Influenza A Virus Induces Autophagosome by Inhibiting LTCC/Calpain 2/LC3A Signaling to Promote Viral Replication
Lu Tian and Xueer Liu contributed equally to this study. The author's order was determined based on their contribution.
ABSTRACT
l-type voltage-gated calcium channels (LTCC), which are accountable for the influx of extracellular Ca2+, have been discovered to play a crucial regulatory role in the process of autophagy. However, the regulatory role of LTCC in autophagy process induced by influenza A virus (IAV) infection remains largely unknown. Here, we found that IAV (H1N1/PR8) induced autophagosome accumulation consistent with previous studies but blocked the fusion of autophagosomes with lysosomes. Meanwhile, viral infection led to a persistent decline of the cytoplasmic calcium signal in A549 cells. Interestingly, activation of LTCC partially restored the cytoplasmic calcium signal, impeded the formation of autophagosomes, and hindered the replication of IAV. Conversely, hindering LTCC or suppressing Cav1.3, the primary isoform of LTCC in A549 cells, significantly enhanced autophagosome formation and IAV replication. Mechanistically, calpain 2, a calcium-dependent cysteine protease, mediated the inhibition of LTCC/Cav1.3 on autophagosome formation and IAV replication by cleaving the carboxyl-terminal (112-118aa) of Microtubule-associated protein 1 light chain 3A(MAP1LC3A). Our findings reveal that IAV infection inhibits the LTCC/Cav1.3-calpain 2-LC3A axis to induce autophagosome formation, contributing to better understanding of viral infection process and providing potential target for combating IAV infection.
1 Introduction
Influenza A virus (IAV) is one of the major pathogens causing respiratory infections in humans and often causes seasonal and global pandemic influenza, posing a great threat to human health and social security [1]. The prevention of IAV infection mainly relies on influenza vaccination to produce virus-specific neutralizing antibodies and induce the protective immune memory response, but lacking protection against sudden emerging virus strains. Therefore, there are limitations in relying on vaccines to prevent a novel influenza virus infection [1, 2]. Anti-influenza viral drugs are widely used to control influenza virus infection and include two main classes of M2 ion channel blockers (amantadine, rimantadine) and neuraminidase inhibitors (oseltamivir, paramivir). The use of M2 ion channel blockers can lead to serious side effects [3], and the current pandemic strains are generally resistant to alkylamines, which are no longer recommended. The neuraminidase inhibitor class of drugs is less toxic. With their widespread use today, IAVs are gradually becoming resistant to them [4]. As a result, we still lack effective therapeutic drugs in the face of new influenza outbreaks.
In recent years, there has been an increasing interest in anti-IAV strategies targeting the autophagy induced by IAV infection. Autophagy has two sides to viral pathogenesis. Whether autophagy promotes viral replication or acts as an antiviral depends on factors such as the virus, the type of infected cells, and the specific stage of infection [5]. On one hand, pathogenic autophagy (Xenophagy) acts as an intrinsic immune responses to clear intracellular pathogens [6]. On the other hand, some viruses, such as SARS-Cov2 and IAV, have evolved strategies to escape autophagic degradation, even hijacking autophagosomes to promote viral replication [7, 8]. IAV infection can trigger autophagy in various cells, enhancing autophagosome aggregation or autophagic flow [9]. H5N1 induces significant autophagic flow in human lung epithelial cells to help itself replicate, H9N2 has a weaker autophagy induction, while H1N1 induces only autophagosome aggregation and blocks autophagosome fusion with lysosomes [8, 10-12]. Whether complete autophagic flow or autophagic accumulation is induced, the essential prerequisite for replicating every subtype of IAV is the induction of autophagy formation.
Voltage-gated calcium channels (VGCCs) regulate the inward flow of extracellular Ca2+ (influx) induced by cell membrane depolarization. VGCCs are divided into two categories based on activation threshold: high-potential activated channels (including L-, P/Q-, N-, and R-type) and low-potential activated channels (T-type) [13]. l-type voltage-gated calcium channels (LTCC) (including Cav1.1, Cav1.2, Cav1.3, and Cav1.4 isoforms) regulates various physiological processes, including muscle contraction, protein secretion, gene expression, and synaptic transmission [14]. Recent studies have emphasized the importance of LTCC in the formation of autophagosomes and the control of various stages of autophagy [15, 16]. In a mouse model of obesity, the LTCC inhibitor verapamil blocks starvation-induced hepatocyte protein aggregation, restores lysosome degradation of the autophagosome, and extends the lifespan of C. elegans by activating autophagy [16, 17]. Williams et al. reported that LTCC antagonists (Verpamil and Nimodipine) induce autophagy in a non-mTOR-dependent manner, whereas LTCC agonizts (BAY K 8644) inhibit autophagy. In Drosophila and zebrafish models of Huntington's disease, the LTCC inhibitor (Verpamil) effectively alleviates Huntington's protein aggregation due to autophagy deficiency [18]. In addition, the induction of autophagy caused by inhibiting LTCC can reduce migration and invasion of ameloblastoma [19]. While whether LTCC is involved in the autophagy process caused by IAV infection has not been explored.
Calpains are a kind of Ca2+-dependent cysteine proteases [20]. Calpains require increased Ca2+ concentration to activate the limited proteolytic function [5] and have been identified as a downstream target of LTCC [18, 21]. The calpain system consists of 15 known isoforms, two small regulatory subunits 1 and 2, and an inhibitor calpastatin, of which μ-calpain 1 (CAPN1) and m-calpain 2 (CAPN2) are the most important and typical [20]. Each subtype of calpain exists in an inactive pre-enzymatic form in cellular solutes [22]. Calpains play a crucial role in various mechanisms of autophagy [23, 24]. Inhibition of calpains induces autophagy in neurodegenerative diseases, and activating calpains inhibits autophagy in fatty livers to ischemia-reperfusion injury [24, 25]. Calpains can cleave autophagy-associated proteins such as ATG5, ATG3, and ATG7 by reducing autophagy levels [25, 26]. However, whether the LTCC/calpains axis is involved in the autophagy process caused by IAV infection has not been demonstrated.
This study explored the role of the LTCC/calpains axis in the autophagosome caused by IAV (H1N1/PR8) and the underlying mechanism. We confirmed that PR8 induced autophagosome accumulation by inhibiting the LTCC (Cav1.3)/calpain 2 axis in pulmonary epithelial cells to promote viral replication. Under PR8 infection conditions, activating the Cav1.3/calpain 2 axis significantly inhibited autophagosome formation via cleaving the carboxyl-terminal of Microtubule-associated protein 1 light chain 3A (MAP1LC3A) and inhibited PR8 replication. Inhibiting the Cav1.3/calpain 2/MAP1LC3A signaling pathway may be a novel strategy for treating IAV.
2 Materials and Methods
2.1 Reagents
The sources of drugs used: BAY K 8644 (Selleckchem, S7924), Rapamycin (Merck, 553211), Bafilomycin A1 (Med Chem Express, HY-100558), 3-MA (Med Chem Express, HY-19312), Nimodipine (Med Chem Express, HY-B0265), MDL-28170 (Med Chem Express, HY-18236), DMSO (Sigma-Aldrich, D8418). The sources of antibodies used: LC3 (Bimake, A5202; 1:1000), H1N1 M1 (Sino Bio, 40010-T62; 1:2000), LTCC (Sigma-Aldrich; 1:1000), Alpha Fodrin (Bimake, A5964; 1:1000), calpain 1 (proteintech, 10538-1-AP; 1:1000), calpain 2 (Bimake, A5571; 1:1000), 6X His tag (GeneTex, GT359; 1:1000), β-Actin (Sigma-Aldrich, A1978; 1:5000).
2.2 Cell Culture and Viruses
Human pulmonary epithelial A549 cells (ATCC, CCL-185), Hela cells (ATCC, CCL-2), Human embryonic kidney cells (HEK293T; ATCC, CRL-11268) were maintained in Dulbecco's modified Eagle medium (DMEM, Gibco); Madin Darby canine kidney cells (MDCK; ATCC, CCL-34) were maintained in Minimal Essential Medium (MEM, Gibco). All media were supplemented with 10% fetal bovine serum (FBS, Gibco) and 100 U/mL penicillin-streptomycin (Gibco) at 37°C with a 5% CO2 incubator.
Cells were infected with the influenza A/Puerto Rico/8/34 (H1N1) virus supplied by Prof. Meilin Jin (Huazhong Agricultural University, China) at an MOI of 2. Cells were infected with viruses for 1 h, and then treated with drugs. The supernatants were collected for the plaque experiment. Cell lysates were collected for Western bolting.
2.3 Western Blotting Analysis
Cell lysates were extracted with RIPA (Beyotime, P0013B) and the protease inhibitor (Beyotime, ST506). The collected proteins were added with 5×SDS-PAGE (Beyotime, P0015L) and boiled for 10 min to denature. Then, the protein samples were separated by gel electrophoresis, transferred to a PVDF membrane (Life Sciences, 09609A), and sealed with 5% skim milk. The PVDF membrane was incubated overnight with the required primary antibodies at 4°C and the homologous secondary antibody at room temperature for 1.5 h the next day. The immune response bands were monitored with ECL Western blotting analysis substrate (Thermo Fisher Scientific, 32209).
2.4 Short Hairpin RNA Generation (shRNA)
The shRNA oligonucleotides targeting Cav1.3, calpain 1, calpain 2, and scrambled (scr) genes were synthesized by IGE Bio (Guangzhou, China). The shRNA sequences are reported in Supporting Information S1: Figure S1. The shRNAs were cloned into a psiRNA-h7SK-GFPzeo vector (InvivoGen, ksirna4-gz21) at the Acc65I/HindIII sites and transferred into cells using lipofectamine 2000 (Invitrogen, 2185347) according to the manufacturer's instructions. Zeocin (50 μg/mL, Beyotime, ST1450) was added to select cells with stably expressed plasmids.
2.5 sgRNA Generation
The plasmids of pMD2.G, pSPAX2, and lentiCRISPRv2 (Addgene, 98290) containing the gene-specific guide RNA (sgRNA) to target LC3A, and scrambled (scr) genes were transfected into 293 T cells using PEI (Polysciences, 24765) to package lentiviral productions. The target sequences are shown in Supporting Information S1: Figure S2. Stable A549 cell lines were screened with 3 μg/mL puromycin (Sangon Biotech, A606719). The single clones were isolated and confirmed by Western blotting analysis.
2.6 Overexpression Plasmids Construction
The GFP-calpain 2 coding sequence was amplified by PCR and inserted into the pCMV vector to generate pCMV-GFP-calpain 2 (pCMV empty vector as control). The primer sequences are reported in Supporting Information S1: Figure S1. 6His tag to the C/N terminal of LC3A coding sequences was amplified by PCR and inserted into the pCMV vector to construct pCMV-C-6His-LC3A, pCMV-N-6His-LC3A. The pCMV-Mut-6His-LC3A plasmid was generated by site mutagenesis PCR to delete the C-terminal cleavage site (112–118aa) from the pCMV-C-6His-LC3A vector. All plasmids were transfected into cells using liposome 2000 (Invitrogen, 2185347).
2.7 Calcium Imaging Experiment
For the calcium imaging experiments, the PN1-GCaMP6m-XC plasmid was constructed and transfected into A549 cells using liposome 2000 (Invitrogen, 2185347). Puromycin (3 μg/mL, Sangon Biotech, A606719) was added to select cells with stably expressed plasmids. The GCaMP6m fluorescence (F) was acquired at 488 nm excitation using the Zeiss LSM 880 laser scanning confocal microscope for 3500 s. The change of GCaMP6m fluorescence was normalized to baseline fluorescence (F0). The index of ΔF/F0 was calculated as the intracellular calcium signal influx (ΔF = F − F0).
2.8 Autophagic Flux Measurement
Hela cells were transfected with PN1-mRFP-GFP-LC3 plasmid using liposome 2000 (Invitrogen, 2185347) and selected with G418(Gibco, 11811031) resistance. After viral infection and the treatment of drugs, cells were fixed with 4% paraformaldehyde at room temperature for 15 min. The antifade mounting medium (Beyotime, P0123) prevents fluorescence quenching. Zeiss LSM 800 laser scanning confocal microscope was used to acquire the fluorescent images. Red fluorescence monitored autolysosomes, and yellow spots combining red with green fluorescence monitored autophagosomes.
2.9 Plaque Assay
MDCK cells were planted in 6-well plates with appropriate density and incubated with dilutions in a 10-fold gradient of the supernatants for 1 h. Each well was incubated with agarose (1%) and viral growth medium for 72 h. Then, cells were fixed with 4% paraformaldehyde and added with 0.1% crystal violet to observe plaques.
2.10 Transmission Electron Microscopy
The male-6-week-old SPF BALB/c mice (Charles River Laboratories, Barcelona, Spain) weighing 17–21 g were used for the experiments. All mouse studies were approved by the Shantou University Medical College for Animal Care and Use Committee. The mice were divided into a control group and a virus-infected group. The mice were intranasally infected with the PR8 virus at 100 PFU/mouse or 50 μL of PBS buffer as a control. Mouse fresh lung tissue was taken at 3 days after viral infection and fixed with 2.5% glutaraldehyde for 2 h at 4°C. Following deprivation in different concentrations of ethanol solution, samples were embedded in Epon-Araldite resin and counterstained with 3% uranyl acetate and 2.7% lead citrate. The HT7800 transmission electron microscope was used to observe the formation of autophagosomes in type II lung epithelial cells.
2.11 Statistical Analysis
The results are expressed as Mean ± SD in at least three independent experiments. Densitometry quantification was performed with ImageJ Software. Data were analyzed using GraphPad Prism 9.0 software with one-way ANOVA or t-test. p < 0.05 was considered statistically significant as indicated: *p < 0.05, **p < 0.01, ***p < 0.001, ns: no statistical significance.
3 Results
3.1 PR8 Induces Autophagosome Accumulation in A549 Cells
We first determined the autophagy levels induced in the human lung epithelial cell line A549, infected with PR8 (Figure 1A,B). PR8 induced a significant turnover from LC3-I to LC3-II compared to the control group. Bafilomycin A1 (Baf A1) is a reversible V-ATPase inhibitor that blocks autophagosome fusion with lysosomes, leading to the impairment of autophagic flux [26, 27]. As shown in the results, the level of LC3-II increased in the treatment with Baf A1. However, the relative of LC3-II protein level was no difference between PR8-infected cells and cells co-treated with Baf A1 and PR8. p62/SQSTM1, served as a substrate for autophagic degradation, interacts with LC3 through the LC3-interacting region domain to promote the formation of autophagosomes, which are ultimately degraded in autolysosomes [28]. p62/SQSTM1 level is inversely proportional to autophagic activity, therefore we also detected its level to evaluate autophagy. The data showed that the expression of p62/SQSTM1 increased in PR8-infected cells same as treated with Baf A1 cells (Figure 1C,D). It suggests the possibility that PR8 infection inhibites the fusion of autophagosomes with lysosomes and promotes the accumulation of autophagosomes as Baf A1 does.
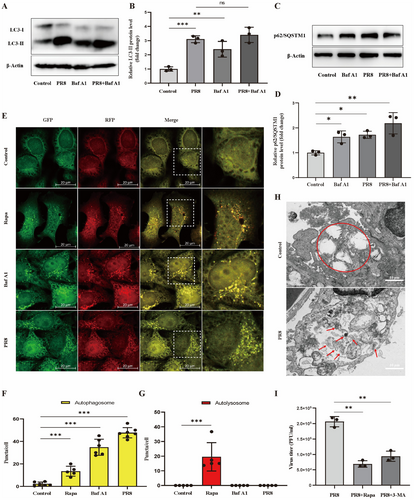
Meanwhile, we constructed a Hela cell line stably expressing a tandem reporter mRFP-GFP-LC3. Green fluorescent protein (GFP) is sensitized and attenuated by a lysosomal acidic environment, while red fluorescent protein (mCherry, mRFP) is insensitive to the low pH lysosomal environment. Therefore, autolysosomes only act as punctate bodies with red fluorescence; autophagosomes are shown as yellow spots by combining red and green fluorescence [11, 27, 29]. As shown in Figure 1E-G, The average number of yellow puncta in PR8-infected A549 cells was remarkably higher than that in the uninfected cells (34.77 ± 2.19 per cell vs. 2.21 ± 1.64 per cell, p < 0.001). These results indicated that PR8 infection mainly induces autophagosome accumulation but not a whole autolysosome flux, as shown in the Rapamycin group stand for a whole autolysosome flux. Then, we infected type II lung epithelial cells with PR8, finding no obvious autophagy in the alveolar epithelial cells of normal control mice. In PR8-infected mice with alveolar epithelial cells, multiple autophagosomes were shown by red arrows, and autophagic aggregation was represented (Figure 1H). This result indicated that PR8 infection of type II lung epithelial cells also induced autophagosome formation. Rapamycin (Rapa) is the most used positive control to induce intact autolysosome flow by simulating starvation. However, 3-MA inhibits PI3P through VPS34 and ultimately inhibits autophagy formation [11, 26, 27]. When PR8 was incubated with Rapa or 3-MA, both RAPA or 3-MA decreased the titer of PR8 compared with PR8 alone (Figure 1I), indicating that PR8 replication was inhibited by inhibiting autophagosomes. Figure 1 indicated that PR8 infection-induced autophagosome formation inhibits autolysosome turnover. Meanwhile, PR8 usedautophagosomes to promote its replication.
3.2 PR8 Infection Inhibits Intracellular Calcium Signal
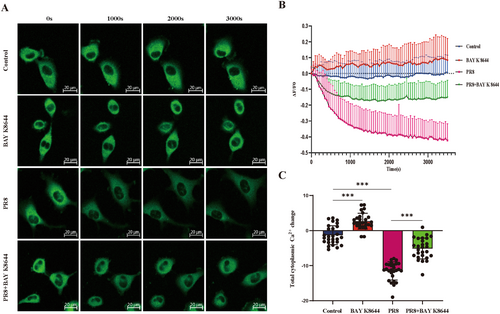
Many studies have emphasized the importance of calcium homeostasis disturbance during autophagy formation, and IAV infection induces oscillations in cytoplasmic Ca2+ in host cells, which significantly attenuates viral internalization and infection [30]. The primary event of influenza virus infection is the attachment of viral granules to the cell host surface, which is mediated by hemagglutinin (HA) binding to salivary cell surface receptors on human and avian respiratory or intestinal epithelial cells, respectively. Yoichiro Fujioka et al. demonstrated that HA binds to voltage-dependent calcium channels, triggering intracellular calcium oscillation and subsequent IAV entry and replication. Calcium channel blockers or Cav1.2 knockdowns can inhibit IAV entry, Calcium channel blockers also inhibit viral replication in the body. They found that Cav1.2 is a salivated host cell surface receptor that binds to HA and is critical for IAV entry into cells [31]. Thus, we determined the intracellular calcium signal in PR8-infected A549 cells by calcium sensor GCaMP6m-Xc during 1 h postinfection. GCaMP6m-Xc is a novel genetically-encoded cytosol Ca2+ indicator incorporating an extra apoCaM-binding motif to protect LTCC-dependent excitation-transcription coupling from perturbations effectively [32]. As shown in Figure 2, compared with Mock groups, PR8 infection induced a significant decrease in intracellular calcium signal than Mock groups. However, An LTCC agonist, BAY K 8644, significantly increased the calcium signal in normal A549 cells and partially alleviated the decrease of intracellular calcium signal caused by PR8-infection. These results suggested that PR8 infection decreases intracellular calcium signal, possibly attributed to decreased Ca2+ influx mediated by LTCC.
3.3 Knockdown of Cav1.3 Promotes Autophagosome Formation and PR8 Replication
LTCCs have been reported to regulate the induction of autophagy [14, 33]. We consider whether LTCC is involved in the autophagosome process caused by PR8 infection. Firstly, we used an agonist of LTCC, BAY K 8644, to induce upregulation of intracellular calcium signal [33]. Consistent with previous results (Figure 2). PR8 infection increased LC3-II levels in A549 cells. BAY K 8644 did not reduce LC3-II levels in normal cells but significantly reduced the upregulation of LC3-II levels and PR8-M1 levels in infectious cells (Figure 3A–C). M1 protein, the most abundant viral protein, connects the viral envelope and the viral structural shell of the nucleocapsid. During viral replication, it is involved in shuttling the viral ribonucleoprotein (vRNP) complex between the nucleus and cytoplasm. The M1 protein provides the structure of the virion, which is used for viral assembly and budding. Therefore, the M1 protein is commonly used to detect influenza viruses [34]. Consistently, in the stable transfected Hela cells with the mRFP-GFP-LC3 fusion protein, the scattered yellow spots indicated autophagosome formation but not autolysosomes in PR8 infectious cells. While the LTCC agonist of BAY K 8644 significantly reduced the autophagosome accumulation induced by PR8 infection (Figure 3D,E). The result indicated that LTCC agonist inhibited autophagosome formation in human pulmonary epithelial cells induced by PR8 infection.
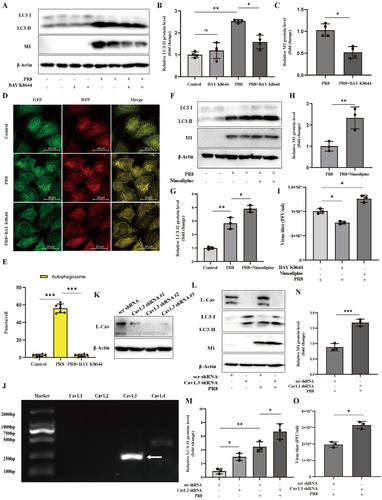
Meanwhile, we further characterized the effect of LTCC on autophagosome using a classic LTCC antagonist, nimodipine, which significantly reduces excessive calcium influx in pathological conditions [33]. As shown in Figure 3F–H, nimodipine upregulated the protein level of LC3-II and PR8-M1 levels compared to PR8 infection alone. The plaque formation assay further suggested that LTCC regulates viral replication (Figure 3I). Thus, LTCC was involved in the autophagosome process caused by IAV infection. Activating LTCC inhibited the formation of autophagosome and PR8 replication, and inhibiting LTCC promoted autophagosome accumulation and PR8 replication in pulmonary epithelial cells.
Next, we further explored which isoforms of LTCC are responsible for these effects. LTCC includes Cav1.1, Cav1.2, Cav1.3, and Cav1.4 isoforms, and Cav1.3 isoforms were mainly expressed in A549 cells, as shown in Figure 3J. We constructed a Cav1.3-KnockDown A549 cells line and corresponding negative control cell line by stably transfecting Cav1.3 shRNA or scrambled shRNA (Figure 3K). Consistently with the effect of LTCC antagonist, knocking down Cav1.3 led to upregulating LC3-II levels and PR8-M1 levels in A549 cells infected with PR8 (Figure 3L–N). Moreover, the lack of Cav1.3 further promoted PR8 replication in A549 cells, as tested by plaque formation assay (Figure 3O). These data indicated that blocking functional LTCC or inhibiting LTCC/Cav1.3 expression both promoted autophagosome accumulation and IAV replication in pulmonary epithelial cells.
3.4 Calpain 2 Mediates the Effect of LTCC/Cav1.3 on Autophagosome and PR8 Replication
Calpains are the downstream target of LTCC and play a crucial role in autophagy [18, 23, 24]. Subsequently, we asked whether calpains are the downstream of LTCC regulating autophagosome in PR8 infected cells. The increased intracellular Ca2+ concentration can activate calpains, which execute a limited proteolytic function [21]. The protein cleavage activity of calpains can be examined by detecting the cleavage of a known calpains substrate, alpha-fodrin, in which the complete natural alpha-fodrin protein (285 kD) is cleaved to produce a small protein fragment (150 kD) [22, 26]. We first examined the cleavage activity of calpain for alpha-fodrin in PR8-infected A549 cells. As shown in Figure 4A–E, calpains had a high cleavage activity for alpha-fodrin in infected A549 cells, while calpain inhibitor MDL-28170 (10 μM) and PR8 infection both significantly decreased the level of cleaved alpha-fodrin protein (150 kD) and increased the level of LC3-II in A549 cells. Meanwhile, compared with the PR8 infection alone, the coexistence of the LTCC agonist BAY K 8644 with PR8 recovered cleaved alpha-fodrin protein (150 kD) and decreased LC3-II and PR8-M1 protein levels. Interestingly, adding the calpain inhibitor (MDL-28170) could abolish the rescue effect of BAY K 8644 on cleaved alpha-fodrin (150 kD), LC3-II, and PR8-M1 in PR8 infectious cells. However, the full-length natural alpha-fodrin protein (285 kD) level had no significant change in the five treatment groups. These data indicated that calpains were downstream of LTCC and involved the regulation of LTCC/Cav1.3 on autophagosome formation and IAV replication.
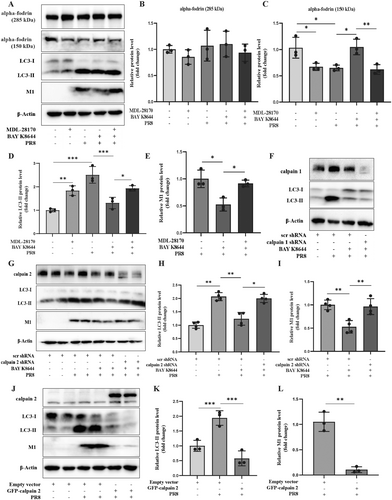
Calpain 1 (u-calpain) and calpain 2 (m-calpain) are widely expressed in most cells [21-23]. Calpain 1 and calpain 2 are the 80 kD catalytic subunits that can form a heterodimer with a 30 kD regulatory subunit calpain 4 to perform limited protein cleavage functions [22, 26]. In the presence of PR8 and BAY K 8644, knocking down calpain 2 but not calpain 1 significantly upregulated LC3-II and PR8-M1 protein levels (Figure 4F–I). Meanwhile, in the presence of PR8 and BAY K 8644, over-expressing GFP-calpain 2 inhibited LC3-II and PR8-M1 protein levels (Figure 4J–L). Therefore, as the downstream of LTCC/Cav1.3, calpain 2 mediated the effect of LTCC/Cav1.3 on autophagosome formation and IAV replication.
3.5 Calpain 2 Inhibits Autophagosome Formation and PR8 Replication by Cleaving LC3A
A previous study has reported that LC3 is required to subvert autophagy and maintain virion stability by interacting with the M2 protein of IAV [35]. Our results showed that over-expressing GFP-calpain 2 significantly inhibited PR8 replication and decreased LC3-II (Figure 4J–L). These results indicated a possibility that LC3 is a potential substrate for calpain 2 to regulate autophagosome formation and virus replication. Autophgay-associated members of the human MAP1LC3/LC3 family include LC3A, LC3B, and LC3C [36, 37], among which LC3A is the main isoform protein expressed in A549 cells. First, we verified the role of LC3A in PR8 replication using a strain of LC3A knockout A549 cell line built by the CRISPR/Cas9 system. Compared with the negative control group, the absence of LC3A significantly inhibited the PR8-M1 protein level and PR8 titer (Figure 5A–C).
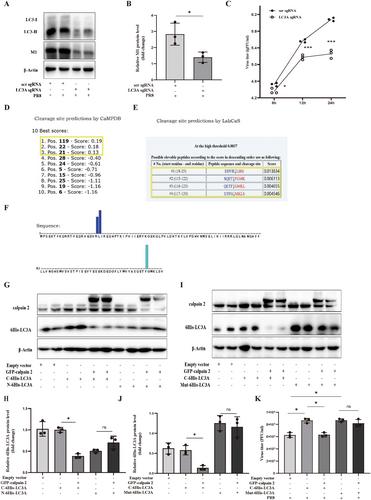
In addition, the potential cleavage sites of calpains in the LC3A protein sequence were predicted using two online software GPS-CCD and LabCas [38, 39]. There were two most likely cleavage sites at the N-terminal (19-24aa) and C-terminal (112-118aa) of LC3A (Figure 5D–F). Subsequently, we constructed two wild-type LC3A expressing vectors, including pCMV-6His-LC3A (adding the 6His tag to the N terminal of LC3A) and pCMV-LC3A-6His (adding the 6His tag to the C terminal of LC3A) to examine which site of LC3A was cleaved by calpain 2. As shown in Figure 5G,H, EGFP-calpain 2 significantly decreased the full-length LC3A-6His but did not change the full-length 6His-LC3A in 293 T cells. Finally, we constructed a mutant of the LC3A with deletion C-terminal cleavage site (112–118aa), the Mut-6His-LC3A. Compared with the cleavage effect of calpain 2 on LC3A-6His, EGFP-calpain 2 did not decrease the protein level of full-length Mut-6His-LC3A (Figure 5I,J). Meanwhile, over-expressing EGFP-calpain 2 significantly reversed the potentiation of LC3A-6His on PR8 titer but did not abolish the potentiation of Mut-6His-LC3A on PR8 titer (Figure 5K). Therefore, all these results suggested that calpain 2 cleaved the C-terminus (112–118aa) of LC3A to inhibit autophagosome formation and IAV replication.
4 Discussion
This study demonstrated that LTCCs were involved in the autophagosome induced by the IAV (H1N1/PR8). Consistent with previous studies, PR8 infection induced autophagosome accumulation but blocked the fusion between autophagosomes and lysosomes. Meanwhile, infection with IAV led to a persistent decline of the cytoplasmic calcium signal in human pulmonary epithelial cells. Activation of LTCC has been found to partially restore the decline in cytoplasmic calcium signal, impede autophagosome formation, and hinder the replication of IAV. Conversely, hindering LTCC or suppressing Cav1.3, the primary isoform of LTCC in A549 cells, significantly enhanced autophagosome formation and IAV replication. Mechanistically, calpain 2, a calcium-dependent cysteine protease, mediated the inhibition of LTCC/Cav1.3 on autophagosome formation and IAV replication by cleaving the carboxyl-terminal (112-118aa) of MAP1LC3A. Our findings proved that the LTCC/Cav1.3-calpain 2-LC3A axis was a novel signal axis for regulating autophagosome caused by IAV infection (Figure 6). Targeted the activation of the LTCC(Cav1.3)/calpain 2/LC3A signal axis provided a novel strategy for anti-IAV infection by inhibiting host autophagosome.
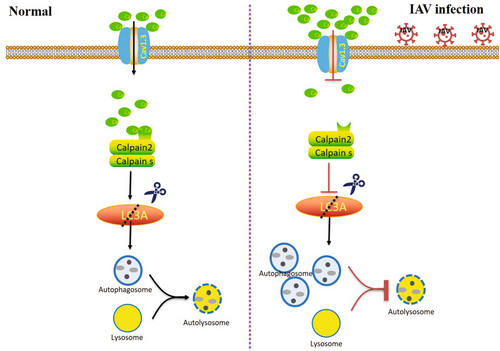
The autophagy process serves as a pro-survival mechanism by selectively breaking down specific cellular components, recycling macromolecules, eliminating damaged organelles and invasive pathogens, thereby facilitating cellular adaptation to adverse conditions such as starvation, injury, stress, infection, and maintaining optimal energy levels and anabolism. The phenomenon of pathogenic autophagy, also known as Xenophagy, serves as an innate immune response to eliminate intracellular pathogens [6]. Nevertheless, certain viruses have developed mechanisms to avoid autophagic disintegration, and in some instances, they even appropriate autophagosomes to facilitate their replication [10, 40, 41]. This behavior is observed in viruses such as IAV and SARS-Cov2. Multiple studies have proved that IAV initiates autophagy in various cell types, promoting its replication. H5N1 virus has been shown to induce a complete autophagy process, while H9N2, H3N2, and H1N1 viruses lead to the accumulation of autophagosomes and hinder autophagosome turnover [8]. In line with prior research, our study revealed that PR8 (H1N1) triggered autophagosome accumulation while impeding their degradation. Other studies have also demonstrated that M2 is involved in initiating the formation of autophagosomes in infected cells through inducing reactive oxygen species (ROS) production [29]. M2 interacts directly with autophagy protein LC3 and promotes LC3 relocalization to the unexpected destination of the plasma membrane. M2 reduces fusion of autophagosomes with lysosomes and insteads traffic to the plasma membrane where it supports IAV budding [35, 42]. Numerous studies have established that curtailing the expression of autophagy-related proteins or impeding the autophagic process through inhibitors can effectively hinder IAV replication [8].
Meanwhile, our results demonstrated the hindrance of PR8 replication after impeding autophagosome formation using 3-MA. It can be inferred that the formation of autophagosome induced by IAV, whether it results in unobstructed autophagic flow or accumulation of autophagosome, is an essential process for replicating all subtypes of IAV. As such, a universal therapeutic approach against IAV may involve targeting infection-triggered autophagosome formation. Interestingly, rapamycin, an inducer of autophagic flow by inhibiting mTOR, also significantly inhibited PR8 replication, which is consistent with a recent work reported by B. Yeganeh et al., in which rapamycin remarkedly increased the expression of viral protein but decreased the replication and release of PR8 virus [43], which may be attributed to the fact that during PR8 infection, rapamycin induces an intact autophagic flow, thus promoting the degradation of intracellular viral components via the autophagy-lysosomes pathway.
LTCC, accountable for the influx of extracellular Ca2+, has been discovered to play a crucial role in regulating numerous physiological processes, such as muscle contraction, protein secretion, gene expression, and synaptic transmission [14, 33]. Recent research has suggested that LTCCs are pivotal in entering IAV into cells during the initial stages of IAV infection, in which a sialylated Cav1.2, a subtype of LTCC, mediates the IAV internalization by binding with the viral hemagglutinin protein [31]. Beyond that, whether LTCC is involved in other processes in IAV infection remains unknown. In accordance with our previous observations on calcium sensor GCaMP6m-Xc, we found a significant reduction in cytosolic Ca2+ levels at early stages during IAV infection. The mechanism for this reduction might be related to hemagglutinin protein(HA). The initial step in IAV infection involves the virus entering the target cells of the respiratory tract. This critical process encompasses several stages: the attachment of the virus to the cell surface, the internalization of IAV into endosomes, and the subsequent fusion of the viral envelope with the host's endosomal membrane. These intricate steps are primarily orchestrated by the viral HA, which plays a pivotal role in mediating the virus's attachment to the cell by binding to sialic acid residues on the cell surface. Furthermore, HA interacts with LTCCs in a sialylation-dependent manner, and LTCC plays a crucial role in the entry of IAV into target cells of the respiratory tract. Additionally, changes in intracellular calcium ion levels can modulate the entry and infection efficiency of IAV [31, 44]. A significant limitation of our study is the lack of dedicated investigation into how IAV modulates the downregulation of Cav1.3. It is worth noting that LTCCs play a crucial role in regulating phagophore expansion [15, 16]. They transmit inward Ca2+ current and trigger Ca2+ release from the sarcoplasmic reticulum/endoplasmic reticulum through calpain-mediated cAMP production and subsequent Ins(1,4,5)P3/InsP3-mediated Ca2+ release, creating a Ca2+-positive feedback loop [15, 16]. The constitutive activation of calpain can impede autophagy by cleaving ATG5 in an unknown manner [45]. Autophagy induced by LTCC antagonists such as verapamil, loperamide, and nimodipine is contingent on activating the Ca2+-calpain pathway in neuroblastoma cells. Conversely, LTCC agonizts like BAY K 8644 have the opposite effect. These prior investigations suggest that excessive LTCC activation may hinder autophagosome formation by promoting calpain-mediated ATG5 cleavage in a Ca2+-dependent fashion.
In our research, we have confirmed the impact of LTCC on autophagy in the context of IAV infection. Additionally, we have presented novel findings that the LTCC-calpain signal axis, specifically Cav1.3-calpain 2, regulated autophagosome formation induced by IAV infection. Our results consistently demonstrated that inhibiting LTCC using nimodipine or knocking down Cav1.3, the primary subtype of LTCC in A549 cells, significantly enhanced autophagosome formation and IAV replication. Conversely, activating LTCC using BAY K 8644 inhibited autophagosome formation by activating calpain 2, suppressing IAV replication. Furthermore, our findings provided insight into the biological significance of the reduced LTCC current observed in other studies following IAV infection [46]. As such, LTCC represents a potential therapeutic target, and LTCC agonizts may serve as promising lead compounds for developing novel anti-IAV infection therapies.
Calpains are host cysteine proteases dependent on Ca2+ and act as negative regulators of autophagic flow by degrading proteins essential for phagophore initiation and nucleation [47]. In neurodegenerative diseases, the upregulation of intracellular Ca2+ via LTCC promotes the cleavage and activation of Gsa by calpains, which increases cAMP levels and inhibits the clearance of intracellular aggregate-prone proteins by autophagic processes, leading to diseases [18]. Our results indicated that the true leading role responsible for calpain-mediated autophagosome and H1N1 (PR8) replication inhibition was MAP1LC3A (LC3A). LC3A, the main isoform of LC3 in human alveolar epithelial A549 cells, is a key protein responsible for autophagosome expansion and maturation and a widely used marker for autophagosome. Ectopic expression of EGFP-calpain 2 fusion protein significantly inhibited full-length LC3A and PR8 replication protein levels, suggesting that LC3A may be cleaved by calpain 2. Owing to their modulatory nature and lack of clear cleavage sequence specificity, calpains appear to have less precise activity and are less amenable to systematic examination than proteases such as caspases and those involved in the ubiquitin-proteasome system [48]. Firstly, we confirmed that MAPLC3A was a novel potential substrate of calpain 2 by two predictive software and experimental verification. Subsequently, we further identified that the cleavage site of calpain 2 was the carboxy terminus of LC3A (112-118aa). Ectopic expression of wild-type LC3A (C-6His-LC3A) and LC3A mutants with deleted potential cleavage sites (Mut-6His-LC3A) promoted PR8 replication. Moreover, ectopic expression of calpain 2 counteracted the PR8 replication enhancement induced by wild-type LC3A but did not alter the enhancement induced by the mutant of LC3A. Mechanistically, the cleavage of LC3A by calpain 2 may disrupt the function of LC3A in subverting autophagosomes and maintaining virion stability by interacting with the M2 protein of IAV, thereby inhibiting IAV replication [35]. Although many studies have tried to dissect the regulatory roles and molecular mechanisms of calpain-dependent cleavage, in fact our understanding of calpain is still fragmentary. There is a limitation that we did not provide experimental data to confirm how calpain 2 recognizes the special C- terminal site of LC3A (112-118aa) and mediates substrate proteolysis. Further investigation is required to elucidate the underlying processes.
In summary, we have identified a new signaling pathway involving LTCC(Cav1.3)/calpain 2/LC3A that regulated autophagosome formation during IAV infection. This finding enhances our understanding of the autophagosome process triggered by IAV infection. IAV infection significantly suppressed the LTCC-mediated intracytoplasmic calcium signal in human alveolar epithelial cells, inhibiting the cleavage of LC3A by activating calpain 2. It should be emphasized that the regulation of autophagosome by LTCC-Capain2-LC3A is mainly on the cleavage of LC3A protein rather than affecting the lipidation of LC3A. In the IAV infection context, LC3A was involved in the LTCC/calpains axis-mediated autophagosome formation, and LC3A was essential for H1N1 (PR8) replication. Therefore, activating the LTCC(Cav1.3)/calpain 2/LC3A signaling pathway could be a promising strategy for combating IAV.
Acknowledgments
This study was supported by the Natural Science Foundation of Guangdong Province (2022A1515012144, 2023A1515012055) and Shantou University Medical College Scientific Research Initiation Grant (510858047).
The authors thank Prof. Xiaodong Liu, Tsinghua University School of Medicine, for providing the PN1-GCaMP6m-XC plasmid.
Open Research
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.




