomly divided into three groups: model (MCAO control), runner with MCAO, and swimmer with MCAO. Swimmers were placed in a circular swimming pool for two trials per day (each trial duration is 60 sec), with an intertrial interval of 30 sec. Models and swimmers were still housed in standard cages. The runners were transferred to cages (48 × 26 × 20 cm) with one running wheel that was freely accessible (four or five animals per cage). Sham animals were treated identically and housed in standard cages.
Design of the experiments. MCAO, middle cerebral artery occlusion; MWMZ, Morris water maze tests; BrdU, bromodeoxyuridine.
After exposed to different exercises, the animals were divided into three consecutive experiments. 1) To determine whether different exercises reverse ischemia-induced cognitive impairment, spatial cognitive performance was tested in the Morris water maze during the last 5 days of the experiment (days 45–49 after focal cerebral ischemia). After the last test, mice were killed for 2,3,5-triphenyltetrazolium chloride (TTC) staining to determine infarct volume or for Fluoro-Jade staining to examine neuron degeneration. 2) To test whether exercise-stimulated spatial memory improvement is dependent on enhanced neurogenesis in the dentate gyrus, mice received bromodeoxyuridine (BrdU; Sigma, St. Louis, MO) injections (50 mg/kg, i.p. seven times at 24-hr intervals) during the third week after surgery (from day 14 to day 20). On day 21, five to seven animals from each group were killed for testing cells proliferation, and, on days 45–49, the remaining mice were subjected to the Morris water maze test and 6 hr later were killed for examining the survival and neuronal identity of newborn cells. 3) To explore the possible signal molecular involved in exercise-induced neurogenesis after stroke, on day 28, three animals from each group were sacrificed for Western blot to measure the levels of phosphorylated CREB (pCREB), and four animals for pCREB staining.
Surgical Preparation
Focal cerebral ischemia was induced by intraluminal MCAO, as described previously (Luo et al., 2005). In brief, with animals under chloral hydrate anesthesia (350 mg/kg, i.p.), a 8/0 surgical nylon monofilament with rounded tip was introduced into the left internal carotid artery through the external carotid stump and advanced 16–17 mm past the carotid bifurcation until a slight resistance was felt. Throughout the procedure, body temperature was maintained at 37°C ± 0.5°C. The filament was left in place for 60 min and then withdrawn. In the sham-operated animals, the occluding filament was inserted only 7 mm above the carotid bifurcation.
Determination of Infarct Volume and Fluoro-Jade Staining
The determination of infarct volume by TTC (Sigma) staining was performed as described previously (Luo et al., 2005). Neuronal degeneration was determined by Fluoro-Jade (Histo-Chem, Jefferson, AK) staining. In brief, sections were washed and mounted on glass slides and dried overnight. The slides were immersed for 3 min in absolute ethanol, for 1 min in 70% ethanol, and for 1 min in distilled water and then transferred to a solution containing 0.01% Fluoro-Jade and 0.1% acetic acid for 30 min on a shaker. After three 10-min washes, the slides were finally coverslipped.
Morris Water Maze Task
For water maze tests, spatial memory performances of mice were evaluated with four trials per day for 5 days (days 45–49). A circular swimming pool measuring 160 cm in diameter and 60 cm in height was filled with room-temperature water made opaque with white nontoxic paint. Prominent posters and objects surrounded the maze. The mouse was placed into a quadrant facing the maze wall with four different start points that varied randomly each day and was required to locate a submerged platform, hidden 1 cm under the surface of water. Each trial lasted either until the mouse had found the platform or for a maximum of 90 sec. All animals were allowed to rest on the platform for 30 sec. Time to reach the platform (latency), length of swim path, and swim speed were recorded semiautomatically by a video tracking system. The platform location was constant during the 5 days' tests. All Morris water maze tests were performed between 08:00 and 12:00 AM.
Immunohistochemistry and Neuronal Identity of BrdU+ Cells
Animals were perfused transcardially with 200 ml 0.05 M sodium phosphate (pH 7.4) containing 0.8% NaCl, followed by 300 ml 4% paraformaldehyde in 0.05 M sodium phosphate (pH 7.4, containing 0.8% NaCl). Brains were removed and postfixed overnight in the same solution. Serial hippocampal sections (40 μm) were made on an oscillating tissue slicer in a bath of physiological saline. BrdU staining has been described previously (Luo et al., 2005). The sections were heated (85°C for 5 min) in antigen-unmasking solution (Vector Laboratories, Burlingame, CA); incubated in 2 M HCl (37°C for 30 min); rinsed in 0.1 M boric acid, pH 8.5, for 10 min; incubated in 1% H2O2 in phosphate-buffered saline (PBS) for 30 min; and blocked in PBS containing 3% normal goat serum, 0.3% (w/v) Triton X-100, and 0.1% bovine serum albumin (BSA) at room temperature for 1 hr, followed by incubation with mouse monoclonal anti-BrdU (1:1,000; Sigma) at 4°C overnight. Subsequently, the sections were developed with the ABC Kit (Vector Laboratories) and detected with diaminobenzidine (DAB; Vector Laboratories). pCREB staining was performed with rabbit antiphospho-CREB-ser133 (1:200; Santa Cruz Biotechnology, Santa Cruz, CA) and reacted with goat anti-rabbit Cy3 (1:200; Chemicon, Temecula, CA). BrdU/NeuN or BrdU/MAP-2 double staining was performed to determine neuronal identity of survival cells. The double-staining procedure before incubation with primary antibodies was similar to the BrdU staining procedure described aboved, except for omitting H2O2 treatment. The sections were incubated with primary antibody at 4°C overnight. The primary antibodies used were as follows: rat anti-BrdU (1:200; Accurate Chemical and Scientific, Westbury, NY), mouse anti-NeuN (1:500; Chemicon), and mouse anti-MAP-2 (1:1,000; Sigma). Subsequently, the sections were incubated with secondary antibodies goat anti-rat Cy3 (1:200; Chemicon) and goat anti-mouse FITC (1:50; Chemicon) for 2 hr at room temperature.
Cell Counting
A single experimenter (C.X.L.) coded all slides from the experiments before quantitative analysis. BrdU+ cells were counted by another experimenter (Q.G.Z.), who was unaware of the experimental conditions for each sample. The analysis was conducted, using a modified version of the optical dissector method, on every sixth section in a series of coronal sections (Gundersen et al., 1988). To determine the total number of BrdU-positive cells per dentate gyrus, the counts from sampled sections were averaged, and the mean values were multiplied by the total number of sections. Fluorescence labeling was analyzed by sampling every section from the experimental animals with a fluorescence microscope as described previously (Luo et al., 2005).
Western Blot Analysis for pCREB
The dentate gyrus of mice was microdissected as described previously (Lu et al., 1998) and homogenized in 100 mM HEPES containing 200 mM NaCl, 10% glycerol, 2 mM Na4P2O7, 2 mM dithiothreitol (DTT), 1 mM EDTA, 1 mM benzamidine, 0.1 mM Na3VO4, 1 μM pepstatin, 10 μg/ml aprotinin, 10 μg/ml leupeptin, and 10 μM phenylmethylsulfonyl fluoride (PMSF) at PH 7.4. After lysis for 15 min in ice, samples were centrifuged at 20,000g for 15 min. The protein content in each supernatant fraction was determined by using Bradford's solution, and samples containing equivalent amounts of protein were applied to 12% acrylamide denaturing gels (SDS-PAGE). The separated proteins were transferred onto nitrocellulose membranes (Amersham, Arlington Heights, IL) using a Bio-Rad mini-protein-III wet transfer unit overnight at 4°C. Blotting membranes were incubated with blocking solution [5% nonfat dried milk powder dissolved in TBST buffer (pH 7.5, 10 mM Tris-HCl, 150 mM NaCl, and 0.1% Tween 20)] for 1 hr at room temperature, washed three times, and then incubated with rabbit antiphospho-CREB-ser133 (1:2,000; Chemicon) or rabbit anti-CREB (1:2,000; Chemicon) in TBST overnight at 4°C. After several washes with TBST buffer, the membranes were incubated for 1 hr with horseradish peroxidase (HRP)-linked secondary antibody (Boshide, Wuhan, China) diluted 1:1,000, followed by washing four times. The membranes were then processed with enhanced chemiluminescence (ECL) Western blotting detection reagents (Pierce, Rockford, IL).
Statistical Analysis
Comparisons among groups were made with one-way ANOVA, followed by post hoc Tukey test. Paired two-tailed Student's t-test was used to compare ipsilateral and contralateral BrdU+ cells number within a group. χ2 test was used to compare survival rate of newly born cells. Data from four trials for each day in the Morris water maze test were averaged, and then were analyzed by a two-way mixed ANOVA, followed by post hoc Tukey test. Pearson's correlation test was used for correlation analysis between the total number of surviving BrdU+ cells in both dentate gyri and mean latency (days 45–49). Data are presented as mean ± SEM, and P < 0.05 is considered statistically significant.
RESULTS
Voluntary Running Restores Impaired Spatial Memory
Spatial cognitive impairment is common after stroke insults. In the first experiment, we examined in mice from day 45 to day 49 after focal cerebral ischemia whether postischemic exposure to different exercises could improve spatial cognitive performance in the Morris water maze. All groups of mice improved in their ability to locate the platform in the Morris water maze over the 5 days. The latency time of mice in the model group was significantly longer than that in the sham group (Fig. 2), suggesting that focal cerebral ischemia impaired spatial cognitive performance. Voluntary wheel running significantly shortened the latency time, compared with the model group (Fig. 2). The reduction of latency time was not likely due to the enhancement of swimming ability of mice, insofar as the swimming velocities measured during the tasks were not significantly different (data not shown). However, exposure to forced swimming did not change the latency time compared with the model group (Fig. 2). These data indicate that postischemic voluntary wheel running reverses spatial cognitive impairment caused by stroke, whereas forced swimming does not.
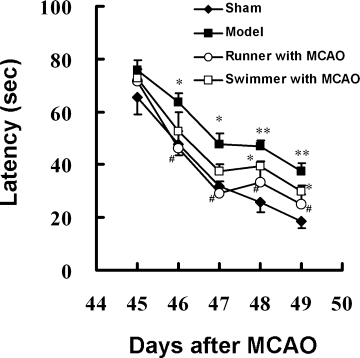
Effect of exercise exposure after focal cerebral ischemia on spatial memory. Spatial memories of sham (n = 7), model (n = 9), runner with MCAO (n = 7), and swimmer with MCAO (n = 6) were tested in the Morris water maze on days 45–49 after MCAO. Data are presented as mean ± SEM. *P < 0.05, **P < 0.01 vs. sham, #P < 0.05 vs. model.
Postischemic Exercises Do Not Attenuate Ischemic Injury
The improved spatial memory is possibly due to reduced ischemic injury after exposure to voluntary exercise. To exclude this possibility, we carried out infarct volume measurement and neuron loss detection. A well-demarcated infarct in the striatum and cortex was detected 49 days after MCAO. No lesions were found in sham-operated animals. In agreement with published data (Ohlsson and Johansson, 1995; Johansson and Ohlsson, 1996), there was no significant difference in infarct volume among groups (Fig. 3A). Moreover, Fluoro-Jade staining showed no animal in any group with ischemic damage in the dentate gyrus (Fig. 3B,C), although focal cerebral ischemia caused a marked neuronal loss in the cortex (Fig. 3D). These data suggest that voluntary or forced exercise does not affect ischemic injury.
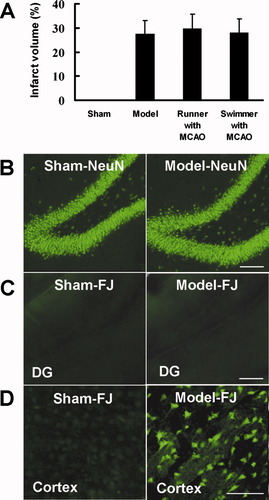
Effect of exercise exposure on ischemic injury. A: Infarct size in sham (n = 3), model (n = 4), runner with MCAO (n = 5), and swimmer with MCAO (n = 3) groups. Data are mean ± SEM. Hippocampal sections were stained with NeuN (B) to show neurons or Fluoro-Jade (FJ; C) to show degenerated neurons in the ipsilateral dentate gyrus in sham (left) and model (right) groups. Cortex sections were stained with FJ (D) to show degenerated neurons in the ipsilateral cortex in model (right) and sham (left) groups. Scale bars = 100 μm in B,C; 50 μm in D. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
The mortality of animals during the first week after MCAO, except for the mice in the sham group, was 44.4% (95/214). During physical exercises, the mortality was 0.0% (0/24) in sham, 27.5% (11/40) in model, 27.0% (10/37) in runner with MCAO, and 40.5% (17/42) in swimmer with MCAO, respectively, suggesting that postischemic exercises do not attenuate ischemic injury.
Postischemic Exercises Do Not Enhance Progenitor Cell Proliferation
To investigate whether improved spatial memory after postischemic exercise was dependent on neurogenesis in the dentate gyrus, progenitor cell proliferation in SGZ was addressed by BrdU labeling of dividing cells for 7 days (from day 14 to day 20 after ischemia) and immunohistochemical analysis 1 day after the last BrdU injection. The number of BrdU+ cells in the ipsilateral dentate gyrus increased substantially in model group compared with sham (Fig. 4A,B,E). Significantly more BrdU+ cells were observed in the ipsilateral dentate gyrus than in the contralateral in model (Fig. 4B,E) and runner with MCAO groups (Fig. 4C,E). The BrdU+ cell number in the ipsilateral in swimmer with MCAO group was also higher than that in the contralateral, though without significant difference (Fig. 4D,E). This result indicates that progenitor cell proliferation induced by ischemia persists during this period. However, there was no significant difference between running and model group in the number of BrdU+ cells in the dentate gyrus (Fig. 4B,C,E). Unexpectedly, the BrdU+ cells in swimming group were significantly lower than in model group (Fig. 4B,D,E). These results suggest that exercises after focal ischemia for 2 weeks do not stimulate progenitor cells proliferation in the dentate gyrus.
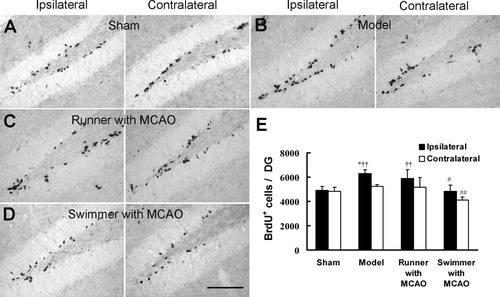
No effect of exercise exposure after focal cerebral ischemia on cell proliferation in the dentate gyrus. The mice received BrdU injection from day 14 to day 20 after MCAO (days 7–13 after exposure to different experiences). Cell proliferation was estimated by BrdU+ cells 1 day after the last BrdU injection. Representatives of the BrdU+ cells in the ipsilateral and contralateral dentate gyrus of sham (A), model (B), runner with MCAO (C), and swimmer with MCAO (D) groups. E: Total number of BrdU+ cells per dentate gyrus in sham (n = 5), model (n = 6), runner with MCAO (n = 6), and swimmer with MCAO (n = 6) groups. Data are mean ± SEM. *P < 0.05 vs. sham, #P < 0.05, ##P < 0.01 vs. model, ††P < 0.01 vs. contralateral dentate gyrus in the same group. Scale bar = 100 μm.
Voluntary Running Enhances Progenitor Cells Survival
Previous study shows that there is increased newborn cell survival in the dentate gyrus in intact animals after voluntary wheel running but not forced swimming (van Praag et al., 1999a). Next, we investigated whether improved spatial memory after postischemic exercise was dependent on the enhanced survival of dividing neuronal progenitor cells in the dentate gyrus. Voluntary wheel running significantly enhanced the number of BrdU+ cells in both ipsilateral and contralateral dentate gyrus 28 days after the last BrdU injection compared with model group (Fig. 5B,C,E). However, forced swimming did not change the number of BrdU+ cells in the dentate gyrus compared with model group (Fig. 5B,D,E). When the number of surviving cells 28 days after the last injection was expressed as a ratio of the number of BrdU+ cells at 1 day after the last injection of BrdU, it was found that the cell survival rate in the dentate gyrus in running group was significantly higher than in model group (Table I). The cell survival rate in the dentate gyrus of swimmer was not significantly different from model group (Table I). These data suggest that voluntary wheel running promotes the survival of progenitor cells in the postischemic dentate gyrus whereas forced swimming dose not. Although there was more BrdU+ cells in the dentate gyrus in model group than in sham at 1 day after the last BrdU injection (Fig. 4), the number of BrdU+ cells in the dentate gyrus 28 days after the last BrdU injection was significantly lower in model group than in sham group (Fig. 5A,B,E). Moreover, the number of BrdU+ cells and cell survival rate were significantly lower in the ipsilateral dentate gyrus than in the contralateral in model and running groups (Fig. 5B,C,E).
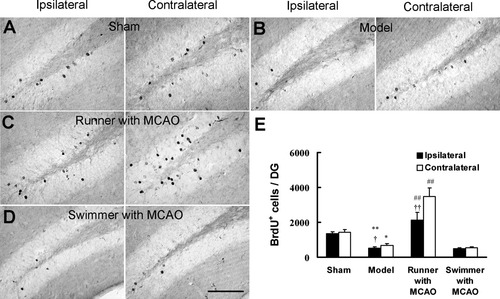
Voluntary wheel running enhances the survival of newborn cells in the dentate gyrus after stroke. The survival of newborn cells was examined 28 days after the last BrdU injection. Representatives of the BrdU+ cells in the ipsilateral and contralateral dentate gyrus of sham (A), model (B), runner with MCAO (C), and swimmer with MCAO (D) groups. E: Total number of surviving BrdU+ cells per dentate gyrus in sham (n = 5), model (n = 7), runner with MCAO (n = 7), and swimmer with MCAO (n = 6) groups. Data are mean ± SEM; *P < 0.05, **P < 0.01 vs. sham, ##P < 0.01 vs. model, †P < 0.05, ††P < 0.01 vs. contralateral dentate gyrus in the same group. Scale bar = 100 μm.
| Survival rate (%) | Sham | Model | Runner with MCAO | Swimmer with MCAO |
|---|---|---|---|---|
| Ipsilateral | 27.2 | 8.4** | 36.2***,**** | 10.0 |
| Contralateral | 29.6 | 13.0* | 67.4*** | 13.5 |
- † Survival rate of newborn cells was expressed as a ratio of BrdU+ cells at 1 day after the last BrdU injection to BrdU+ cells at 28 days after the last BrdU injection.
- * P < 0.05 vs. sham.
- ** P < 0.01 vs. sham.
- *** P < 0.01 vs. model.
- **** P < 0.01 vs. contralateral.
To determine whether BrdU+ cells in the dentate gyrus became neurons, we used antibodies against neuronal marker NeuN and MAP-2. We found that the majority of BrdU+ cells in the GCL were colabeled with NeuN or MAP-2 (Fig. 6A,B) 4 weeks after the last BrdU injection. These findings provided evidence that most of the newborn cells in the dentate gyrus matured into granule neurons. Therefore, voluntary wheel running after focal ischemia could promote neurogenesis in the dentate gyrus by increasing the number of new neurons.
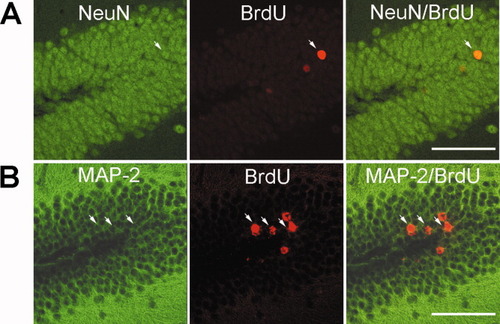
Neuronal identity of BrdU+ cells in the dentate gyrus 28 days after the last BrdU injection. The hippocampal sections were doubly stained with BrdU (red) and NeuN (green; A) or MAP-2 (green; B) to show neuronal phenotype of newborn cells. Arrows indicate double labeled cells. Scale bars = 50 μm.
As shown in Figure 7, there was a negative correlation between the total number of surviving BrdU+ cells in both dentate gyri 49 days after ischemia and the mean latency to reach the platform in the Morris water maze 45–49 days after ischemia. These data indicate that the survival of dividing progenitor cells in the dentate gyrus is associated with the improved spatial cognitive performance and that wheel running may improve spatial memory by enhancing the survival of dividing progenitor cells in the dentate gyrus.
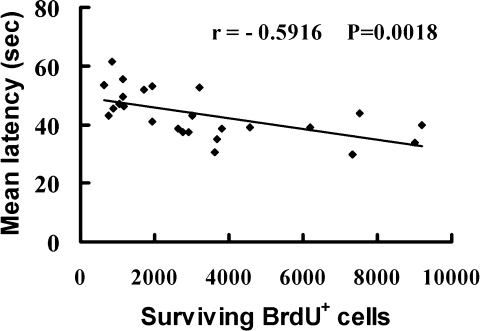
Scatterplot for correlation between the total number of surviving BrdU+ cells in both dentate gyri and mean latency (day 45–49) in all groups after focal cerebral ischemia. r = −0.5916, P = 0.0018.
CREB Phosphorylation Mediates the Enhanced Progenitor Cell Survival
Because the CREB phosphorylation regulates the survival of newborn neurons (Nakagawa et al., 2002b; Zhu et al., 2004), we determined whether the running-induced survival of the dividing progenitor cells in the dentate gyrus results from the up-regulation of CREB activity. Western blots showed a substantial increase of pCREB level in both ipsilateral and contralateral dentate gyrus in running group, but not in swimming group, compared with model group (Fig. 8A). However, there was no significant difference between groups with regard to protein content of CREB. Consistent with the Western blots, staining of the hippocampal sections with anti-pCREB antibody demonstrated that the number of pCREB labeled nuclei was markedly increased in running group 28 days after focal cerebral ischemia compared with model group (Fig. 8B). The pCREB-positive cells aligned in the lowest part of GCL, adjacent to or including the SGZ, of dentate gyrus. The pCREB protein content and the number of pCREB-labeled nuclei in ipsilateral dentate gyrus were lower than in contralateral in model and running group (Fig. 8A,B). These data suggest that voluntary wheel running after focal cerebral ischemia up-regulates the level of pCREB in the dentate gyrus.
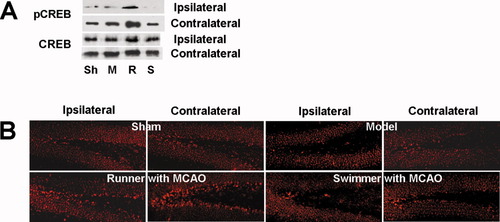
Voluntary wheel running up-regulates CREB phosphorylation in the dentate gyrus after focal cerebral ischemia. Western blot using antibodies against pCREB or CREB (A) and immunofluorescence using an antibody against pCREB (B) to show the effect of different exercises after focal ischemia on the phosphorylation of CREB in dentate gyrus. Similar results were observed in each of three experiments. Sh, sham; M, model; R, runner with MCAO; S, swimmer with MCAO. The pCREB-positive cells aligned in the lowest part of GCL, adjacent to or including the SGZ, of dentate gyrus. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
DISCUSSION
Voluntary exercise (Stummer et al., 1994) could enhance the functional recovery after stroke, including spatial learning and memory. However, little is known about the mechanisms underlying these beneficial effects. Here we report that voluntary wheel running promotes the survival of newborn cells in the dentate gyrus by up-regulating CREB phosphorylation and, consequently, reverses spatial memory impairment after focal cerebral ischemia.
Previous study has shown that there is increased neurogenesis (both cells proliferation and survival) in the dentate gyrus in intact animals after voluntary exercise (van Praag et al., 1999a). Our present study provides the first evidence that exposure to voluntary wheel running after focal cerebral ischemia promoted newborn cells survival but had no effect on cell proliferation in the dentate gyrus. This discrepancy may be due to progenitor cells in the ischemic brain being insensitive to beneficial stimuli because of low brain-derived neurotrophic factor (BDNF) level (Lee et al., 2004).
Our data show that voluntary exercise was beneficial, whereas forced exercise was harmful, after stroke. Forced swimming decreased cell proliferation in the dentate gyrus. The reduced proliferation in swimmer following ischemic insults is possibly caused by aversive stress and/or too much physical exercise (tolerable physical exercise for intact mice might be too much for ischemic mice), insofar as it has been found that daily repeated psychosocial stress results in a decrease of 29% of the proliferation rate in the hippocampus (Czeh et al., 2002), and too much physical exercise may be stressful and hence reduce proliferation (Ra et al., 2002). Thus exposure to various types of physical exercises after ischemic stroke may have various effects on neurogenesis in the dentate gyrus.
A tendency in swimmers to display an improved spatial memory was observed in spite of low levels of pCREB and neurogenesis (Fig. 2, 4E, 5E, 8A). This may be because these mice were familiar with the water maze; they had swum in the same water maze without a task for 2 min everyday before the water maze test. Either “unmasking” of existing neuronal networks (e.g., dendritic sprouting, synaptogenesis) or establishment of new neuronal networks (e.g., neurogenesis) contributed to brain plasticity (Mohammed et al., 2002). It is likely that the spatial memory in the swimmers with MCAO group was ameliorated through something other than pCREB-neurogenesis-memory improvement.
Cognitive impairment is common after stroke insults. Environmental enrichment and running improve cognitive functions in intact and ischemic brain (van Praag et al., 1999b; Gobbo and O'Mara, 2004; Dahlqvist et al., 2004). Hippocampus is very important for spatial memory (Morris et al., 1982). Enhanced neurogenesis in the intact adult dentate gyrus has been associated with improved spatial memory performance (Kempermann et al., 1997; van Praag et al., 1999b; Drapeau et al., 2003). In the current study, we found that voluntary wheel running significantly promoted the survival of newborn cells in the dentate gyrus and restored impaired spatial cognitive performance and that there was a significant correlation between newborn cells survival in dentate gyri and spatial memory. Thus, our data provide the first evidence that improved spatial cognitive performance by postischemic voluntary wheel running was, at least in part, due to the running-induced survival of newborn cells in the dentate gyrus after stroke. Moreover, as exposure to postischemic enriched environment (Ohlsson and Johansson, 1995; Dahlqvist et al., 2004), voluntary wheel running after stroke did not change infarct volume and neuron degeneration in the dentate gyrus, suggesting that the improved spatial cognitive performance in running mice is not related to the reduce of infarct volume or neuron degeneration in the dentate gyrus.
The signal transduction pathways involved in mediating exercise-induced neurogenesis in the dentate gyrus are so far unknown. The cAMP-CREB pathway regulates the survival of newborn neurons in adult hippocampus (Nakagawa et al., 2002a, b). We recently found that CREB activation is a necessary and sufficient condition for the survival of new neurons in the dentate gyrus after ischemia (Zhu et al., 2004). Our present data showed that running up-regulated CREB phosphorylation in the dentate gyrus. However, forced swimming had no effect on the survival of newborn cells and CREB phosphorylation. These results suggest that CREB phosphorylation may be involved in exercise-induced neurogenesis in the dentate gyrus after ischemic insults. BDNF has been identified as a target gene of CREB (Young et al., 1999; Zhu et al., 2004). It is well understood that BDNF promotes neuronal survival (Young et al., 1999; Zhu et al., 2004). Increasing evidence indicates that environmental enrichment and physical exercise up-regulate BDNF level in adult hippocampus (Neeper et al., 1995, 1996; Young et al., 1999) and ischemic brains 4 weeks after insult (Gobbo and O'Mara, 2004). The N-methyl-D-aspartate (NMDA) receptor might also be involved in this signal transduction pathway, because its activation has been found to regulate CREB phosphorylation in vivo (Nijholt et al., 2002) and in vitro (Leutgeb et al., 2005). Moreover, the expression level of NR2B receptor subunit increased 38.3% in the dentate gyrus of runners (Farmer et al., 2004), and the enhanced neurogenesis with running was suppressed in mice lacking the NMDA receptor α1 subunit (Kitamura et al., 2003). Overall, we hypothesize that postischemic voluntary wheel running potentiates NMDA receptor functions, which then elevates the levels of phosphorylated CREB and up-regulates BDNF expression in the dentate gyrus and consequently promotes the newborn cell survival.
CREB activity determination by examining phosphorylation of CREB at Ser133 has been widely used (Nakagawa et al., 2002b; Zhu et al., 2004). However, recent studies found that CREB-mediated gene expression occurs in the absence of Ser133 phosphorylation (Conkright et al., 2003; Iourgenko et al., 2003), indicating phosphorylation-independent CREB activity. Thus, the measurement of CREB phosphorylation at Ser133 may not be good enough for determining CREB activity. CRE-bingding assay and CRE-dependent reporter gene assay at a given gene promoter may be more precise for the measurement of CREB activity (Blendy, 2006).
Consistent with reduced survival rate of newborn cells after cerebral ischemia (Yagita et al., 2001; Takasawa et al., 2002; Zhu et al., 2003) is our finding of fewer surviving newborn cells and lower survival rate in the ipsilateral dentate gyrus than in contralateral. One of the possible reasons may be low BDNF level in the ipsilateral dentate gyrus at the chronic recovery phase of ischemia, because of the excessive consumption of BDNF at early phases of ischemia and/or infarct-induced cell loss. Supporting evidence comes from the findings that exercise elevates BDNF and trkB proteins in the contralateral hemisphere after ischemia (Kim et al., 2005) and that BDNF mRNA decreases consistently from 1 week to 8 weeks in both CA1 and cortical areas following stroke (Lee et al., 2004). Furthermore, our data showed that the phosphorylated CREB was markedly reduced in the ipsilateral dentate gyrus 28 days after MCAO. Our future studies will seek to reveal the biochemical events underlying the low survival rate of newborn cells in the ipsilateral dentate gyrus.




