Postacute Sequelae of COVID-19 Across 12 Major Health Domains and 141 Diseases in Individuals With Mental Illness Among COVID-19 Survivors: A Population-Based Cohort Study in South Korea
Jiseung Kang, Jaeyu Park, and Yejun Son contributed equally to this study as the first authors.
ABSTRACT
Understanding whether individuals with mental illness, who face challenges related to healthcare barriers, are more vulnerable to postacute sequelae of COVID-19 is limited. Here, we investigated the potential association between pre-existing mental illness and postacute sequelae of COVID-19 across 12 major health domains and 141 specific diseases in COVID-19 survivors. The large-scale, population-based cohorts from South Korea (K-COV-N cohort) used in the study included 8 632 221 individuals aged 20 years or older who were infected with SARS-CoV-2 between January 1, 2020, and December 31, 2022. The risk of postacute sequelae of COVID-19 was assessed in the 1:2 propensity score-matched cohorts, comprising 12 major health domains and 141 diseases based on the ICD-10 code, following mental illness among patients with COVID-19. We assessed the time attenuation effect of major health outcomes after 30 days following SARS-CoV-2 infection. Multiple subgroup analyses were conducted by severity of mental illness, COVID-19 severity, vaccination, and SARS-CoV-2 strain. After 1:2 exposure-driven propensity score matching, we identified 1 341 320 participants with mental illness (mean age, 49.51 [SD, 13.82] years; 62.27% female) and 2 653 597 controls (mean age, 48.78 [SD, 13.75] years; 62.03% female). Individuals with mental illness exhibited significantly higher risks across all 12 major health domains, including: infectious and parasitic events (adjusted hazard ratio [aHR], 1.36 [95% CI, 1.33–1.38]), blood and immune-related events (1.21 [1.17–1.26]), endocrine, nutritional, and metabolic events (1.21 [1.18–1.24]), nerve-related events (2.13 [2.07–2.19]), eye-related events (1.29 [1.25–1.34]), ear and mastoid events (1.52 [1.50–1.54]), circulatory events (1.25 [1.17–1.35]), respiratory events (1.26 [1.24–1.29]), digestive events (1.41 [1.40–1.41]), skin-related events (1.34 [1.30–1.38]), musculoskeletal events (1.42 [1.41–1.43]), and genitourinary events (1.54 [1.18–2.01]). Of the 141 postacute sequelae of COVID-19, 133 showed significantly increased risks. The association was strongest within the first 6–12 months after SARS-CoV-2 infection, with risks progressively attenuating beyond 12 months and nearly disappearing after 18 months. Subgroup analysis revealed that individuals with mild mental illness exhibited higher aHRs for 11 of the 12 health outcome domains compared with those with severe mental illness. Altogether, our findings show the increased risk of postacute sequelae of COVID-19 across 12 major health domains in individuals with mental illness among COVID-19 survivors. These findings highlight the need for targeted monitoring and intervention strategies to address the vulnerabilities of this population, particularly during the post-COVID-19 period.
1 Introduction
The COVID-19 pandemic has resulted in unprecedented morbidity and mortality, with approximately 800 million confirmed cases and 7 million deaths globally [1]. The global incidence and mortality rates of COVID-19 are declining [2]. However, the long-term consequences of SARS-CoV-2 infection, commonly referred to as “postacute sequelae of COVID-19”, “long COVID”, or “post-COVID-19 conditions”, remain a significant concern [3]. Individuals infected with SARS-CoV-2 face increased risks of post-COVID-19-related mortality and various comorbidities, including cardiovascular, metabolic, neuropsychiatric, and gastrointestinal disorders [4]. Furthermore, approximately 45% of individuals globally report experiencing postacute sequelae of COVID-19, and the growing prevalence of these conditions is likely to exacerbate economic burdens [5, 6].
Previous studies have emphasized the importance of understanding predisposing factors in elucidating the pathophysiology and phenomenology of postacute sequelae of COVID-19 [7]. However, less work has been performed on the prevalence of postacute sequelae of COVID-19 at a population level and how the incidence of postacute sequelae of COVID-19 may vary by specific underlying conditions, particularly mental illness. Individuals with mental illness often face significant barriers to healthcare utilization, including unmet medical needs, reduced access to timely medical interventions, and poorer treatment prognoses [8-10]. Therefore, we here have approached this question by utilizing a large-scale population-based cohort, including people who are living with mental illness.
This study aims to investigate potential associations between predisposing mental illness and the development of postacute sequelae of COVID-19, as well as to compare the incidence of various postacute sequelae of COVID-19 in individuals with mental illness to controls (those in infected SARS-CoV-2 without predisposing mental illness). The primary objective is to determine whether an underlying diagnosis of mental illness predisposes individuals to develop postacute sequelae of COVID-19 across 141 health outcomes and 12 organ domains.
2 Methods
2.1 Study Design and Participants
This study is a longitudinal, propensity score-matched cohort study utilizing a large-scale, general population-based cohort representative of South Korea (K-COV-N cohort; total n = 8 632 221). The universal healthcare system of South Korea encompasses the entire population and integrates COVID-19-related records into a single database, enabling the analysis of relationships among SARS-CoV-2 infection, vaccination, and health outcomes based on the International Classification of Diseases 10th (ICD-10) codes [11, 12]. The study included only patients with confirmed SARS-CoV-2 infection. It was approved by the Kyung Hee University Institutional Review Board (KHSIRB-23-241[EA]), the Korea Disease Control and Prevention Agency (KDCA), and the National Health Insurance Service (NHIS; KDCA-NHIS-2024-1-280). In accordance with the approval conditions, anonymized administrative data were used, and informed consent from participants was waived.
2.2 Data Source
The K-COV-N cohort, established through a collaboration between the NHIS, KDCA, and Statistics Korea, includes comprehensive data from January 1, 2018, to December 31, 2022 for adults aged 20 and older. It covers national health screenings, prescriptions, mortality records, COVID-19 vaccination details, SARS-CoV-2 test results, and related health outcomes. This cohort is designed to track individuals across hospitals, multiple healthcare facilities, and even cases of deaths occurring outside the healthcare system. The look-back period for evaluating prior diagnostic history spanned from 2018 to the index date, while the follow-up period covered 2020–2022. According to a previous validation study, the diagnostic records in claims data exhibited an overall positive predictive value of 82% [11, 12].
2.3 Exposure
The primary exposure of this study was the mental illness among patients with COVID-19 [13, 14]. SARS-CoV-2 infection was verified based on results from real-time reverse transcription polymerase chain reaction tests or nasal and throat swab antigen tests approved by the KDCA. In this study, mental illness was defined as exposure based on ICD-10 codes and classified into nonaffective disorders and affective disorders. A more detailed ICD-10 code-based definition is described in Supporting Information S1: Table S1 [13, 14].
2.4 Outcomes
The primary outcomes of this study included 12 major health domains, while the secondary outcomes comprised 141 specific diseases defined based on ICD-10 codes [13, 14]. Outcomes were defined as incident events occurring after 30 days following SARS-CoV-2 infection. The 12 major health domains included: (1) infectious and parasitic diseases, (2) blood and immune-related disorders, (3) endocrine, nutritional, and metabolic diseases, (4) nervous system disorders, (5) eye and adnexa disorders, (6) ear and mastoid process disorders, (7) circulatory diseases, (8) respiratory diseases, (9) digestive diseases, (10) skin and subcutaneous tissue disorders, (11) musculoskeletal disorders, and (12) genitourinary disorders. A detailed list of specific conditions within each category is provided in Supporting Information S1: Table S1. Participants were censored at the time of reinfection, defined as a positive SARS-CoV-2 test occurring at least 90 days after the initial infection [15, 16]. Follow-up ended at the incidence of the outcome, death, or December 31, 2022, whichever came first [17].
2.5 Covariates
The demographic characteristics of the study participants were collected from the national health insurance database. These include sex, age (20–39, 40–59, and ≥ 60 years), household income level (low income [0–39th percentile], middle income [40–79th percentile], and high income [80–100th percentile]), and residential area (urban and rural) [18].
Body mass index (BMI) was categorized as underweight (< 18.5 kg/m²), normal weight (18.5–22.9 kg/m²), overweight (23.0–24.9 kg/m²), and obese (≥ 25.0 kg/m²), according to the Asian-Pacific guidelines [11, 12]. Glomerular filtration rate was categorized as < 60, 60–89, and ≥ 90 mL/min/1.73 m2, and unknown. Blood pressure was classified as systolic blood pressure < 140 mmHg and diastolic blood pressure < 90 mmHg, or systolic blood pressure ≥ 140 mmHg and/or diastolic blood pressure ≥ 90 mmHg, and unknown. Fasting blood glucose levels were divided into < 100 mg/dL, ≥ 100 mg/dL, and unknown [11, 12]. All these metrics were based on fasting serum samples obtained during the national health examination. The missing indicator method was used to address missing variables across all covariates.
In addition, medical history, including the Charlson comorbidity index (CCI) and treatment for diabetes and hyperlipidemia, hypertension medication use, smoking status (never, ex-, and current smoker), weekly alcohol consumption frequency (< 1 day, 1–2 days, 3–4 days, and ≥ 5 days, and unknown), and aerobic physical activity level (sufficient and insufficient). Sufficient aerobic physical activity was defined as aerobic exercise totaling ≥ 600 min per week in Metabolic Equivalent Task scores, based on the guidelines provided by the World Health Organization on physical activity [19]. The representative population in our study is depicted in Supporting Information S1: Table S2.
2.6 Propensity Score Matching
To ensure the reliability of the primary outcomes and enhance generalizability, exposure-driven propensity score matching was performed to compare individuals with mental illness to those without mental illness among COVID-19 survivors [17, 20]. Propensity scores were calculated using a logistic regression model adjusted for covariates, including age, sex, residential area, BMI, CCI, history of medication use (diabetes, hyperlipidemia, and hypertension), smoking status, alcohol consumption, aerobic physical activity, and COVID-19-related variables (SARS-CoV-2 strain type, severity of COVID-19, and COVID-19 vaccination) [17, 20].
We constructed a 1:2 matched cohort using a greedy nearest-neighbor matching algorithm without replacement to reduce confounder bias. A caliper width of 0.001 standard deviations of the logit of the propensity score was applied [21]. To compare the two groups, covariate balance between the matched groups was assessed using standardized mean differences (SMDs), which quantify the difference in means or proportions between groups relative to the pooled standard deviation. As shown in Table 1, for all matched covariates, the SMD was less than 0.1, which was considered indicative of adequate covariate balance. Unmatched covariates were incorporated into the Cox proportional hazards regression model to adjust for potential residual confounding [21, 22].
| Matching covariates | 1:2 Propensity score-matched discovery cohort (n = 3 994 917) | ||
|---|---|---|---|
| Mental illness (n = 1 341 320) | Controls (n = 2 653 597) | SMDa | |
| Mean age (SD), years | 49.51 (13.82) | 48.78 (13.75) | 0.043 |
| Age, n (%) | 0.024 | ||
| 20–39 years | 359 407 (26.80) | 717 817 (27.05) | |
| 40–59 years | 566 571 (42.24) | 1 127 616 (42.49) | |
| ≥ 60 years | 415 342 (30.97) | 808 164 (30.46) | |
| Sex, n (%) | < 0.001 | ||
| Male | 506 033 (37.73) | 1 007 603 (37.97) | |
| Female | 835 287 (62.27) | 1 645 994 (62.03) | |
| Region, n (%) | 0.003 | ||
| Urban | 594 760 (44.34) | 1 180 924 (44.50) | |
| Rural | 746 560 (55.66) | 1 472 673 (55.50) | |
| Household income, n (%) | 0.018 | ||
| Low household income level | 494 242 (36.85) | 970 391 (36.57) | |
| Middle household income level | 518 310 (38.64) | 1 030 230 (38.82) | |
| High household income level | 328 768 (24.51) | 652 976 (24.61) | |
| Charlson Comorbidity Index, n (%) | < 0.001 | ||
| 0 score | 1 180 575 (88.02) | 2 359 498 (88.92) | |
| 1 score | 99 853 (7.44) | 182 156 (6.86) | |
| ≥ 2 score | 60 892 (4.54) | 111 943 (4.22) | |
| BMI, n (%) | 0.027 | ||
| Underweight (< 18.5 kg/m2) | 57 261 (4.27) | 111 735 (4.21) | |
| Normal weight (18.5–22.9 kg/m2) | 512 897 (38.24) | 1 015 123 (38.25) | |
| Overweight (23.0–24.9 kg/m2) | 296 939 (22.14) | 587 655 (22.15) | |
| Obesity (≥ 25.0 kg/m2) | 474 223 (35.35) | 939 084 (35.39) | |
| Smoking status, n (%) | < 0.001 | ||
| Nonsmoker | 1 251 242 (93.28) | 2 476 677 (93.33) | |
| Ex-/Current smoker | 90 078 (6.72) | 176 920 (6.67) | |
| Alcohol consumption, n (%) | 0.009 | ||
| Rarely | 887 685 (66.18) | 1 750 466 (65.97) | |
| Sometimes | 310 718 (23.17) | 619 915 (23.36) | |
| Everyday | 107 765 (8.03) | 214 358 (8.08) | |
| Unknown | 35 152 (2.62) | 68 858 (2.59) | |
| Aerobic physical activity, n (%) | 0.001 | ||
| Insufficient | 716 642 (53.43) | 1 413 056 (53.25) | |
| Sufficient | 624 678 (46.57) | 1 240 541 (46.75) | |
| Medical history, n (%) | |||
| Medication use for diabetes | 83 176 (6.20) | 164 563 (6.20) | < 0.001 |
| Medication use for hyperlipidemia | 99 157 (7.39) | 155 976 (5.88) | 0.018 |
| Medication use for hypertension | 210 838 (15.72) | 366 530 (13.81) | 0.018 |
| SARS-CoV-2 strain type, n (%) | 0.037 | ||
| SARS-CoV-2 infection in the pre-delta era | 7880 (0.59) | 15 268 (0.58) | |
| SARS-CoV-2 infection in the delta era | 20 014 (1.49) | 38 650 (1.46) | |
| SARS-CoV-2 infection in the omicron era | 1 313 426 (97.92) | 2 599 679 (97.97) | |
| Severity of COVID-19, n (%) | 0.010 | ||
| Mild COVID-19 | 1 337 451 (99.71) | 2 647 598 (99.77) | |
| Moderate to severe COVID-19 | 3869 (0.29) | 5999 (0.23) | |
| COVID-19 vaccination, n (%) | 0.043 | ||
| Incomplete vaccination (< 2 doses) | 80 372 (5.99) | 156 645 (5.90) | |
| Complete vaccination (2 doses) | 321 069 (23.94) | 638 451 (24.06) | |
| Booster vaccination (≥ 3 doses) | 939 879 (70.07) | 1 858 501 (70.04) | |
| Unmatched covariatesb, n (%) | |||
| Blood pressure | 0.143 | ||
| SBP < 140 mmHg and DBP < 90 mmHg | 1 187 989 (88.57) | 2 350 156 (88.56) | |
| SBP ≥ 140 mmHg or DBP ≥ 90 mmHg | 147 199 (10.97) | 298 988 (11.27) | |
| Unknown | 6132 (0.46) | 4453 (0.17) | |
| Glomerular filtration rate, n (%) | 0.003 | ||
| < 60 mL/min/1.73 m2 | 35 275 (2.63) | 64 517 (2.43) | |
| 60–89 mL/min/1.73 m2 | 556 796 (41.51) | 1 100 423 (41.47) | |
| ≥ 90 mL/min/1.73 m2 | 740 108 (55.18) | 1 478 423 (55.71) | |
| Unknown | 9141 (0.68) | 10 234 (0.39) | |
| Fasting blood glucose, n (%) | 0.111 | ||
| < 100 mg/dL | 354 808 (26.45) | 689 369 (25.98) | |
| ≥ 100 mg/dL | 980 282 (73.08) | 1 959 699 (73.85) | |
| Unknown | 6230 (0.46) | 4529 (0.17) | |
- Abbreviations: BMI, body mass index; DBP, diastolic blood pressure; SBP, systolic blood pressure; SD, standard deviation; SMD, standardized mean difference.
- a SMD is used to assess covariate balance between the matched groups. SMD < 0.1 indicates no significant imbalance. All SMDs were < 0.1 in the propensity score-matched cohorts, except for unmatched covariates.
- b Unmatched covariates (e.g., blood pressure, glomerular filtration rate, fasting blood glucose) were directly included in the matching process but were adjusted for in the outcome regression models to address potential residual confounding.
2.7 Statistical Analysis
This study analyzed the association of mental illness and 141 diseases categorized into 12 major health domains among patients with COVID-19 using the Cox proportional hazard regression model [13, 14]. Subgroup analyses were conducted based on the severity of mental illness. Severe mental illness were defined as nonaffective disorders or affective disorders with psychotic features, while mild mental illness included all other mental illness. Supporting Information S1: Table S3 shows information on additional stratification analyses that were performed based on the age, sex, BMI, household income, number of vaccinations (unvaccinated or single dose [incomplete], 2 doses [complete], and ≥ 3 doses [booster]), COVID-19 severity (mild and moderate-severe), and SARS-CoV-2 strain type (original, delta, and omicron) [12]. The study categorized original SARS-CoV-2 cases as those with an index date between the first infection and July 31, 2021. The Delta era was defined as cases with an index date between August 1, 2021, and December 31, 2021 [23]. The Omicron era included cases with an index date from January 1, 2022, until the end of the observation period [24]. Moderate to severe COVID-19 cases were defined as patients requiring admission to the intensive care unit or treatment with oxygen therapy, extracorporeal membrane oxygenation, renal replacement, or cardiopulmonary resuscitation. All other cases were categorized as mild [12, 18]. COVID-19 vaccination status was determined by the number of doses received before SARS-CoV-2 infection. The Johnson & Johnson/Janssen single-dose vaccine was considered equivalent to two doses [12, 18]. All statistical analyses were performed using SAS software (version 9.4; SAS Institute Inc.), with two-sided p values < 0.05 considered statistically significant.
2.8 Patient and Public Involvement
The Korean government implemented data anonymization measures to protect patient confidentiality by removing personal identifiers from the dataset. While individuals could not be directly identified due to the absence of names, the dataset retained all essential variables for analysis. This study relied on a retrospective review of a restricted administrative dataset, with no direct participant engagement or public involvement. The study was independently designed and carried out without external input. Although South Korea currently lacks a formal system for incorporating patients or the public into the research process, the findings from this study will be officially documented and reported to the NHIS, which manages healthcare data in South Korea.
2.9 Role of the Funding Source
This study was supported by grants from the National Research Foundation of Korea, funded by the Korean Government (MSIT; RS–2023–00248157) and the MSIT (Ministry of Science and ICT), Korea, under the ITRC (Information Technology Research Center) support program (IITP-2024-RS-2024-00438239) supervised by the IITP (Institute for Information & Communications Technology Planning & Evaluation). The funders played no role in the study design, data collection, data analysis, data interpretation, or manuscript writing. All authors confirm that they had full access to all the data in the study and had final responsibility to submit for publication.
3 Results
A total of 8 632 221 participants with COVID-19 (mean age, 46.3 [SD, 13.4] years; 51.8% female) were utilized as primary cohort (Supporting Information S1: Table S4 and Figure 1). In a 1:2 propensity score-matched cohort, we identified 1 341 320 participants with mental illness (mean age, 49.51 [SD, 13.82] years; 62.27% female) and 2 653 597 controls (mean age, 48.78 [SD, 13.75] years; 62.03% female; Table 1 and Figure 1). After 1:2 propensity score matching, the cohorts of participants were well matched in terms of baseline characteristics, as evidenced by SMD values below 0.1, indicating no significant imbalance (Table 1).

After 30 days after the SARS-CoV-2 infection, individuals with mental illness among COVID-19 survivors had an increased risk of being diagnosed to have developed Postacute sequelae of COVID-19 with all 12 major health domains (Figure 2). The adjusted hazard ratios (aHRs) for these domains included: infectious and parasitic events (aHR, 1.36 [95% CI, 1.33–1.38]), blood and immune-related events (aHR, 1.21 [95% CI, 1.17–1.26]), endocrine, nutritional, and metabolic events (aHR, 1.21 [95% CI, 1.18–1.24]), nerve-related events (aHR, 2.13 [95% CI, 2.07–2.19]), eye and adnexa events (aHR, 1.29 [95% CI, 1.25–1.34]), ear and mastoid process events (aHR, 1.52 [95% CI, 1.50–1.54]), circulatory events (aHR, 1.25 [95% CI, 1.17–1.35]), respiratory events (aHR, 1.26 [95% CI, 1.24–1.29]), digestive events (aHR, 1.41 [95% CI, 1.40–1.41]), skin and subcutaneous tissue events (aHR, 1.34 [95% CI, 1.30–1.38]), musculoskeletal events (aHR, 1.42 [95% CI, 1.41–1.43]), and genitourinary events (aHR, 1.54 [95% CI, 1.18–2.01]).
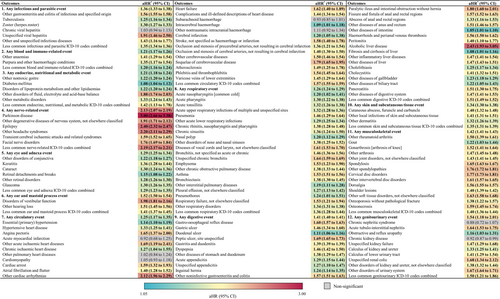
Of the 141 postacute sequelae of COVID-19, 133 events had significantly elevated risks in individuals with mental illness (Figure 2 and Supporting Information S1: Table S5). The symptoms with the largest aHRs were Parkinson disease (aHR, 3.00 [95% CI, 2.66–3.38]), alcoholic liver disease (aHR, 2.43 [95% CI, 1.93–3.05]), epilepsy (aHR, 2.40 [95% CI, 2.32–2.47]), other headache syndromes (aHR, 2.20 [95% CI, 2.11–2.29]), other cardiac arrhythmias (aHR, 2.12 [95% CI, 1.96–2.29]), disorders of vestibular function (aHR, 1.98 [95% CI, 1.81–2.16]), other degenerative diseases of nervous system not elsewhere classified (aHR, 1.91 [95% CI, 1.71–2.12]), unspecified viral hepatitis (aHR, 1.91 [95% CI, 1.46–2.50]), and other disorders of fluid, electrolyte and acid–base balance (aHR, 1.34 [95% CI, 1.30–1.38]).
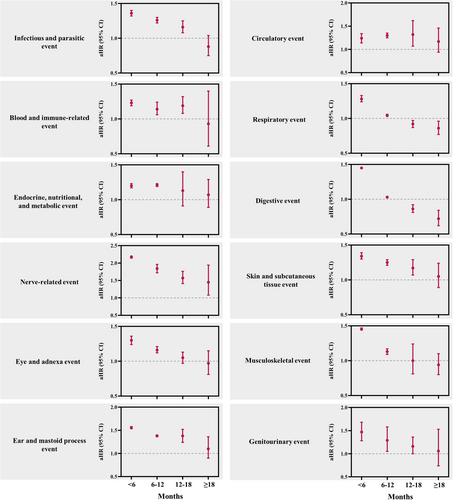
The association between underlying mental illness and the 12 major health domains was more pronounced within the first 6 months and during the 6–12-month period following the index date. The aHRs decreased over time, with the effect size progressively diminishing beyond 12 months and nearly disappearing after 19 months (Figure 3 and Supporting Information S1: Table S6).
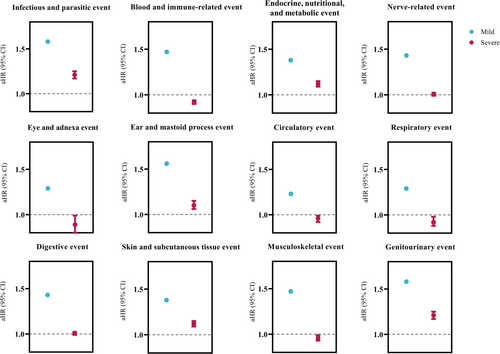
Subgroup analysis by severity of mental illness revealed higher aHRs for 10 categorized health domains, including infectious and parasitic diseases, blood and immune-related disorders, nervous system disorders, eye and adnexa disorders, ear and mastoid process disorders, respiratory diseases, digestive diseases, skin and subcutaneous tissue disorders, musculoskeletal disorders, and genitourinary disorders, in individuals with mild mental illness compared with those with severe mental illness (Figure 4 and Supporting Information S1: Table S7).
Further stratification analysis identified several sociodemographic and clinical risk factors, including the severity of mental illness, COVID-19 severity, vaccination, and the SARS-CoV-2 strain, that were significantly associated with the incidence of postacute sequelae of COVID-19 in individuals with mental illness (Supporting Information S1: Table S8).
4 Discussion
4.1 Findings and Explanation
Among survivors of COVID-19, we investigated the association between underlying mental illness and the incidence of postacute sequelae of COVID-19 and obtained several key findings. First, individuals with mental illness exhibited a significantly higher risk of developing postacute sequelae of COVID-19 across all 12 major health domains. Specifically, the highest risks were observed for nerve-related, genitourinary, and musculoskeletal events. Second, among the 141 postacute sequelae of COVID-19, 133 showed significantly elevated risks in individuals with mental illness. Third, the association between mental illness and postacute sequelae of COVID-19 was largest within the first 6 months and for 6–12 months following the index date. The effect size progressively decreased beyond 12 months and nearly disappeared after 18 months, suggesting a time-dependent attenuation of risks. Fourth, subgroup analysis showed that individuals with mild mental illness had higher aHRs for 11 of the 12 major health domains compared with those with severe mental illness, suggesting differential risks based on the severity of underlying mental illness. Lastly, stratification analysis identified several sociodemographic and clinical risk factors significantly associated with the incidence of postacute sequelae of COVID-19 in individuals with mental illness.
4.2 Comparison With Other Studies
Several cohort studies have identified mental illness as components of postacute sequelae of COVID-19 [25-27]. However, these studies primarily focused on the risk of developing neurological and psychiatric outcomes as postacute sequelae of COVID-19 following SARS-CoV-2 infection. In contrast, this study uniquely examines the risks of developing all postacute sequelae of COVID-19 in individuals with underlying mental illness, representing a distinct and novel contribution to the field. Previous research, such as studies on the prevalence of long COVID in individuals with diabetes mellitus in UK cohorts [7], provides valuable insights for specific populations but lacks focus on individuals with mental illness. This knowledge gap is significant, as mental illness are associated with unmet healthcare needs, limited healthcare accessibility, and shared underlying mechanisms with conditions such as metabolic diseases and impaired behaviors [28-30]. Our study, therefore, provides novel evidence for the association between underlying mental illness and risks of postacute sequelae of COVID-19, using a nationwide, population-based cohort with a more substantial and representative sample size (N = 8 632 221).
4.3 Possible Mechanisms
Individuals with mental illness often face significant barriers to accessing healthcare, including unmet healthcare needs, lower accessibility to timely medical interventions, and poorer prognosis for treatments [8]. A previous study found that approximately 27% of adults experiencing symptoms of depression or anxiety during the pandemic reported unmet healthcare needs [31]. These challenges may be compounded by the stigma associated with mental health conditions, socioeconomic disparities or burdens, insufficient community support, and limited healthcare systems [9, 10]. Such stigma or inequities can result in delayed diagnosis and suboptimal management of both predisposing conditions and new complications, such as postacute sequelae of COVID-19 [9, 10]. Furthermore, the pandemic exacerbated these issues, as disruptions or constriction to healthcare services and lockdown measures disproportionately impacted vulnerable populations, including those with mental illness [32].
The association between predisposing mental illness and postacute sequelae of COVID-19 may be derived from distinct characteristics of brain-body health in individuals with mental illness. A previous cross-sectional study mapped multisystem health profiles of four common neuropsychiatric disorders using a population-based cohort and suggested normative models of brain and physical function across the lifespan [33]. Findings revealed that individuals with mental illness not only exhibited deviations from normative brain phenotypes but also showed significantly poorer physical health across multiple systems compared with healthy controls [33]. Consistently low health scores were observed in metabolic, hepatic, and immune systems among those with mental illness. Poor metabolic health aligns with reports of increased risks for metabolic diseases such as diabetes, metabolic syndrome, and obesity in this population, partially attributed to antipsychotic side effects and chronic stress [33, 34]. Chronic psychological stress, commonly associated with mental illness, disrupts hypothalamic-pituitary-adrenal axis regulation, further impairing endocrine and metabolic systems [35]. In addition, physiological baseline vulnerabilities may be exacerbated by SARS-CoV-2 infection, increasing susceptibility to postacute sequelae of COVID-19 through triggered systemic inflammation and immune dysregulation [36].
The differential risks between individuals with mild and severe mental illness may be attributed, in part, to variations in healthcare engagement. Those with severe mental illness are more likely to receive regular medical supervision due to the complexity of their condition, including routine health evaluations and targeted care, even during the pandemic. Conversely, individuals with mild mental illness, who are less frequently engaged with healthcare systems, may face more significant neglect during healthcare disruptions, such as those experienced during the COVID-19 pandemic lockdowns [37]. This neglect can lead to inadequate management of both underlying conditions and emerging post-COVID complications, increasing their vulnerability. Consistent with our findings, these disparities emphasize how systematic gaps in treatment and oversight disproportionately increase the risks for individuals with mild mental illness, suggesting their increased susceptibility compared with those with severe disorders.
The association between predisposing mental illness and risks of postacute sequelae of COVID-19 was most pronounced within the first 6 months, followed by a time-dependent attenuation [38]. This pattern may be explained by the resolution of acute inflammation triggered by SARS-CoV-2 infection, as the pro-inflammatory cytokine surge (e.g., IL-6, TNF-α) diminishes over time, reducing systemic and neuroinflammatory responses [12, 39]. In addition, as pandemic-related disruptions subside, improved healthcare accessibility and management of both underlying conditions and emerging complications may contribute to the reduction in risks over time [40]. Behavioral and environmental factors, such as increased healthcare engagement and stabilization of care systems, likely play a significant role in mitigating long-term outcomes.
4.4 Strengths and Limitations
This study benefitted from a large sample size, minimizing selection bias and providing robust statistical power. By utilizing a nationwide population-based cohort, we found that predisposing mental illness were associated with an increased risk of postacute sequelae of COVID-19, with a time-dependent attenuation of effect size. These findings remained consistent after adjusting for potential confounders, including age, sex, and sociodemographic factors. In addition, the national health insurance system in South Korea, which covers 98% of the population, integrates all COVID-19-related records into a unified database; therefore, this system enabled the investigation of dynamic relationships between SARS-CoV-2 infection and ICD-coded health outcomes [41].
Despite its strengths, this study has several limitations. First, the findings, based on an Asian population, may have limited generalizability to other racial or ethnic groups due to variations in COVID-19 risk and response across races or ethnicities. Second, our health insurance claims database needed more data on certain socioeconomic status indicators, such as education level, occupational status, and pandemic-related stress, as well as other social determinants of health, potentially omitting key contextual factors. Third, missing data within the K-COV-N cohort posed challenges for causal inference, although the missing indicator method was applied to mitigate bias. Fourth, while our findings allow for causal inferences, precisely identifying specific causal relationships remains challenging. Fifth, there is a possibility of undiagnosed COVID-19 cases, including asymptomatic infections or individuals who did not undergo polymerase chain reaction testing. Finally, the observational design precludes definitive causal relationships, and the K-COV-N cohort, while substantial, only partially represents part of the South Korean population.
4.5 Policy Implications
Based on the findings of this cohort study, it is noticeable that predisposing mental illness had detrimental associations with increasing the risk of postacute sequelae of COVID-19 among COVID-19 survivors. Policymakers should prioritize addressing unmet healthcare needs and improving access to mental health services, particularly during pandemics and public health crises. Enhancing treatment accessibility and flexibility through remote care options, extended service hours, and improved operational systems can increase access to mental health services, reduce dropout rates, and improve attendance [37]. Telemedicine for psychiatric care offers a viable alternative to in-person services, providing effective treatment outcomes while overcoming barriers related to physical access. Enhancing healthcare systems to ensure continuity of care during crises is also critical. This includes guaranteeing uninterrupted access to essential mental health services via telemedicine, mobile clinics, and community-based resources, thereby mitigating the disproportionate impact of service disruptions on individuals with mental illness. By implementing these measures, policymakers can reduce healthcare inequities and improve outcomes for vulnerable populations with mental illness, particularly in times of crisis.
5 Conclusion
In this large-scale, population-based cohort study, predisposing mental illness in patients with COVID-19 were significantly associated with the 133 postacute sequelae of COVID-19 across multiple organ systems. The association was strongest within 6 months of SARS-CoV-2 infection and declined over time. Individuals with mild mental illness showed higher risks for postacute sequelae of COVID-19 compared with those with severe mental illness. Additional future research is needed to describe the underlying mechanisms and pathophysiology of clinical outcomes. These findings highlight the higher vulnerability of individuals with mental illness to postacute sequelae of COVID-19 and underscore the need for targeted monitoring and intervention strategies to support individuals with mental illness and postacute sequelae of COVID-19.
Author Contributions
Dr. Dong Keon Yon had full access to all of the data in the study and took responsibility for the integrity of the data and the accuracy of the data analysis. All authors approved the final version of the manuscript before submission. Study concept and design: Jiseung Kang, Jaeyu Park, Yejun Son, Hayeon Lee, and Dong Keon Yon. Acquisition, analysis, or interpretation of data: Jiseung Kang, Jaeyu Park, Yejun Son, Hayeon Lee, and Dong Keon Yon. Drafting of the manuscript: Jiseung Kang, Jaeyu Park, Yejun Son, Hayeon Lee, and Dong Keon Yon. Critical revision of the manuscript for important intellectual content: all authors. Statistical analysis: Jiseung Kang, Jaeyu Park, Yejun Son, Hayeon Lee, and Dong Keon Yon. Study supervision: Dong Keon Yon. Dong Keon Yon and Hayeon Lee supervised the study and served as guarantors. Jiseung Kang, Jaeyu Park, and Yejun Son contributed equally as the first authors. Masoud Rahmati, Hayeon Lee, and Dong Keon Yon contributed equally as corresponding authors. The corresponding author attests that all listed authors meet the authorship criteria and that no one meeting the criteria has been omitted.
Acknowledgments
This study was supported by the Institute of Information & Communications Technology Planning & Evaluation (IITP) grant funded by the Korea government (RS-2024-00509257, Global AI Frontier Lab) and the MSIT (Ministry of Science and ICT), Korea, under the ITRC (Information Technology Research Center) support program (IITP-2024-RS-2024-00438239) supervised by the IITP (Institute for Information & Communications Technology Planning & Evaluation). The funders had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Ethics Statement
It was approved by the Kyung Hee University Institutional Review Board (KHSIRB-23-241[EA]), the Korea Disease Control and Prevention Agency (KDCA), and the National Health Insurance Service (NHIS; KDCA-NHIS-2024-1-280).
Consent
In accordance with the approval conditions, anonymized administrative data were used, and informed consent from participants was waived.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data are available upon request. Study protocol and statistical codes: available from Dong Keon Yon ([email protected]). Dataset: available from the Korea Disease Control Agency (KDCA) through data use agreements.




