Spatiotemporal changes of apolipoprotein E immunoreactivity and apolipoprotein E mRNA expression after transient middle cerebral artery occlusion in rat brain
Abstract
Apolipoprotein E (ApoE) is a constituent of lipoprotein and plays an important role in the maintenance of neural networks. However, spatiotemporal differences in ApoE expression and its long-term role in neural process after brain ischemia have not been studied. We investigated changes of ApoE immunoreactivity and ApoE mRNA expression both in the core and in the periischemic area at 1, 7, 21, or 56 days after 90 min of transient middle cerebral artery occlusion. Double stainings for ApoE plus NeuN or plus ED1 were performed in order to identify cell type of ApoE-positive stainings. The maximal increase of ApoE expression was observed at 7 days in the core and at 7 and 21 days in the periischemic area. In the core, ApoE plus NeuN double-positive cells increased at 1 and 7 days, without ApoE mRNA expression, whereas they increased in the periischemic area, with a peak at 21 days, with ApoE mRNA expression in glial cells but not in neurons. On the other hand, ApoE plus ED1 double-positive cells increased only in the core, with a peak in number at 7 and 21 days and marked ApoE mRNA expression in macrophages. The present study suggests that ApoE plays various important roles in different type of cells, reflecting spatiotemporal dissociation between degenerative and regenerative processes after brain ischemia, and that ApoE is profoundly involved in pathological conditions, such as brain ischemia. © 2003 Wiley-Liss, Inc.
Apolipoprotein E (ApoE) is a 34-kDa glycoprotein and is a constituent of lipoprotein (Mahley, 1988). In the CNS, ApoE is synthesized and secreted mainly by astrocytes and microglia and is involved in lipid transport, playing a pivotal role in the maintenance of cell membranes, myelin, and neural networks under normal conditions (Boyles et al., 1985; Ignatius et al., 1986). In fact, ApoE-containing lipoprotein promotes synapse development in cultured neurons (Mauch et al., 2001). ApoE is the most abundant component of high-density lipoprotein (HDL)-like lipoproteins in human cerebrospinal fluid (Pitas et al., 1987).
Under pathological conditions, ApoE shows a protective effect against neuronal insults through its antioxidant effect (Miyata and Smith, 1996; Horsburgh et al., 2000), antiinflammatory effect (Laskowitz et al., 1998), or neurotrophic effect (Nathan et al., 1994). ApoE is involved in regeneration process after epileptic seizure (Poirier et al., 1993; White et al., 2001) and peripheral nerve injury (Boyles et al., 1989). ApoE also plays an important role after cerebral ischemia, being involved in tissue clearance and repair via macrophages (Trieu and Uckun, 2000; Kitagawa et al., 2001). Recent studies reported that ApoE-gene-knockout mice showed a worse outcome than wild-type mice after brain ischemia (Sheng et al., 1999; Horsburgh et al., 1999, 2000; Trieu and Uckun, 2000; Kitagawa et al., 2001).
Although ApoE protein is scarcely present in neurons of normal brain (Han et al., 1994; Beffert et al., 1998), it is induced after transient brain ischemia both in neurons and astrocytes of rat and gerbil (Kida et al., 1995; Horsburgh and Nicoll, 1996; Ishimura et al., 1996). However, the changes were observed only at several days after the ischemia. Although tissue clearance and repair usually take several weeks after brain ischemia, alternations in ApoE have not been studied in relation to long-term neural processes after brain ischemia. In this study, therefore, changes of ApoE immunoreactivity and ApoE mRNA expression were examined for the long term, up to 8 weeks after transient middle cerebral artery occlusion (tMCAO).
MATERIALS AND METHODS
Animal Model
Male Wistar rats at age 11 weeks (body weight 250–280 g) were used in this study. The animals were anesthetized with an intraperitoneal injection of pentobarbital (10 mg/250 g rat). A burr hole (diameter 2 mm) was carefully made in the skull for measurement of cerebral blood flow (CBF), with the dura matter preserved at this time. The location of the burr hole was 3 mm dorsal and 5 mm lateral to the right from the bregma, which is located in the upper part of the MCA territory.
On the next day, the animals were anesthetized with an nitrous oxide/oxygen/isoflurane mixture (69%:30%:1%) during surgical preparation. The right MCA was occluded by insertion of a 4-0 surgical nylon thread with silicon coating through the common carotid artery according to our previous report (Kitagawa et al., 1999). Body temperature was maintained at 37°C ± 0.3°C using a heating pad during the surgical procedure for MCAO. Ninety percent of the operated animals showed gait disturbance following MCAO. Asymptomatic rats were excluded from the experiment. After 90 min of tMCAO, CBF was restored by removing the nylon thread. Regional CBF (rCBF) of the right frontoparietal cortex region was measured before, during, and after MCAO through the burr hole by using a laser blood flowmeter (Flo-C1; Omegawave, Tokyo, Japan) according to our previous report (Kitagawa et al., 1999). Sham control animals were treated in the same way but without MCAO. After the incision was closed, the animals recovered and were allowed free access to water and food at ambient temperature until sampling. The experimental protocol and procedures were approved by the Animal Committee of the Graduate School of Medicine and Dentistry, Okayama University.
Tissue Preparation
Eighty percent of the symptomatic rats survived and were available for this experiment. One, seven, twenty-one, or fifty-six days (n = 6 in each) after the reperfusion, rats were anesthetized with an overdose of pentobarbital and perfused through the heart with 200 ml of ice-cold heparinized saline, followed by 300 ml of 4% paraformaldehyde in 0.1 M phosphate buffer (PB; pH 7.4). Their brains were removed and postfixed by immersion in the same fixative (1 day, 4°C) and rapidly frozen after cryoprotection. For histochemistry and in situ hybridization study, frozen coronal sections were cut at 20 μm on a cryostat at −20°C and collected on glass slides.
ApoE Single Staining
The brain sections were incubated in 0.3% H2O2 in methanol to quench endogenous peroxide for 30 min. After phosphate-buffered saline (PBS) wash, nonspecific binding was blocked with 5% normal bovine serum in PBS. The anti-ApoE rabbit serum prepared against purified ApoE from rat blood was used to detect rat ApoE immunohistochemically (Shimano et al., 1991; Igeta et al., 1997), and the brain sections were incubated with the antibody (1:800) overnight at 4°C. The sections were then incubated with biotinylated rabbit IgG (1:200; Vector Laboratories, Burlingame, CA), followed by incubation with avidin-biotin-peroxidase complex (Vectastatin ABC Kit; Vector Laboratories) for 30 min. Diaminobenzidine tetrahydrochloride (DAB) was used as a color substrate. A set of sections was stained in a similar way without the primary antibody. The sections were examined with light microscopy (Olympus BX-51; Olympus Optical Co., Ltd., Tokyo, Japan).
Western Blotting
For Western blot analysis, 4 sham-operated rats, 16 rats with ischemia (at 1, 7, 21, 56 days after tMCAO; n = 4 at each time point) were studied. After decapitation under deep pentobarbital anesthesia, each brain was removed. A set of sections was stained with hematoxylin and eosin and examined by light microscopy for anatomic orientation of the ischemic lesion. On the basis of this staining, the brain tissues were collected from the ischemic core and the periischemic area, respectively (see Fig. 1). The area of the ischemic core contains the primary somatosensory area and the caudate putamen. The periischemic area contains mainly the hind limb area of the primary somatosensory and does not contain the anterior cingulate cortex or the motor cortex. The tissue samples were homogenized and centrifuged at 3,500g for 10 min at 4°C, and the supernatants (S1 fraction) were collected. Protein concentrations of the S1 samples were determined by Lowry assay (Bio-Rad, Hercules, CA). For the cell extracts of platelet-derived growth factor-treated or nontreated controls (Cell Signaling Technology, Beverly, MA), 5 μg of total protein for each sample were then electrophoresed in a 12.5% sodium dodecyl sulfate-polyacrylamide gel and transferred to a polyvinylidine fluoride membrane (Millipore, Bedford, MA). The membrane was blocked with 5% bovine serum albumin in TBST (20 mM Tris-HCl buffer, pH 7.4, 150 mM NaCl, 0.1% Tween) for 1 hr, followed by incubation with the rabbit anti-ApoE antibody (1:3,000) at 4°C overnight. On the next day, immunoblots were incubated with horseradish peroxidase-conjugated goat anti-rabbit IgG (1:2,000; Amersham, Buckinghamshire, United Kingdom) at room temperature, followed by detection using a chemiluminescence Western blotting detection system kit (ECLTM-RPN 2106; Amersham Pharmacia Biotech, Uppsala, Sweden). The results were scanned and quantified using Scion Image software (NIH). Statistical evaluation of each sample was carried out by one-way analysis of variance (ANOVA), followed by the Bonferroni/Dunn post hoc test, with significance set at P < .05.
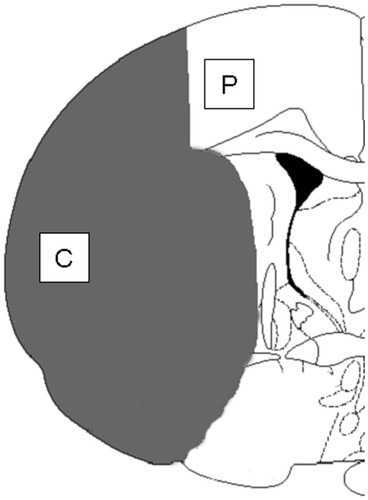
Schematic representation of the topographical distribution of ischemic damage (shaded area). Coronal section of rat brain is at the level of bregma −0.26 mm based on the rat brain atlas of Paxinos and Watson. Two areas [ischemic core (C), periischemic area (P)] for analyses of immunohistochemistry, immunoblotting, and in situ hybridization are illustrated.
Double Staining for ApoE Plus NeuN
An anti-NeuN monoclonal antibody recognizes vertebrate neuron-specific nuclear protein found on most neuronal cell types. For double staining of ApoE plus NeuN, the sections were incubated together with the above-mentioned anti-ApoE antibody and mouse anti-NeuN antibody (1:1,000; Chemicon, Temecula, CA) overnight at 4°C. The sections were then incubated with rhodamine-labeled goat anti-rabbit IgG (1:250; Chemicon) and fluorescein isothiocyanate (FITC)-labeled horse anti-mouse IgG (1:250; Vector Laboratories) for 1 hr at room temperature. A set of sections was stained in a similar way without the primary antibodies. Signals were examined using a confocal microscope (Carl Zeiss LSM 510; Carl Zeiss Co., Ltd., Tokyo, Japan). The FITC and rhodamine fluorospheres were imaged simultaneously using the 488-nm argon and 543-nm HeNe laser lines, with filters allowing emission detection in the 510–530 nm range (FITC) and at >570 nm (rhodamine).
Double Staining for ApoE Plus ED1
An anti-ED1 polyclonal antibody recognizes a single-chain glycoprotein of 90–100 kDa antigen expressed on lysosomal membrane of both activated microglia and blood-derived macrophages (Damoiseaux et al., 1994). For double staining of ApoE plus ED1, the sections were incubated together with the above-mentioned anti-ApoE antibody and mouse anti-ED1 antibody (1:400; MCA314R; Serotec) overnight at 4°C. The sections were then incubated with rhodamine-labeled goat anti-rabbit IgG (1:250; Chemicon) and FITC-labeled horse anti-mouse IgG (1:250; Vector Laboratories) for 1 hr at room temperature. A set of sections was stained in a similar way without the primary antibodies. Signals were examined using a confocal microscopy as mentioned above.
Analysis of Histochemistry
Based on the hematoxylin-eosin staining, the numbers of double-positive cells for ApoE plus NeuN or ApoE plus ED1 in 0.25 mm2 of three random applicable areas were counted in a blind manner and averaged in the ischemic core and the periischemic area, respectively. The difference in total number of double-positive cells between the groups was statistically analyzed by one-way ANOVA, followed by the Bonferroni/Dunn post hoc test, with significance set at P < .05.
In Situ Hybridization
The tissue sections were processed using 35S-labeled oligonucleotide antisense probes for ApoE mRNA (oligonucleotide 3′-end labeling system; Perkin-Elmer, Oak Brook, IL). The oligonucleotide probe, based on the reported sequences of ApoE, is 5′-TTTATTAAGCAAGGGCCACCAGAGGGCCAGAGTGGTGCTTGGGAC-3′ (Page et al., 1998). The hybridization and washing procedures were performed as described previously (Sato et al., 1996). Briefly, the sections were fixed with 4% paraformaldehyde in PBS for 5 min, followed by two 3-min washes with PBS. Then, they were acetylated with 0.1 M triethanolamine/0.9% NaCl (pH 8.0) and 0.25% acetic anhydride for 10 min and dehydrated with an ethanol series and chloroform. After prehybridization, 100 μl hybridization buffer containing 1 × 106 dpm 35S-labeled probe were placed onto each section. The hybridization buffer consisted of 4× SSC (600 mM NaCl, 60 mM sodium citrate, pH 7.0), 50% (v/v) deionized formamide, 10% (w/v) dextran sulfate, 1× Denhardt's solution (0.2% polyvinylpyrrolidone, 0.2% Ficoll, 0.2% bovine serum albumin), 100 mM dithiothreitol, and 0.1% (w/v) salmon sperm DNA (pH 8.0). Hybridiztion was performed overnight at 37°C in a humid chamber. The slides were rinsed four times for 20 min each at 37°C and four times for 20 min each at 25°C with 1× SSC, and air dried. These slides were dipped in a Kodak NTB2 emulsion diluted 1:1 with distilled water for 7 days at 4°C, then developed with Kodak D-19 developer and fixed with Kodak Unifix. After development, the slides were counterstained with hematoxylin and eosin. To ensure that the in situ hybridization technique was specific for each probe, a parallel control study was carried out, in which a set of sections was incubated with each radioactive probe in the presence of a 50-fold excess of the same, unlabeled probe.
RESULTS
Physiological Parameters
Five animal groups for sham control (SC) and 1, 7, 21, and 56 days showed the same body temperatures (37°C ± 0.3°C) during the surgical procedure for MCAO. No significant differences were noted in mean regional CBF (rCBF) among ischemic groups before, during, and just after MCAO (data not shown).
Hematoxylin and Eosin Staining
Acute neuronal damage, shrunken angular cells with pyknotic neclei, and vacuolation of the neuropil were observed in the striatum and the cortex at 1 day after tMCAO. Macrophage infiltration occurred in the infarction area and peaked at 7–21 days and decreased in number at 56 days after tMCAO (data not shown). These histological changes were detected in the ischemic core at each time point, and we defined the area adjacent to the ischemic core as the periischemic area (Fig. 1).
Single ApoE Staining After tMCAO
In SC brain, a weak immunoreactivity was observed in some astorcyte-like cells in the whole brain (Fig. 2a,f). After tMCAO, many ApoE-positive cells were observed with moderate intensity and triangular shape at 1 day in the ischemic core (Fig. 2b, arrows). At 7 days, ApoE-positive cells reached the maximum in the numbers with triangular (Fig. 2c, arrows) and with round shapes (Fig. 2c, arrowheads), and immunoreactivity in the neuropil also increased in the whole ischemic core. The number of ApoE-positive cells decreased at 21 days, and most of them were not triangular but round (Fig. 2d, arrowheads). The number of ApoE-positive cells greatly decreased at 56 days in the core (Fig. 2e, arrowheads).
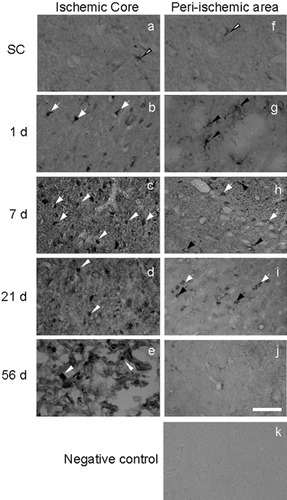
Changes of ApoE single immunostaining in the ischemic core (a–e) and the periischemic area (f–j) in the sham control (SC; a,f) and at 1, 7, 21, and 56 days after 90 min of transient middle cerebral artery occlusion (tMCAO; b–e,g–j). A weak immunoreactivity for ApoE was observed in SC brain (a,f). In the ischemic core, the number and the intensity of ApoE-positive cells reached a maximum at 7 days, with triangular (c, arrows) or round (c, arrowheads) shapes. In the periischemic area, ApoE immunoreactivity was observed in star-like cells at 1 and 7 days (g,h, arrowheads) and small, angular (h,i, black arrows) or triangular (h,i, white arrows) cells at 7 and 21 days, with a peak at 21 days. The brain section from 7 days without the first antibody showed no staining (k). Scale bar = 50 μm.
In the periischemic area, both the number and the intensity of ApoE immunoreactivity increased at 1 day after tMCAO, mainly in star-like cells estimated to be reactive astrocytes (Fig. 2g, arrowheads). At 7 days, the number of ApoE-positive cells further increased, with a slight reduction in the intensity. In addition to the astrocyte-like cells (Fig. 2h, arrowhead), cells with small, angular (Fig. 2h, black arrows) and triangular (Fig. 2h, white arrows) shapes also became immunoreactive, estimated to be neurons. At 21 days, ApoE-positive cells reached the maximum in number with small, angular (Fig. 2i, black arrows) or triangular (Fig. 2i, white arrows) shapes, but there were no more star-like astrocytes. At 56 days, the number of ApoE-positive cells decreased markedly (Fig. 2j). Brain sections without the first antibody showed no staining (Fig. 2k).
Western Blotting
Densitometric analysis of the Western blot images revealed a significant increase in ApoE expression in the ischemic core at 7, 21, and 56 days after brain ischemia in comparison with that in the SC animals. Maximal increase of ApoE expression occurred in the ischemic core at 7 days after tMCAO. In the periischemic area, the ApoE expression reached significant differences at 7 and 21 days after brain ischemia compared with that in the SC animals. A representative blot and the results of the densitometric analysis are depicted in Figure 3.
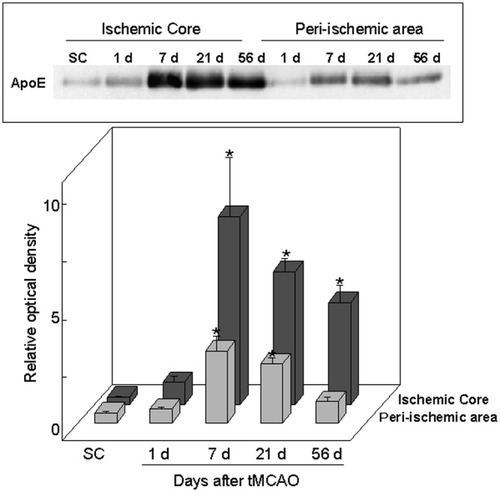
Western blot analysis of ApoE in the ischemic core and the periischemic area in the sham control and at 1, 7, 21, and 56 days after 90 min of tMCAO. Note the significant increase in ApoE immunoreactivity at 7, 21, and 56 days in the ischemic core and at 7 and 21 days in the periischemic area. The maximal increase in ApoE level occurred at 7 days in the ischemic core. Mean ± SD. *P < .05 compared with sham control animals.
Double Staining for ApoE Plus NeuN
In the SC brain, ApoE immunoreactivity was not observed (Figs. 4a, 5a) in NeuN-positive cells (Figs. 4b,c, 5b,c, 7SC). After tMCAO, the number of double-positive cells increased from 1 to 56 days in the ischemic core (Fig. 7). At 1 day, most ApoE was positive in the cytoplasm of NeuN-positive cells with a triangular shape (Fig. 4d–f, arrows). However, at 7 days, ApoE-positive but NeuN-negative cells with a large, round shape greatly increased in number, which were estimated to be macrophage (Fig. 4g,i,k). Although the number of ApoE plus NeuN double-positive, triangular cells did not change at 7 days, both intensities were reduced with reduction in size (Fig. 4h,j,l, arrows). At 21 and 56 days after tMCAO, ApoE plus NeuN double-positive cells were no longer observed in the ischemic core (Figs. 4m–r, 7).
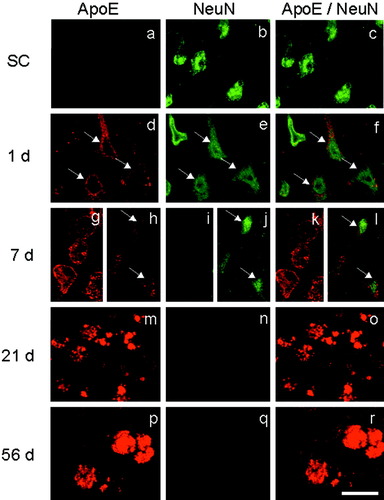
Double-immunofluorescence study with ApoE plus NeuN in the ischemic core. Single ApoE (left column), single NeuN (middle column), and the merged image (right column) in the sham control (SC; a–c) and at 1, 7, 21, and 56 days (d–r) after 90 min of tMCAO. Note that the number of ApoE plus NeuN double-positive cells increased at 1 day (f, arrows) with reduced intensity and size of cells at 7 days (l, arrows). ApoE-positive but NeuN-negative cells estimated to be macrophages began to be seen at 7 days (g,i,k) and predominated at 21 and 56 days (m–r). Scale bar = 20 μm.
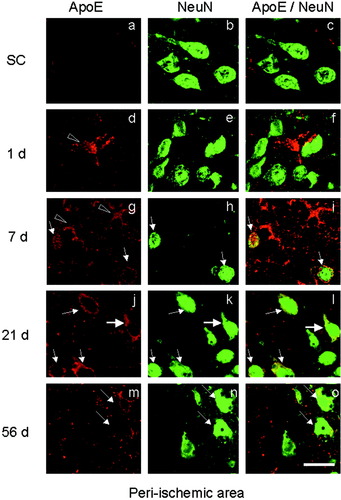
Double-immunofluorescence study with ApoE plus NeuN in the periischemic area. Single ApoE (left column), single NeuN (middle column), and the merged image (right column) in the sham control (SC; a–c) and at 1, 7, 21, and 56 days (d–o) after 90 min of tMCAO. Note that double-positive cells for ApoE plus NeuN began to be observed at 7 days (i, arrows), with a peak in the number at 21 days (l, arrows) and a decrease at 56 days (m–o, arrows). ApoE single-positive astrocytic cells were observed at 1 and 7 days (d,g, arrowheads). Scale bar = 20 μm.
In the periischemic area, ApoE plus NeuN double-positive cells were not observed at 1 day (Figs. 5d–f, 7), and most ApoE-positive cells were star-like astrocytes (Fig. 5d, arrowhead). At 7 days, double-positive cells with an angular shape increased in number (Fig. 5g–i, arrows; Fig. 7), and ApoE-positive but NeuN-negative cells were also found that could be estimated to be reactive astrocytes (Fig. 5g, arrowheads). The number of double-positive cells reached the maximum at 21 days, and most ApoE-positive cells were also NeuN-positive, with angular (Fig. 5l, thin arrows) or triangular (Fig. 5l, thick arrow) shapes; these were estimated to be neurons (Figs. 5j–l, 7). At 56 days, a few double-positive cells were observed in the periischemic area of only three among six animals (Fig. 5m–o, arrows; Fig. 7). Brain sections without the first antibody showed no staining (not shown).
Double Staining for ApoE Plus ED1
In the SC brain, ApoE plus ED1 double-positive cells were not observed (Fig. 7SC). After tMCAO, most ApoE-positive cells were not doubly positive for ED1 at 1 day in the ischemic core (Figs. 6a–c, 7). The number of ApoE plus ED1 double-positive cells peaked at 7 and 21 days and decreased at 56 days in the ischemic core (Figs. 6d–l, 7). However, the intensity of ApoE in ED1-positive cells became gradually stronger from 7 to 56 days (Fig. 6f,i,l). In the periischemic area, on the other hand, few ApoE plus ED1 double-positive cells were observed until 56 days after tMCAO (Fig. 7). Brain sections without the first antibody showed no staining (not shown).
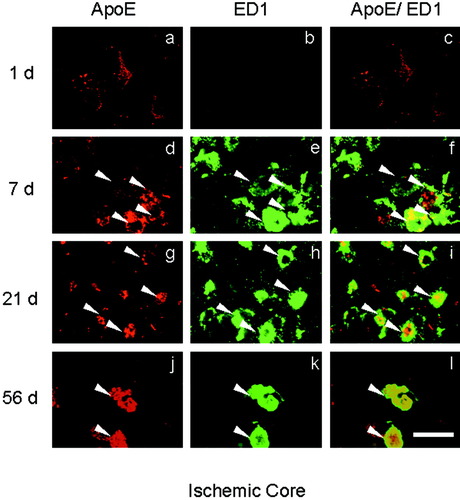
a–l: Double-immunofluorescence study with ApoE plus ED1 in the ischemic core. Single ApoE (left column), single ED1 (middle column), and the merged image (right column) at 1, 7, 21, and 56 days (d–l) after 90 min of tMCAO. Note the increase in number of double-positive cells at 7 and 21 days (f,i, arrowheads), with a decrease at 56 days (l, arrowheads), whereas the staining strength of double-positive cells progressively increased from 7 to 56 days (f,i,l). Scale bar = 20 μm.
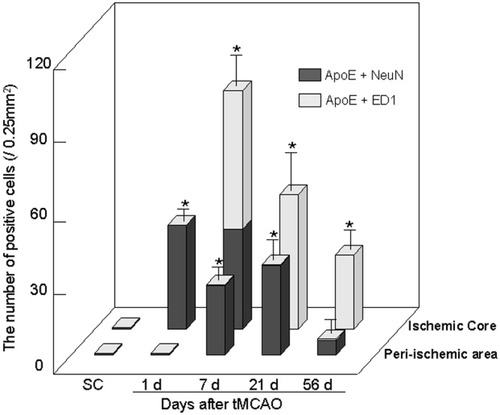
Changes of the number of double-positive cells for ApoE plus NeuN (black bars) or plus ED1 (gray bars) in the ischemic core and the periischemic area after 90 min of tMCAO. The number of ApoE plus NeuN double-positive cells increased at 1 and 7 days in the core and at 7 and 21 days in the periischemic area. On the other hand, the number of ApoE plus ED1 double-positive cells increased at 7 and 21 days in the core, whereas there were only a few in the periischemic area. Total number of double-positive cells reached the maximum at 7 days in the core but at 21 days in the periischemic area. Mean ± SD. *P < .05 compared with sham control animals.
In Situ Hybirdization
In the SC brain, ApoE mRNA was not expressed in neurons or glial cells (Fig. 8a). After tMCAO, the ApoE mRNA expression was not observed in the shrunken neurons with pyknotic nuclei at 1 day in the ischemic core (Fig. 8b). Marked ApoE mRNA expression was observed in macrophages, with a large, round shape in the ischemic core (Fig. 8c), and in glial cells with small, round nuclei in the periischemic area (Fig. 8d) at 7, 21, and 56 days after brain ischemia.
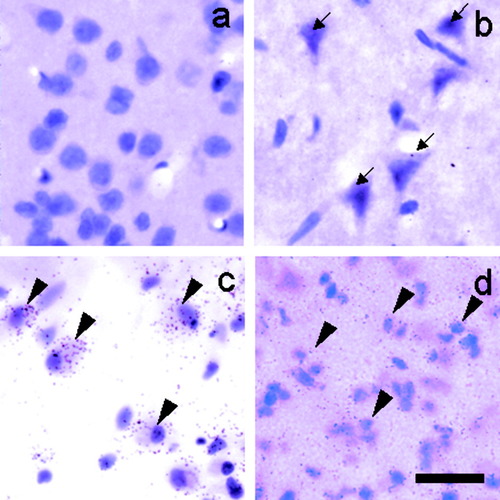
Representative photomicrographs of emulsion-dipped sections hybridized with 35S-labeled ApoE probe and counterstained with hematoxylin and eosin. ApoE mRNA was not observed in neurons in the SC brains (a) or at 1 day in the core after tMCAO (b, arrows). Marked expression of ApoE mRNA was observed in the macrophages at 7 days in the core (c, arrowheads) and in glial cells at 21 days in the periischemic area (d, arrowheads). Scale bar = 20 μm.
DISCUSSION
This is the first report on the long-term changes of ApoE immunoreactivity and ApoE mRNA expression after tMCAO. The important findings of this study are as follows. 1) The maximal increase of ApoE expression was observed at 7 days in the core and at 7 and 21 days in the periischemic area. 2) The number of ApoE plus NeuN double-positive cells increased at 1 and 7 days in the ischemic core, without ApoE mRNA expression in neurons. 3) In the periischemic area, the number of ApoE plus NeuN double-positive cells increased at 7 days and peaked at 21 days, with ApoE mRNA expression in glial cells but not in neurons. 4) ApoE plus ED1 double-positive cells increased only in the core, with a peak in number at 7 and 21 days and a peak in intensity at 56 days, with marked ApoE mRNA expression in macrophages.
ApoE was expressed in astrocyte-like cells in SC brains (Fig. 2a,f), probably reflecting the fact that astrocytes principally secrete ApoE in normal CNS (Pitas et al., 1987). The number of ApoE single-positive cells and the ApoE immunoreactivity peaked at 7 days in the ischemic core (Figs. 2a–e, 3), which coincides with a previous report on mouse brain after permanent MCAO (Kitagawa et al., 2001). Because reactive astrocytes appear in the periischemic area from 1 to 7 days after focal ischemia (Yamashita et al., 1996; Van Beek et al., 2000), positive ApoE immunoreactivity in the star-like astrocytic cells (Fig. 2g) suggests an enhanced secretion of ApoE in the periischemic area after brain ischemia. Double staining for ApoE plus glial fibrillary acidic protein (GFAP) would confirm the identity of these cells, but we did not examine this in the present study. ApoE was up-regulated in relation to both synaptic loss and synaptic regeneration after entorhinal cortex injury (White et al., 2001). Thus, the first finding in the present study suggests that the up-regulation of ApoE is related to degeneration processes in the core but to regeneration processes in the periischemic area. In addition, the shape of single ApoE-positive cells changed temporally and spatially in this study (Fig. 2b–e,g–j), suggesting that the function and mechanism of ApoE up-regulation are different among cell types after brain ischemia. Therefore, we examined the type of ApoE-positive cells by double staining with NeuN or ED1.
The second finding suggests that ApoE was taken up by degenerating neurons in the ischemic core during 1–7 days and that the level of ApoE protein was reduced with neuronal cell death in the core (Fig. 4h). ApoE ameliorated acute neuronal damage after transient global ischemia through its antioxidant effect but did not mitigate glutamate toxicity or block apoptosis (Horsburgh et al., 2000; Kitagawa et al., 2002). Thus, ApoE was mainly taken up by neurons of the core during 1–7 days (Figs. 4, 7) and works as an antioxidant molecule. Lipids internalized into neurons may be used for regeneration, but ApoE taken up by neurons failed to protect them in the core. ApoE plus NeuN double-positive cells were not observed at 21 and 56 days in the core (Fig. 4o,r). Because even NeuN single-positive cells were not observed at this stage (Fig. 4n,q), almost all neuronal cells in the core have already died at 21 days after brain ischemia.
Because ApoE plus NeuN double-positive cells were not found at the acute stage (1 day) in the periischemic area (Figs. 5d–f, 7), the third finding suggests that ApoE was synthesized by glial cells and taken up by neurons of the periischemic area at later stages, 7–21 days after tMCAO, probably to help with rearrangement of partially injured cell membranes and synapses. Because ApoE containing lipid enhances neurite outgrowth and promotes synaptogenesis (Handelmann et al., 1992; Nathan et al., 1994; Mauch et al., 2001), ApoE may be involved in lipid transport, maintenance of cell membranes, and synaptic plasticity at later stage in the periischemic area (Figs. 5, 7, 9), as observed in cerebral cortical and peripheral nerve injuries (Ignatius et al., 1986; Boyles et al., 1989; Poirier et al., 1993; Stroemer et al., 1995; White et al., 2001).
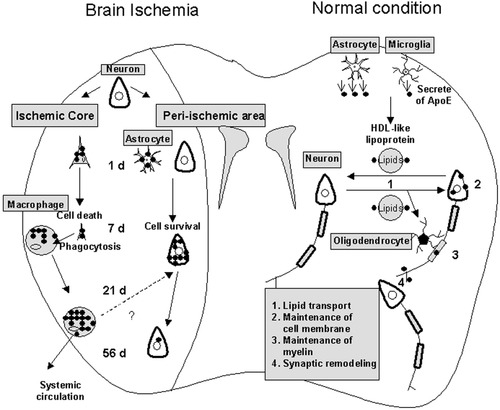
Schematic illustration of the role and changes of ApoE under normal conditions and after brain ischemia. Under normal conditions, ApoE is synthesized and secreted by astrocytes and microglia and is involved in lipid transport, maintenance of cell membrane and myelin, and synaptic remodeling (1–4). After brain ischemia, ApoE was taken up by degenerating neurons at 1–7 days but was expressed within macrophages at 7–56 days in the core. Most ApoE-positive macrophages may move from the ischemic core back into the systemic circulation. On the other hand, ApoE was taken up by neurons at 7–56 days in the periischemic area.
The fourth finding suggests that macrophages infiltrated into the ischemic core after the acute stage to phagocytose lipids derived from necrotic tissue (Figs. 6, 7, 9). Because brain tissue did not develop necrosis in the periischemic area, macrophages did not infiltrate that area (Fig. 7). Progressive enhancement of ApoE immunoreactivity from 7 to 56 days in each ED1-positive cell (Fig. 6f,i,l) suggests a concentration of ApoE in macrophages at later stage after tMCAO, which could be explained by an unlimited uptake of oxidized lipoprotein in the cytoplasm of macrophages (Kudo and Nagayama, 1988; Chait and Heinecke, 1994). The number of ApoE plus ED1 double-positive cells decreased from 7 to 56 days after tMCAO (Fig. 7), indicating a return of macrophages from the ischemic core back into the systemic circulation. In addition to phagocytosis, the level of ApoE in macrophages could also be up-regulated with ApoE mRNA synthesis by cholesterol loading and a cytokine such as transforming growth factor-β that is expressed at later stage after brain ischemia (Mazzone et al., 1989; Rouis et al., 1990; Lehrmann et al., 1998; Larkine et al., 2000). Because ApoE carries cholesterol out of macrophages, ApoE that is expressed in macrophages in the core at later stages of reperfusion (Figs. 6, 7) might also participate in cholesterol redistribution in the periischemic area to recycle cholesterol in the local ischemic lesion for neural reorganization (Rosenfeld et al., 1993; Larkine et al., 2000).
In conclusion, this study suggests that ApoE plays various important roles in different type of cells, reflecting spatiotemporal dissociation of neural process after brain ischemia. In the core, ApoE was taken up mainly by degenerating neurons at 1–7 days for neuroregeneration, whereas ApoE was expressed predominantly in macrophages at 7–56 days, probably for clearance of necrotic tissue after brain ischemia. On the other hand, ApoE was synthesized by glial cells and taken up principally by neurons at 7–56 days in the periischemic area to play an important role in tissue repair after brain ischemia (Figs. 7, 9). Although ApoE plays important roles in maintaining normal brain structure and function, the present study shows that ApoE is also profoundly involved under pathological conditions such as brain ischemia through different cellular, temporal, and spatial aspects.
Acknowledgements
This work was partially supported by Grants-in-Aid for Scientific Research (B) 12470141, (C) 13670649, and (Hoga) 12877211 from the Ministry of Education, Science, Culture and Sports of Japan, Cell Biology Science Foundation, and by grants (K. Tashiro, Y. Itoyama, and S. Tsuji) and Comprehensive Research on Aging and Health funds (H11-Choju-010, No. 207; A. Koizumi) from the Ministry of Health and Welfare of Japan. We thank Dr. N. Yamada (Ibaragi, Japan) for his generous gift of the anti-ApoE antibody.




