Acquisition of an appetitive behavior prevents development of stress-induced neurochemical modifications in rat nucleus accumbens
Abstract
In rats, exposure to chronic unavoidable stress produces a decrease in dopamine output in the nucleus accumbens shell that is accompanied by a decreased density of the dopamine transporter and an increased activity of the dopamine-D1 receptor complex. These modifications have been hypothesized to be adaptive to decreased dopamine output in stressed rats. We investigated whether the learning of an appetitive behavior sustained by palatable food, which is associated with increased dopamine output in the nucleus accumbens shell as measured by microdialysis experiments, would affect the modifications induced by chronic stress exposure on dopamine transporter density and dopamine-D1 receptor complex activity in the nucleus accumbens. Rats exposed to chronic unavoidable stress after acquisition of the appetitive behavior showed a higher dopamine extraneuronal release in the nucleus accumbens shell than that of stressed animals, and similar to that of control rats. Moreover, previous acquisition of the appetitive behavior prevented development of a stress-induced decrease in dopamine transporter density, measured by [3H]-WIN 35428 binding, a stress-induced increase in dopamine-D1 receptor density, measured by binding of [3H]-SCH 23390, and SKF 38393-stimulated adenylyl cyclase activity in the nucleus accumbens. These results support the hypothesis that changes induced in pre- and postsynaptic dopaminergic transmission by chronic stress exposure are related to decreased dopamine output. © 2003 Wiley-Liss, Inc.
Substantial evidence suggests that both acute and chronic stress exposure in rats influence different behavioral patterns, such as locomotor activity (Zebrowska-Lupina et al., 1990), consumption of palatable food (Griffith et al., 1992), sexual activity (Sato and Kumamoto, 1992; Wang et al., 1995; Sugiura et al., 1997), and sensitization to drugs of abuse (Sorg and Kalivas, 1991, 1993). The dopaminergic system plays a crucial role in these effects. Dopaminergic neurons of the mesolimbic system are activated rapidly by environmental stimuli, including different stressors (Kaneyuki et al., 1991). Stress exposure induces a biphasic modification of dopamine (DA) transmission. Acute exposure to different stressful events increases DA release in the prefrontal cortex (PFC) (Sorg and Kalivas, 1993; Finlay et al., 1995; Yoshioka et al., 1996), and to a lesser extent in the nucleus accumbens shell (NAcS) (Rougé-Pont et al., 1993; Kalivas and Duffy, 1995; Tidey and Miczek, 1996). Exposure to chronic unavoidable stress produces sequelae of adaptive modifications in dopaminergic transmission, such as decreased synaptic DA release in the medial PFC (mPFC) and NAcS (Mangiavacchi et al., 2001), downregulation in the number of DAT binding sites, upregulation in the number of D1 binding sites, and increased D1-stimulated adenylyl cyclase activity in the NAc (Scheggi et al., 2002). It was suggested that both reduced DAT density and increased DA-D1 receptor complex activity reflect a compensatory mechanism that is apt to maintain adequate dopaminergic D1 transmission despite decreased DA output. An analogous condition of significantly decreased DAT density in the NAc is present in rats withdrawn from cocaine (Pilotte et al., 1994) at a time when extraneuronal DA levels in the same area are reduced significantly (Parsons et al., 1991; Rossetti et al., 1992). To test further the hypothesis that stress-induced decreases in DAT density and increases in DA-D1 receptor complex activity in the NAc are secondary to the reduction in DA output, we utilized a behavioral manipulation, avoiding the use of pharmacologic tools such as antidepressant drugs.
We had demonstrated previously that the acquisition of an instrumental appetitive behavior (vanilla appetitive behavior, VAB) partially protects rats from both behavioral and neurochemical effects of chronic stress. Rats trained to consume palatable food when exposed to unavoidable stress rapidly develop an escape deficit when tested in a shock-escape paradigm, but their performance in an appetitive task and DA output in the NAcS remain unmodified (Masi et al., 2001). We regarded this composite behavioral model of stress-induced avoidance deficit with no modification of DA output as a useful tool for testing our hypothesis. Thus, we investigated whether long-term stress exposure would induce similar or different changes in DAT density and DA-D1 receptor complex activity in the NAc of control rats and rats trained previously to learn a motivated behavior apt to obtain palatable food.
Abbreviations used
DA, dopamine; DAT, dopamine transporter; mPFC, medial prefrontal cortex; NAcS, nucleus accumbens shell; PFC, prefrontal cortex; VAB, vanilla appetitive behavior.
MATERIALS AND METHODS
Animals
Experiments were carried out on male Sprague-Dawley rats (Charles River, Calco, Italy) weighing 125–150 g at their arrival in the vivarium. Animals were housed five per cage (59 × 38.5 × 20 cm) for the entire duration of the experiments. They were moved to a different cage or apparatus only for the time required for behavioral manipulation. They were maintained in an environment of constant temperature and humidity, with free access to food and water. We used a 12-hr reverse light/dark cycle (7:00 AM lights off, 7 PM on). Experiments were carried out under a red light and controlled noise conditions. Rats were allowed at least 1 week of habituation to the animal colony. The rats weighed 200–225 g when experimental procedures began.
All procedures used in this study were in strict accordance with European legislation on use and care of laboratory animals (EEC Council Directive 86/609), with the guidelines of the National Institutes of Health on the care and use of laboratory animals, and were approved by the University of Siena Ethics Committee.
Chronic Stress Procedure
Escape test apparatus.
A plexiglas cage (30 × 60 × 30 cm; D. Gambelli, Siena, Italy) with dark walls and a floor fitted with stainless steel rods spaced 1 cm apart was divided into two equal chambers by a dark plexiglas partition with a 10 × 10 cm sliding door. One compartment was connected to a S48 Grass stimulator (Grass Instruments, Astro-Med Inc., West Warwick, RI) (electrified chamber) and the other was disconnected from it (neutral chamber).
Before exposure to unavoidable stress, animals spent 30 min/day for at least 3 days in the escape-test apparatus with the sliding door open to habituate to the test environment. Rats were then exposed to a 50-min unavoidable stress session. Each rat was immobilized with a flexible wire net, an electrode was applied to the distal third of the tail that was left uncovered, and about 80 electric shocks (1 mA × 5 sec at 30-sec intervals) were administered in a cage without bedding. Twenty-four hours later, rats were tested in a shock-escape paradigm in the apparatus described. An electrode connected to the S48 Grass stimulator was applied to the tail and the animal was placed in the electrified chamber of the apparatus. After a 5-min habituation period, the rat in the electrified chamber received 30 consecutive electric shocks (1 mA × 5 sec), at 30-sec intervals. During the delivery of each shock, the door connecting the electrified chamber to the neutral one was open. Animals that succeeded in escaping were replaced gently in the electrified chamber, at the end of the 5-sec shock period. More than 95% of rats exposed to the unavoidable stress session 24 hr before the test developed an escape deficit, defined by a score that ranged from 0–3 escapes per 30 shocks. In our experimental conditions, rats that had not been exposed to unavoidable stress (Naive) scored between 22–30 escapes in 30 trials.
To maintain the escape deficit after the escape test, each rat: 1) was restrained in a flexible wire net for 10 min starting 48 hr after the escape test; 2) received 10 min of restraint plus 10 min of unavoidable shocks 48 hr after (1); and 3) spent 20 min in the cage where the unavoidable shock had been administered previously, 48 hr after (2).
By repeating this alternate-day stress procedure, the escape deficit was maintained in all animals. No significant differences in the amount of daily food and water consumption or in the curve of body weight increase was observed between controls and rats exposed to 3 weeks of chronic stress.
Induction of Vanilla Sugar-Sustained Appetitive Behavior
Apparatus.
Rats fed ad lib with a standard diet were used. Two dark plexiglas boxes (Box 1 and 2) were separated by a 10 × 10 cm sliding door. Box 2 formed the straight arm of a Y-maze (15 × 40 × 20 cm for each of the three arms). A vanilla sugar pellet used as a reinforcer was placed in the maze at the end of one of two divergent arms. Vanilla sugar pellets were made daily; standard food pellets were crushed by mortar and pestle, and fragments weighing approximately 150 mg were dampened with water and rolled in vanilla sugar (≈40 mg) to obtain regular pellets.
Training procedure.
The experimental procedure has been described previously in detail (Ghiglieri et al., 1997). The day before the first training session, rats were allowed a first run in the Y-maze, which had one of the two arms closed. Each animal was placed in Box 1 and 10 sec later, a 5-sec cue light signaled the opening of the sliding door. Rats were given 2 min to enter Box 2 and reach the end of the open arm where a vanilla sugar pellet was earned. Training sessions began 24 hr later.
Training sessions 1–3.
The rat was placed in Box 1 and the cue light signaled the opening of the sliding door. If the rat did not enter Box 2 within 60 sec, it was returned to the home cage for 15 min. If it entered Box 2, it was allowed 60 sec to reach the end of one of the diverging arms. Either the right or the left arm was designated correct, balanced among the animals. If the rat entered the empty arm, the rat was returned to the home cage for 15 min before the next trial. If the rat entered the baited arm, the rat was allowed to consume the vanilla sugar pellet and then returned to the home cage for 15 min before the next trial. The two intervals (time to leave Box 1 and time to reach the end of an arm) were reduced progressively throughout training sessions and at session 10, they were fixed at 10 and 20 sec, respectively.
One training session was administered every other day. Each rat underwent 10 complete trials for each session, at 15-min intervals, and the number of correct runways was recorded. At the end of the training, rats underwent a final session in the Y-maze to assess the final score. In the final session, the shorter times to leave Box 1 and reach the end of an arm were applied. A control trained rat consistently made 6–8 correct runways in 10 trials at each session, and this ratio of correct responses was reached within 10 sessions. In the remaining 2–4 trials, rats did not necessarily reach the end of the non-baited arm.
Microdialysis Procedure
Anesthetized animals (pentobarbital, 50 mg/kg, plus scopolamine, 0.4 mg/kg, intraperitoneally[i.p.]) were placed in a stereotaxic instrument and a vertical probe was lowered into the NAcS (AP + 1.7 mm, L ±1.2 mm, V −8.0 mm) according to Paxinos and Watson (1986). Concentric microdialysis probes were made from semipermeable dialysis tubing (internal diameter: 0.22 mm; external diameter: 0.31 mm; AN 69, Hospal, Bologna, Italy). The length of the permeable portion of the membrane was 2.0 mm. The probe was fixed to the skull with dental cement and stainless steel screws, and the skin was sutured. After surgery, rats were housed individually in a plexiglas microdialysis box (20 × 30 × 30 cm) with a grid floor and an open top. A period (24 hr) of recovery and habituation to the chamber was allowed before the beginning of microdialysis. On the day of the experiment, a Ringer solution (147 mM NaCl, 2.2 mM CaCl2, and 4 mM KCl) was infused at a flow rate of 1 μl/min through the probe. After a 2-hr equilibration period, dialysate samples were collected every 15 min. At least 4–5 samples were obtained for the estimation of basal levels, and then, to evaluate DA output, rats received an injection of cocaine (5 mg/kg, i.p.) and four samples were collected. The acute inhibition of DAT produced by cocaine, which does not interfere with DA release (Di Chiara and Imperato, 1988; Hurd and Ungerstedt, 1989), induces an extraneuronal accumulation of the monoamine proportional to the amount taken up by nerve terminals. In fact, the basal value of extraneuronal DA is the result of a functional equilibrium between DA output and reuptake. Thus, DA measured by microdialysis after acute administration of cocaine is a percentage of the monoamine that has reached the interneuronal space and should represent a rather precise index of DA output (Gambarana et al., 1999a, b).
Dialysate samples were analyzed immediately by reverse phase high performance liquid chromatography (HPLC) with electrochemical detection. DA was eluted on a C-18 reverse phase column (Supelcocosil LC18 DB, Supelco, Bellefonte, PA). The detector was an ESA Coulochem II with an ESA 5014 A analytical cell (ESA Inc., Chelmsford, MA). The potential of the first electrode was set at −175 mV, and that of the second (the recording electrode) at +175 mV. The mobile phase consisted of an aqueous solution containing 33 mM NaH2PO4, 0.1 mM Na2EDTA, 1 mM sodium octyl sulfate, 20% methanol (vol/vol), and 15% acetonitrile (vol/vol). A flow rate of 1.0 ml/min was used.
Data were collected by PC using EZChrom 6.6 software (Scientific Software Inc., San Ramon, CA) and quantified based on peak area compared to a standard curve run before and after each experiment. For consistency, basal values were expressed as pg/10 min dialysis, and accumulation values after cocaine administration as pg/60 min.
At the end of the experiment, rats were killed to verify probe placement. The brain was removed quickly, dipped briefly into cold 2-methylbutane (−25°C) to preserve gross morphology, and frozen on dry ice. Coronal sections (50 μm) were cut on a cryostat, stained with 0.4% cresyl violet solution, and probe placement was verified microscopically. Microdialysis data was utilized only when correct probe placement had been confirmed.
Tissue Preparation for Binding and Adenylyl Cyclase Assay
Rats utilized for evaluation of DAT binding, DA-D1 receptor binding, and activity of adenylyl cyclase were killed 2 days after the end of behavioral manipulations. The brains were removed quickly and the nucleus accumbens was dissected out from 1-mm coronal slices on an ice-cooled plate.
[3H]-WIN 35428 binding.
DA transporter binding was evaluated in nucleus accumbens membranes essentially according to the method of Kaufman et al. (1991). The utilized ligand [3H]-WIN 35428 is a cocaine analog used widely to measure DA transporter levels (Flores et al., 1998). The nucleus accumbens was homogenated with a Teflon/glass pestle in 20 vol of ice-cold 50 mM Tris-HCl buffer, pH 7.4, and centrifuged (38,000 × g, 20 min). The resultant pellet was resuspended in the initial volume of the buffer and centrifuged twice. The final pellet was resuspended in 50 mM Tris-HCl buffer, pH 7.4, containing 120 mM NaCl and 5 mM KCl. For the binding assay, 400 μl aliquots of the membrane preparations were incubated with different concentrations (2.5–40 nM) of [3H]-WIN 35428 (specific activity 84 Ci/mmol; New England Nuclear, Boston MA) in a final volume of 500 μl. After 60 min of incubation at 0–4°C, the reaction was stopped by adding 4 ml of ice-cold 50 mM Tris-HCl buffer, pH 7.4. The membranes were filtered through Whatman GF/C filters (Whatman Ltd., Maidstone, UK) that had been soaked previously for at least 6 hr with 0.1% polyethyleneimine, and rinsed twice with 4 ml of the same ice-cold buffer using a Brandel Cell Harvester (Biomedical Research, Gaithersburg, MD). Nonspecific binding was determined in the presence of 100 μM cocaine, which inhibits DA reuptake by binding to DA transporter. The maximum number of binding sites (Bmax) and the apparent dissociation constant (Kd) were calculated by linear regression analysis of Scatchard plots corresponding to the saturation curves of specific [3H]-WIN 35428 binding.
[3H]-SCH 23390 binding.
DA-D1 receptor density was assayed in nucleus accumbens membranes using the tritiated ligand [3H]-SCH 23390, which is a potent and selective antagonist of DA-D1 receptors (Iorio et al., 1983). The nucleus accumbens was homogenated with a Teflon/glass pestle in 100 volumes of ice-cold 50 mM Tris-HCl buffer, pH 7.4, and centrifuged (48,000 × g, 10 min). The resultant pellet was resuspended in the initial buffer volume and centrifuged twice. The final pellet was resuspended in 50 mM Tris-HCl buffer, pH 7.4, containing 120 mM NaCl, 5 mM KCl, 2 mM CaCl2, and 1 mM MgCl2. The binding assay was carried out according to the method of Billard et al. (1985). Briefly, 400 μl aliquots of the membrane preparations were incubated with different concentrations (0.03–10 nM) of [3H]-SCH 23390 (specific activity 80 Ci/mmol; New England Nuclear) in a final volume of 500 μl. After 30 min of incubation at 37°C, the reaction was stopped by adding 4 ml of ice-cold 50 mM Tris-HCl buffer, pH 7.4, and the membranes were filtered through Whatman GF/B filters and rinsed twice with 4 ml of the same ice-cold buffer using a Brandel Cell Harvester. Nonspecific binding was determined in the presence of 10 μM of the DA receptor blocker cis-flupentixol. The maximum number of binding sites (Bmax) and the apparent dissociation constant (Kd) were calculated by linear regression analysis of Scatchard plots corresponding to the saturation curves of specific [3H]-SCH 23390 binding.
Adenylyl cyclase assay.
Adenylyl cyclase activity was assayed in membranes prepared from nucleus accumbens using the method of Olianas et al. (1983). The concentration of SKF 38393 required to induce half-maximal activation of adenylyl cyclase activity (ED50), and the maximal increment obtained by the stimulation (Vmax) was calculated by linear regression analysis of Eadie-Hofstee plots obtained from concentration-response curves. Protein content was determined with the method of Lowry et al. (1951).
Experimental Protocols
To examine whether acquisition of vanilla sugar-sustained appetitive behavior would prevent the pre- and postsynaptic neurochemical modifications induced by chronic stress exposure, rats that underwent behavioral and neurochemical experiments were divided into 5 groups of 24 animals each. Group 1 rats were handled daily by experimenters and used only for the neurochemical experiments (CTR). Group 2 rats were handled daily by experimenters for the duration of the experimental protocols and then underwent the escape test (Naive). Group 3 rats were handled daily for 3 weeks, exposed to the unavoidable stress session and escape test sequence, and then underwent the chronic stress protocol for an additional period of 3 weeks and were tested finally for escape (Stress). Group 4 rats were handled daily for 3 weeks and then trained in the Y-maze (3 weeks) until they reached the criterion score of 6–8 successes/10 trials (VAB). Group 5 rats were trained in the Y-maze (3 weeks) and then exposed to the unavoidable stress session and escape test sequence when they had reached the criterion score. These rats received the stress procedure or Y-maze training on alternate days for an additional period of 3 weeks (VAB + Stress).
Two days after the end of the behavioral assessments, 6–8 animals from each group underwent surgery for probe implantation in the NAcS and the remaining animals were killed for the determination of DAT binding, DA-D1 receptor binding, and adenylyl cyclase activity in the nucleus accumbens. The timeline of the experiments is summarized in Table I.
| Group | n | 3 Weeks + 3 Weeks | Y-maze test | Escape test | ||
|---|---|---|---|---|---|---|
| CTR | 24 | Handling | Handling | No | No | Neurochemistry |
| Naive | 24 | Handling | Handling | No | Yes | Neurochemistry |
| Stress | 24 | Handling | Chronic stress | No | Yes | Neurochemistry |
| VAB | 24 | Handling | Y-maze test | Yes | No | Neurochemistry |
| VAB + Stress | 24 | Y-maze training | Chronic stress + Y-maze training | Yes | Yes | Neurochemistry |
Drugs
Pentobarbital was dissolved in a mixture of 12% ethanol, 38% propylene glycol, and 50% deionized/distilled water (vol/vol), and was injected in a volume of 4 ml/kg rat body weight. Scopolamine and cocaine were dissolved in deionized/distilled water and injected i.p. in a volume of 1 ml/kg rat body weight. All chemicals were purchased from commercial sources; cocaine was purchased from SALARS (Como, Italy).
Statistical Analysis
Statistical analyses were carried out using commercially available software (Instat 2.01 for Macintosh, GraphPad Software Inc., San Diego, CA). Statistical comparisons between two experimental groups were made by unpaired t-test. When data from more than two experimental groups were examined, one-way analysis of variance (ANOVA) was used, followed by post hoc Bonferroni's test, when applicable (P < 0.05), unless otherwise specified.
RESULTS
Behavioral Experiments
At the end of Y-maze training and stress exposure, performance of rats in the VAB + Stress and VAB groups was compared. The number of successful runways scored by rats in the two groups was similar. When the two groups of rats exposed to stress (Stress and VAB + Stress) and the Naive rats were tested for escape, analysis by one-way ANOVA showed significant differences between the groups (F2,27 = 46.159, P < 0.001). Chronic exposure to unavoidable stress induced a clear-cut escape deficit as shown in Table II.
| Group | n | Number of correct runways | Number of escapes |
|---|---|---|---|
| VAB | 10 | 7.3 ± 0.5 | — |
| VAB + Stress | 10 | 6.9 ± 0.5 | 6.1 ± 2.1* |
| Stress | 10 | — | 3.0 ± 1.0* |
| Naive | 10 | — | 22.4 ± 1.2 |
- † Rats were handled (Naive) or received repeated stress (Stress), VAB training (VAB), or VAB training + stress exposure (VAB + Stress) as described in the experimental protocols. In the VAB, test scores are expressed as the mean ± SEM of successful runways in 10-trial block. In the escape test, data are expressed as the mean number of escapes ± SEM in 30 consecutive trials.
- * P < 0.001 compared to the score of the Naive group (ANOVA with Bonferroni multiple comparison test).
Effect of Appetitive Behavior Acquisition on DA Output in the NAcS
In the NAcS, analysis of basal DA levels by one-way ANOVA showed significant differences between the groups (F4,25 = 115.88, P < 0.001). Post hoc analysis demonstrated that DA levels (Table III) in the Stress group were significantly lower than in the CTR and Naive groups. Levels of extraneuronal DA in the VAB and VAB + Stress groups were significantly higher than in the Stress, Naive, and CTR groups. When baseline levels had been determined, rats received an injection of cocaine (5 mg/kg, i.p.) and four samples were collected. Because of significant difference in basal levels of extraneuronal DA between groups, accumulation after acute uptake inhibition was calculated as the sum of the absolute amounts of the monoamine (measured values minus the mean basal value) in each of four samples collected after cocaine administration. Analysis by one-way ANOVA showed a significant difference between the groups (F4,25 = 48.85, P < 0.001). Bonferroni's test (Table III) demonstrated that DA output in the Stress group was significantly lower than that in the CTR, Naive, VAB, and VAB + Stress groups. In the VAB + Stress group, DA output was similar to that in the CTR and Naive groups. In the VAB group, DA output was significantly higher than that in the CTR, Naïve, and VAB + Stress groups.
| Group | n | Basal DA values (pg/10 min) | DA accumulation (pg/60 min) |
|---|---|---|---|
| CTR | 6 | 11.26 ± 0.25 | 27.32 ± 1.44 |
| Naive | 6 | 10.83 ± 0.32 | 28.31 ± 1.28 |
| Stress | 6 | 4.40 ± 0.24* | 2.83 ± 0.29* |
| VAB | 6 | 14.36 ± 0.43** | 43.90 ± 2.63** |
| VAB + Stress | 8 | 13.52 ± 0.51** | 21.70 ± 2.63 |
- † Rats were handled (CTR and Naive) or received repeated stress (Stress), VAB training (VAB) or VAB training + stress exposure (VAB + Stress) as described in the experimental protocols. At the end of the behavioral experiments, 6–8 animals from each group underwent surgery for probe implantation in the NAcS. Basal values are the average of 4–5 samples for each rat. When baseline levels had been determined rats received an injection of cocaine (5 mg/kg i.p.) and four samples were collected. DA accumulation was calculated as the sum of its absolute amount (measured values minus mean basal value) in each of four samples collected after cocaine administration. Data are expressed as mean ± SEM of at least six experiments.
- * P < 0.001 Stress vs. CTR, Naive, VAB, and VAB + Stress groups.
- ** P < 0.001 VAB vs. CTR and Naive groups (ANOVA with Bonferroni multiple comparison test).
Effect of Appetitive Behavior Acquisition on DAT Binding in Nucleus Accumbens
Binding of [3H]-WIN 35428 to DAT in the nucleus accumbens was examined. Analysis of binding data by one-way ANOVA showed a significant difference between the groups (F4,25 = 8.87, P < 0.001). Bonferroni's test revealed that the number of binding sites (Fig. 1) in the Stress group was significantly lower than in the CTR, Naive, VAB, and VAB + Stress groups, whereas the number of binding sites in the VAB and VAB + Stress groups was similar to that in the CTR and Naive groups. No significant difference was found in the apparent affinity between groups, as indicated by their Kd values (CTR, 21.68 ± 2.6 nM; Naive, 18.57 ± 2.2 nM; Stress, 10.54 ± 1.48 nM; VAB, 26.17 ± 4.26 nM; VAB + Stress, 23.75 ± 6.29 nM).
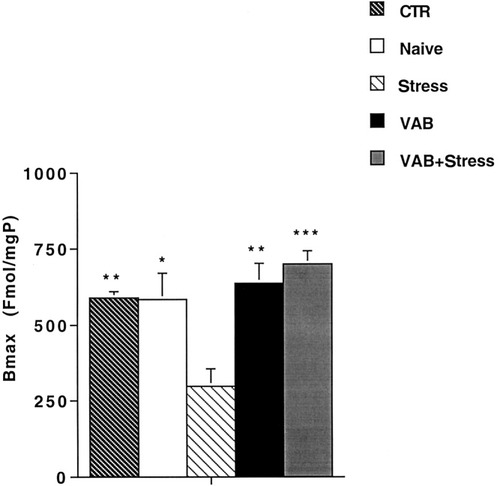
[3H]-WIN 35428 Bmax in the nucleus accumbens after exposure to VAB learning and a 3-week stress procedure. Animals were handled or were exposed to the behavioral procedures as described. They were killed 2 days after the behavioral tests, the nucleus accumbens was dissected out on ice and processed for determination of DAT binding. Values are expressed as the mean ± SEM of at least five experiments. *P < 0.05, Naive group vs. Stress group; **P < 0.01, CTR and VAB groups vs. Stress group; ***P < 0.001, VAB + Stress group vs. Stress group (ANOVA with Bonferroni multiple comparison test).
Effect of Appetitive Behavior Acquisition on 3H-SCH23390 Binding and Adenylyl Cyclase Activity in Nucleus Accumbens
Analysis by one-way ANOVA of saturation curves obtained from the binding of [3H]-SCH 23390 to DA-D1 receptors in the nucleus accumbens showed a significant difference between groups (F4,23 = 7.07, P < 0.001). Post hoc analysis by Bonferroni's test revealed that the Bmax value (Fig. 2) was significantly higher in the Stress group than in the CTR, Naive, VAB, and VAB + Stress groups with no significant difference between the Bmax values of the CTR, Naive, VAB, and VAB + Stress groups. The apparent affinity was not different between groups, as indicated by their Kd values (CTR, 2.48 ± 0.2 nM; Naive, 2.04 ± 0.3 nM; Stress, 2.15 ± 0.5 nM; VAB, 2.71 ± 0.5 nM; VAB, + Stress, 2.51 ± 0.4 nM).
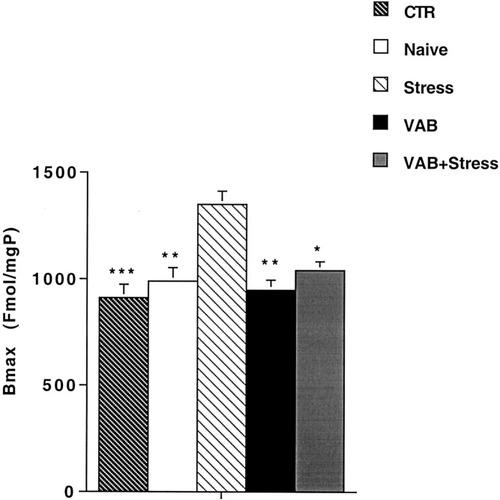
DA-D1 receptor density in the nucleus accumbens after exposure to VAB and 3-week stress procedure. Animals were handled or were exposed to the behavioral procedures as described. They were killed 2 days after the escape test, the nucleus accumbens was dissected out on ice and processed for determination of DA-D1 receptor binding. Values are expressed as the mean ± SEM of at least five experiments. *P < 0.05, VAB + Stress group vs. Stress group; **P < 0.01, Naive and VAB groups vs. Stress group; ***P < 0.001, CTR group vs. Stress group (ANOVA with Bonferroni multiple comparison test).
Basal levels of adenylyl cyclase were not significantly different among groups (data not shown). Analysis by one-way ANOVA showed in SKF 38393-stimulated adenylyl cyclase a significant difference between the groups (F4,36 = 4.68, P < 0.01). Post hoc analysis indicated an increase in the Vmax of the enzyme (Fig. 3) in the Stress group compared to the CTR, Naive, and VAB + Stress groups with no significant difference between the Vmax values of the CTR, Naive, VAB, and VAB + Stress groups. The ED50 value for SKF 38393 stimulatory activity was not different in the five groups (CTR, 3.25 ± 0.6 μM; Naive, 3.33 ± 0.8 μM; Stress, 3.31 ± 0.5 μM; VAB, 3.44 ± 0.7 μM; VAB + Stress, 2.34 ± 0.6 μM).
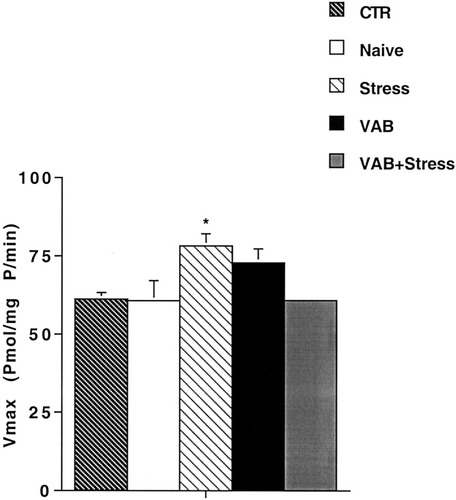
SKF 38393-stimulated adenylyl cyclase activity in the nucleus accumbens after exposure to VAB and 3-week stress procedure. Animals were handled or exposed to the behavioral procedures as described. They were killed 2 days after the escape test, the nucleus accumbens was dissected out on ice and processed for determination of adenylyl cyclase activity. Values are expressed as the mean ± SEM of at least five experiments. *P < 0.05, Stress group vs. CTR, Naive and VAB + Stress group (ANOVA with Bonferroni multiple comparison test).
DISCUSSION
The present data seem to support the hypothesis that a stress-induced decrease in DAT density and an increase in DA-D1 receptor complex activity in the nucleus accumbens is secondary to the reduction in DA output. Accordingly, when exposed to repeated stress, rats that had learned previously vanilla sugar-sustained appetitive behavior developed a clear-cut escape deficit, maintained a DA output in the nucleus accumbens similar to that of control animals, and showed no adaptive modifications at the dopaminergic synapse. That is, chronic unavoidable stress exposure in these animals had an evident behavioral effect, but no effect on the number of DAT binding sites or of DA-D1 receptors in the nucleus accumbens. This dissociation in sequelae of repeated stress exposure can be alternatively related to retention of the appetitive behavior, to DA output in the nucleus accumbens, to both these phenomena, or to neither of them. Acute exposure to avoidable stress had no persisting effects on DA output and it did not induce adaptive modifications at the dopaminergic synapse, as observed in Naive rats 2 days after escape test administration.
Rats are naturally attracted by a palatable food such as vanilla sugar even when fed ad lib, and they easily learn an appetitive behavior aimed at earning vanilla sugar pellets in a Y-maze apparatus (Ghiglieri et al., 1997). The acquisition of vanilla sugar-sustained appetitive behavior is associated with a steady increase in DA output in the NAcS and mPFC (Masi et al., 2001, 2003). When rats are exposed to repeated unavoidable stress during Y-maze training, they develop a deficit in avoiding aversive stimuli, never learn the appetitive behavior (Ghiglieri et al., 1997), and present a significantly decreased DA output in the NAcS and mPFC (Masi et al., 2001). Chronic stress exposure consistently induces avoidance deficit in rats that have learned previously the appetitive behavior, but in these animals it does not modify Y-maze performance and it has no effect on DA output in the NAcS (Masi et al., 2001). Appetitive behavior learning also antagonizes the behavioral and neurochemical effects of a long-term lithium treatment (Masi et al., 2000). Chronic lithium administration induces a decrease in DA output in the NAcS and a deficit in avoidance similar to that observed after chronic stress exposure (Gambarana et al., 1999a), and both of these effects are reverted by acquisition of appetitive behavior but not by the mere daily consumption of vanilla sugar meals (Masi et al., 2000). Thus, it seems to be the cascade of molecular events that underpins the learned ability to earn vanilla sugar that antagonizes the effects of lithium and, very likely, also those induced by stress exposure.
Dopaminergic transmission in mesolimbic areas plays a crucial role in the competence to learn vanilla sugar-sustained appetitive behavior, as only rats that show an intense dopaminergic response after consuming a vanilla sugar meal for the first time have the capacity to associate its hedonic taste with an otherwise neutral stimulus, such as the baited arm (Masi et al., 2003). Thus, in agreement with other authors (Fenu et al., 2001; Di Chiara, 2002; Salamone and Correa, 2002), we regard DA in the mesolimbic areas as crucial for learning and maintaining an instrumental appetitive behavior. Moreover, we consider acquired appetitive behavior to be a condition that sustains mesolimbic DA output at a level that guarantees stress resistance in terms of performance in the Y-maze and in terms of unchanged values of DAT density and DA-D1 receptor complex activity. Our results allow for the conclusion that a direct correlation exists between DA output and observed pre- and postsynaptic modifications in the nucleus accumbens. Although we attribute maintenance of DA output in the range of control values to previous acquisition of appetitive behavior, however, we cannot exclude that acquisition of any other instrumental behavior could have antagonized a stress-induced decrease in nucleus accumbens DA output.
Acknowledgements
We thank Ms. C. Pisaneschi for language editing of the article.




