Serological Response to Mpox and Direct Virus Detection in Asymptomatic Patient Prior to the First Diagnosed Case: A Retrospective Study of the 2022 Montpellier Epidemic
ABSTRACT
The Mpox (formerly monkeypox) outbreak in 2022 presented unprecedented challenges, including widespread transmission in non-endemic regions, particularly among men who have sex with men. This study examines Mpox infections in Montpellier, France, focusing on diagnostic testing, serological profiles, and potential asymptomatic cases, based on data from Montpellier University Hospital. We retrospectively analyzed results from 91 patients tested positive for Mpox DNA. Antibody responses were monitored using novel Mpox virus peptide-based serological assays. Our findings highlight that MPXV antibodies can develop early in infection, peaking within 2 weeks to 3 months, though responses varied by antigen type. Additionally, asymptomatic Mpox infections were suggested, with virus detected in blood, urine, anal, oral and genital swabs screened Sexual Transmitted Infection samples. Notably, viral presence was confirmed in Montpellier samples as early as mid-May 2022, before the first known symptomatic case in Hospital, in the same period as the first official case in France. This study underscores the need for expanded screening in high-risk clinics to control Mpox spread and supports the potential utility of serological assays for broad immune profiling. Enhanced diagnostic tools and proactive surveillance in sexual health settings are recommended to improve outbreak response and prevent further transmission.
1 Introduction
The emergence of Mpox (formerly known as monkeypox) as a globally significant public health threat in 2022 prompted a rapid response from health authorities worldwide [1]. The disease has historically been associated with endemic regions in Africa, mostly the Democratic Republic of the Congo (DRC) for clade I and West Africa such as Nigeria for clade II with more moderate cases [2]. Mpox virus (MPXV) transmitted by contact with infected animals such as rodents, or human-to-human transmission through close contact and direct skin exposure to symptomatic patient. The 2022 outbreak exhibited unprecedented characteristics [3], including widespread transmission in non-endemic countries primarily affecting men who have sex with men with many presenting symptoms mainly localized to the anogenital region [4]. Recently, a new clade I virus emerged in DRC with exacerbate number of infections in the country and evidence of supportive sexual transmissions [5]. This new MPXV poses the threat of a new epidemic worldwide, with potentially more serious complication than the clade IIb 2022 outbreak.
According to World Health Organization (WHO) report, there have been 106,310 confirmed cases of Mpox globally from the beginning of 2022 to August 31, 2024 [6]. France was the second European country most affected by the 2022 Mpox outbreak [7], with Occitanie region emerging as a secondary epicenter after Ile-de-France [8]. 4,975 cases were reported in 2022 in the country [9], 48 cases in 2023 [10], and 167 cases between January 1 and September 24, 2024 [11].
With first case reported at the beginning of May 2022, MPXV infections in France increased until a significant peak in the middle of the summer. Health authorities then urged large-scale vaccination with 3rd-generation vaccines initially designed to combat smallpox (derived from vaccinia virus conferring cross-immunity against Orthopoxviridae) to halt the epidemic [12]. The epidemic followed a similar pattern to other affected countries, with a rapid initial rise in cases followed by a gradual decline. However, the persistence of sporadic cases and occasional clusters highlights the ongoing challenges in controlling the disease [13].
Some patients exhibit only minimal signs or may even be asymptomatic, making detection and diagnosis more challenging. Previous studies have predominantly focused on symptomatic Mpox cases, which may not fully capture the extent of the disease [14, 15]. A growing body of evidence suggests that asymptomatic or minimally symptomatic Mpox cases could be underestimated [16-23]. A proportion of infections may go undiagnosed, contributing to the continued spread of the disease. Moreover, classic clinical picture may also be altered by concomitant presence of multiple sexually transmited infections (STI) which further complicate diagnosis [24, 25]. On the other hand, symptoms may be misattributed to other viral infections, such as herpes simplex virus (HSV) or varicella-zoster virus (VZV) infections. A better understanding of the markers of MPXV infection, especially in asymptomatic, paucisymptomatic or coinfected individuals, could be useful to adapt testing strategies and control the Mpox epidemic. The kinetics of diagnostic markers remain incompletely understood, although viral DNA has been detected in saliva, semen, and anogenital samples [26, 27].
An urgent need to develop rapid and reliable diagnostic tools has arisen as a result of these threats. Molecular diagnostic for Orthopoxviridae and specific MPXV have long been available [28] but the evolution of the virus requires new adaptations [29]. Investigating the detection of MPXV DNA in blood and the serological response according to the time since symptom onset is crucial. A few of serological tests have been developed over the last 2 years [30-34]. These tests, which detect antibodies specific to the Mpox virus, make it possible to confirm past infections, even in the absence of symptoms, and to assess the immunity acquired following vaccination or natural infection. Their sensitivity and specificity vary according to the technological platforms used (ELISA, immuno-fluorescence, etc.) and the viral antigens targeted. Thanks to this, anti-MPXV antibodies have also been detected in people with no known history of the disease [35, 36], suggesting that seroprevalence surveys could be of interest to investigate the asymptomatic burden of cases.
In this study, we report data from cases diagnosed and managed in a French referral hospital, focusing on MPXV molecular test results in blood, urine, and genital swabs, as well as the serological profile. The second part of the study investigates the presence of asymptomatic cases at the onset of the epidemic among individuals consulting at a sexual health clinic.
2 Methods
2.1 Patients and Samples Collection
We retrospectively analyzed results from all patients tested for Mpox DNA for a suspicion of MPXV infection care at Montpellier University Hospital, in the Infectious and Tropical Diseases department (ITD). These patients presented with cutaneous-mucosal vesicular eruptions suggestive of MPXV infection, possibly associated with fever and pain. Biological diagnosis was performed on a swab of skin or mucosal lesions, and serum by real time PCR Moreover, subjects without any suspicion of Mpox were included as a control group: patients who consulted for access to MPXV vaccination, patients with a negative MPXV PCR assay, and samples from 2021 before the emergence of MPXV in Europe.
In the second part of the study, we analyzed genital samples collected for STI diagnosis from patients consulting at a sexual health and HIV testing center (CeGIDD), as well as HIV-1-infected outpatients from the ITD department. Indications for sampling were either for diagnostic in cases of symptoms consistent with gonorrhea or chlamydia, or systematic screening in person living with HIV or MSM using HIV pre-exposure prophylaxis. In the sexual health and HIV testing center, screening could also have been performed in a prevention context among young adults. Oral, intra-urethral, anorectal, vaginal swabs, and urinary first-jet samples, were collected between March and June 2022 in cobas PCR Cell Collection tubes and stored at 4°C until analysis (Roche, Switzerland).
2.2 MPXV Molecular Test
Nucleic acid extraction was carried out on the EZ1 Advanced XL extractor using the QIAmp DSP virus kit (Qiagen, Germany) for lesion swabs (biological diagnostic of symptomatic patient) and blood samples. For bacterial STI screening samples, we used high throughput extractor MGISP-960 with MGI Automatic Virus DNA/RNA Extraction kit (MGI Tech, China).
PCR was performed on the QuantStudio 5 Real-Time PCR System (Thermo Fisher Scientific, USA) using a specific MPXV assay [28] and an orthopoxvirus PCR assay [37] validated by both the French Orthopoxvirus national reference center (Institut de Recherche Biologique des Armées IRBA, Bretigny-sur-Orge, France) and Centers for Disease Control and Prevention. The test was considered positive for the presence of MPXV if both PCRs led to amplification to avoid nonspecific amplicons, otherwise the test was considered negative. For biologically diagnosed patient, a multiplex PCR assay detecting HSV-1, HSV-2 and VZV DNA was performed simultaneously using RealStar alpha Herpes virus PCR kit (Altona Diagnostics, Germany).
2.3 Serology Assay
A micro-bead multiplex assays based on synthetic peptides from Right terminal region B of the MPXV (B21R.179/180, B21R.185/186 and B22R.64/65) [30] were used to evaluate antibody reactivity, as previously described [38]. Another MPXV peptide, H3L, known as a strong immunogenic surface protein highly conserved in the Poxviridae family was also included [34, 39].
Briefly, peptides were conjugated to bovine serum albumin, then covalently coupled on fluorescent magnetic beads (Luminex Corp, USA) and stored in storage buffer (Bio-Rad, France) at 4°C in the dark. The bead mixture containing Phosphate Buffered Saline (PBS) with 0.75 mol/NaCl, 5% heat-inactivated fetal bovine serum (Gibco-Invitrogen, France), 1% bovine serum albumin and 0.2% Tween-20 (Sigma-Aldrich, France) was added to each well of the 96-well flat-bottom chimney plates (Greiner bio one, Germany). Blood samples were diluted at 1/200 then incubated in the plate, after washing biotin-labeled anti-human IgG (BD-Pharmingen, France) was added. Finally, streptavidin-R-phycoerythrin (Fisher Scientific/Life Technologies, France) was added before antigen-antibody reaction was read by the Magpix equipment (Bio-Rad, France).
Median Fluorescence Intensity (MFI) per 100 beads was measured for all samples. For MPXV specific peptides (B21R.179/180, B21R.185/186 and B22R.64/65) a panel of 89 Mpox negative samples was also tested which allow to set a cut-off value for each peptide calculated as the mean value plus three standard deviations. As vaccination status was not available for this panel, cut-off value for H3L antigen was determined using the likelihood ratio from the Receiver Operating Characteristic (ROC) curve (Supporting Information S1: Figure S1). The results were expressed as index by dividing the MFI values by the associated cut-off for each peptide (an index greater than 1 indicates a positive test). Since reactivity to only one MPXV peptide is enough to conclude that the test is positive [30], we decided to consider the maximum index value between the three antigenic MPXV peptides for each samples. H3L indexes were analyzed separately due to cross-reactivity with vaccine antibodies.
2.4 Statistical Analysis
We used GraphPad Prism 9.0.0 software (Graphpad Prism, USA) to perform statistical analysis and ROC curve. Mean values were compared using a t-test, proportions using the chi-square test or Fisher test if the validity conditions were not met (expected numbers greater than or equal to 5). We also used a mixed-effects model (REML) with Geisser-Greenhouse correction to test time and antigen factors of serology kinetics results, and a two-way ANOVA test to compare different group subjects, both followed by multiple comparisons test (Fishers Least Significant Difference). All tests were considered significant when the p value was less than 0.05.
3 Results
3.1 MPXV Testing in Symptomatic Patient
From June 22, 2022 to the end of May 2024, 213 patients were tested for the presence of MPXV in lesion swab. More specifically, 160 (75%) of these patients were sampled during the epidemic period in France from June to October 2022 with a significantly higher positivity rate compare to the tests performed afterwards (p < 0.0001) (Table 1). A large majority of patients were men having sex with men, lot of them presenting papulovesicular eruptions or vesicular rashes sometimes associated with fever. Eruptions induced by HSV and VZV were the main differential diagnosis with 22.67% of negative Mpox patients during the first period and 36.17% afterwards; two patients were tested positive for both HSV1 and MPXV DNA in lesion swab. Smallpox vaccination status did not differ between MPXV positive and negative patient nor between the epidemic period and afterwards, and infected individuals were significantly more often males in the epidemic period (p < 0.01).
| Epidemic (June 2022–October 2022) | Postepidemic (November 2022–May 2024) | Mpox epidemic versus postepidemic | |||||
|---|---|---|---|---|---|---|---|
| MPXV PCR positive | MPXV PCR negative | p value | MPXV PCR positive | MPXV PCR negative | p value | p value | |
| Number | 85 | 75 | N/A | 6 | 47 | N/A | (< 0.0001)**** |
| Male | 97.7% (83/85) | 81.33% (61/75) | (< 0.001)*** | 100% (6/6) | 93.61% (44/47) | nsa | nsa |
| Mean age (years) | 38.14 (27.31; 48.97) | 32.49 (17.93; 47.06) | (< 0.01)** | 36.67 (25.56; 47,78) | 32.53 (19.87; 45.19) | ns | ns |
| Childhood smallpox vaccine | 16.47% (14/85) | 16% (12/75) | ns | 16.67% (1/6) | 8.51% (4/47) | nsa | nsa |
| HSV/VZV associated vesicles | 2.35% (2/85) | 22.67% (17/75) | (< 0.001)*** | 0% (0/6) | 36.17% (17/47) | nsa | nsa |
| Viral bloodstream detection | 57.14% (24/42) | N/A | N/A | 40% (2/5) | N/A | N/A | nsa |
| Mean cycle threshold by PCR in blood samples | 35.26 (33.45; 37.07) | N/A | N/A | 35.64 (32.30; 38.98) | N/A | N/A | ns |
- Note: p values were calculated using the Student's t-test for comparisons of means. Chi-square test for comparisons of proportions was used when the criteria for test validity were met (theoretical numbers greater than or equal to 5), otherwise Fischer test was performed (p value annotated a). Tests were considered statistically significant when p value was less than 0.05.
We have detected MPXV viremia in 55.32% of patients (26/47) with available blood samples around the time of diagnosis. The mean threshold cycle value was 34.76 (31.91; 37.60) with a minimum Ct of 32.92 and a maximum Ct of 39.97. Both PCR methods, MPXV assay and Orthopoxvirus assay, gave similar results with a mean Ct difference 0.33 for the same sample.
3.2 MPXV Serological Test Setup
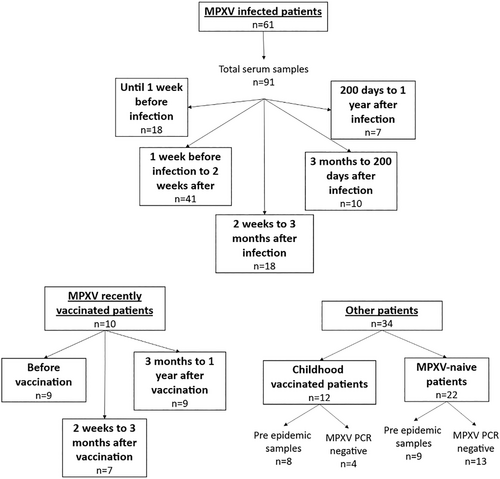
We evaluated humoral response over time. Since the date of onset of symptoms was rarely available, we classified the samples collected for each patient according to the time since the day of MPXV-diagnosis by PCR. 94 samples from 61 patients with PCR confirmed Mpox infection were tested (Figure 1), including 18 samples collected before diagnosis (until 7 days before diagnosis), 41 samples collected at the onset of infection (from 7 days before diagnosis to 14 days afterwards), 18 samples collected a short time after the resolution of the infection (from 14 to 90 days after), 10 samples collected from 90 to 200 days after diagnosis, and 7 samples from at least 200 days after MPXV positive PCR (maximum 335 days).
For H3L peptide, we used 27 single patient samples collected 14 days to 325 days after diagnosis, as well as 36 negative samples from patients born after 1980 (presumed unvaccinated against smallpox): 13 were MPXV PCR negative, 9 were collected in 2021 before the emergence of the virus in Europe, 14 contracted MPXV several weeks to several months later and were therefore considered naïve at the time of collection. We obtained a sensitivity of 0.89 and a specificity of 0.97 and Area Under Curve (AUC) for the ROC was estimated to 94.74% (95% CI: 87.77%–100.0%) (Supporting Information S1: Figure S1A). Using the maximum index strategy with MPXV specific peptides, we obtained a sensitivity of 0.70 and specificity of 0.91, based on the measurement of 27 single patient samples collected 14 days to 325 days after diagnosis and 123 negative control (89 from the MPXV negative samples panel, 17 from Mpox-like symptomatic patients with negative PCR screening and 17 from pre epidemic samples from CeGIDD and ITD departments collected in 2021). AUC for the ROC was estimated to 88.74% (95% CI: 81.10%–96.38%) (Supporting Information S1: Figure S1B).
3.3 MPXV Seroconversion and Persistence Over Time
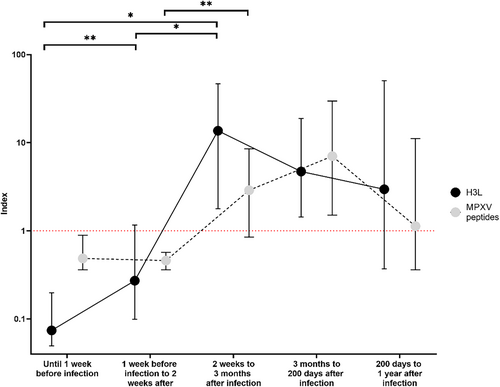
The evolution of the antibody index for both MPXV peptides and H3L following infection at the population level was represented in Figure 2. As these matched data had a lot a missing value among the 5 times categories, and small number of measures, we used a mixed-effects model (REML) with Geisser-Greenhouse correction. Time effect, antigen type and the interaction factor between time and antigen type were found statistically significant (respectively p = 0.0266, p = 0.0133 and p = 0.0246). When comparing by antigen type, only one significantly difference was found for MPXV peptides between the “near diagnosis” samples and the early stage of the postinfection (p = 0.0097) with Fisher's less significant difference test. For H3L, same tests revealed significant differences between the first three time categories: until 1 week before infection versus at the onset of infection (p = 0.008), until 1 week before the infection vs between 2 weeks and 3 months after infection (p = 0.0137), and 1 week before infection to 2 weeks after versus 2 weeks to 3 months after infection (p = 0.0137). According to these, we observed seroconversion that began within the first 2 weeks of infection and peaked between 2 weeks and 3 months postinfection for H3L antigen, whereas it was delayed using MPXV-specific antigens and with generally lower indexes.
At the individual level (Supporting Information S1: Figure S2A,B), some patients did not appear to have a humoral response to infection (arrows pointing horizontally or downwards), but in the majority of patients we did observe an increase in antibody titer (arrows pointing upwards). This dichotomy illustrates the wide median confidence intervals observed in Figure 2.
We detailed results over time before and after MPXV event as a heatmap including Mpox patients and a MPXV recently vaccinated group of subject (Supporting Information S1: Figure S3). Graphically, B21R 180/186 seemed to be the more immunogenic peptide between the 3 MPXV peptides. On another hand, we observed that childhood vaccinated subjects could have enhanced antibody reaction after MPXV infection or vaccination. We also represented serological kinetic of the recently MPXV vaccinated group for both antigen types (Supporting Information S1: Figure S4).
3.4 Anti-MPXV Antibody Between Different Groups of Patients
To assess antibody production in relation to type of exposure, we compared the indexes of patients between 2 weeks and 3 months postinfection with those of subjects who had recently received the third generation smallpox/Mpox vaccine (indicated for patients at risk of MPXV exposure or recently post-exposed), those who had been vaccinated against smallpox in childhood (born before 1980) or those presumed unexposed (no known infection or vaccine) (Figure 3). Using a two-way ANOVA test, antigen type and group subject were a significant source of variation (p < 0.001 for both factor, interaction factor p = 0.0158).
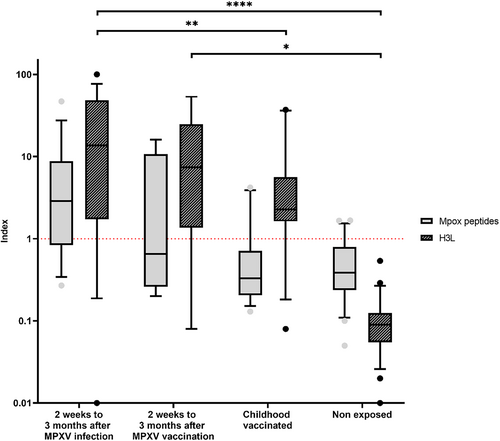
H3L antigen showed significant differences between the post MPXV infection group and both childhood vaccinated subjects (p = 0.0017) and nonexposed subjects (p < 0.0001). Other H3L results included no difference between MPXV vaccinated patients and childhood vaccinated or post-Mpox groups, while their H3L antibody index differed significantly from that of the unexposed subjects (p = 0.0096). Regarding MPXV peptide antibodies, no significant differences were found between groups including between indexes measured after infection or vaccination.
3.5 Screening for Mpox Infections in Patients Consulting at a Sexual Health Center and Infectious and Tropical Diseases Department in the Early Phase of the Epidemic Period
We have screened all bacterial STI screening samples from patients seen at CeGIDD and IDT department from Mars to June 2022 (respectively 1,678 samples and 488 samples). These samples include first void urine sample, oral swab, anal swab, vaginal swab and intra urethral swab. We have detected 11 MPXV-positive samples from 8 different patients (Table 2). One of these patient was seen in IDT department and had all 4 samples positive for MPXV (oral, anal and urethral swab, as long as first catch urine sample) and a Mycoplasma genitalium infection detected by PCR. The other ones were CeGIDD patients and only one MPXV positive sample, either anal swab, first catch urine sample, or vaginal swab for the only female patient screened positive for MPXV. None of it included coinfection with Neisseria gonorrhea or Chlamydia trachomatis, but one patient presented an active syphilis detected by serology test. We also looked for MPXV presence in bloodstream in 6 of 8 patients and detected 2 viremias (33%).
| Patient | Origin | Sex | Age (years) | Sample date | MPXV+ samplesa | Cycle threshold | MPXV- samplesa | Mpox symptoms | NGb and CTc PCR | Other IST |
|---|---|---|---|---|---|---|---|---|---|---|
| Patient 1 | CeGIDD | Male | 37 | 5/13/22 | FCU | 36.21 | S | No | Negative | — |
| Patient 2 | CeGIDD | Male | 23 | 5/24/22 | FCU | 38.81 | AS, OS | No | Negative | — |
| Patient 3 | CeGIDD | Male | 42 | 6/20/22 | AS | 21.69 | OS, FCU | No | Negative | Syphilis (serology) |
| Patient 4 | ITD | Male | 40 | 6/22/22 | AS | 34.15 | S | Yes | Negative | Mycoplasma genitalium (AS) |
| OS | 24.83 | |||||||||
| US | 20.01 | |||||||||
| FCU | 24.2 | |||||||||
| Patient 5 | CeGIDD | Male | 30 | 6/22/22 | AS | 39.22 | OS, FCU | No | Negative | — |
| Patient 6 | CeGIDD | Female | 23 | 6/22/22 | VS | 38.78 | S | No | Negative | — |
| Patient 7 | CeGIDD | Male | 34 | 6/23/22 | AS | 15.68 | — | Yes | Negative | — |
| S | 34.1 | |||||||||
| Patient 8 | CeGIDD | Male | 37 | 6/23/22 | AS | 22.31 | — | Yes | Negative | — |
| S | 32.74 |
- a Sample type: AS, anal swab; FCU, first catch urine; OS, oral swab; S, serum; US, urethral swab; VS, vaginal swab.
- b NG, Neisseria gonorrhoeae.
- c CT, Chlamydia trachomatis.
While six of eight samples date back to the end of June (start of peak incident), two samples were found positive for MPXV at least 1 month earlier (May 13 and May 24). For three of the eight patients, Mpox was suspected at the time of bacterial STI screening or within a few days of it because of clinical symptoms; diagnosis was confirmed by molecular biology on lesion swabs. In contrast, the other five patients never had any symptoms suggestive of MPXV infection during the sampling period. This may suggest that these five patients were asymptomatic or minimally symptomatic Mpox cases.
4 Discussion
In this study, we report valuable insights into the MPXV epidemiology and immune response patterns in the Montpellier region. This predominance occurred despite initial constraints on testing due to strict MOT regulations in France and a subsequent reliance on clinical expertize over PCR diagnostics in the postepidemic period when infectiologists were trained enough to recognize typical Mpox symptomatic presentation. Notably, we observed no atypical clinical presentations, such as severe or chronic infections, within our cohort. The detection of low-level viremia near the time of diagnosis in 56.32% of patients with MPXV infection is consistent with other studies which have shown viral clearance in the blood to be around 10–20 days after infection [40-43]. Although infectivity of the virus in the blood remains to be demonstrated, this period of systemic dissemination raises concerns about infectious risks associated with blood donation [44-46].
The retrospective design of our study imposed limitations on sample collection, preventing systematic acquisition of blood samples at key stages across the infection timeline. We also tested only a small number of patients, particularly in the group of subjects who had been vaccinated recently (n = 10) or during childhood (n = 12) (Figure 1). As a result, we could not fully document the progression of immune responses from initial exposure (naïve immunity) through seroconversion and into the establishment of long-term or waning immunity. Nevertheless, our findings underscore the likely protective role of antibodies in response to Mpox infection, as suggested by the increase in the antibody index after vaccination (Figure 2) and the presence of H3L reactive antibodies in childhood vaccinated subjects (Figure 3). Smallpox vaccine is well-known to induce a long-term humoral immunity [47, 48]. We have also seen in this study that some individuals may not have responded correctly to vaccination, highlighting that screening for antibody responses could be advantageous for identifying immune status across populations, particularly in higher-risk groups or individuals who may benefit from additional monitoring or intervention.
Our approach employed the H3L peptide assay which demonstrated satisfactory diagnostic sensitivity and specificity, enabling us to effectively detect humoral immunity responses following MPXV infection. However, H3L is a highly conserved antigen within the Poxviridae family and it has the capacity to elicit cross-reactive antibody responses, including those generated by vaccination. This implies that a serological assay based on H3L cannot reliably differentiate between antibodies induced by natural MPXV infection and those arising from prior vaccination. Consequently, such an assay may not be optimal for identifying MPXV infection within vaccinated populations. However, it remains a valuable tool for seroprevalence studies in regions with low vaccination coverage, providing insights into the spread of Mpox in populations where natural infection is more likely to be the primary source of immunity.
On the opposite, MPXV B21R and B22R are specific proteins absent in smallpox virus and vaccinia viruses preventing any cross-reaction with antibodies from the vaccination. Yet, we found no significant differences using these antigens between the post-MPXV infection and post-MPXV (Figure 3). Three of seven samples from recently vaccinated with third generation vaccine overcome the positivity threshold (15.99, 10.77, 5.93) and for the first two patients, a prevaccination sample was available showing an already positive test (respectively 16.67 and 19.90) (Data not shown). Although the performance of the test with MPXV peptides is lower than that of the H3L test, and that our study lacks statistical power, these results could be explained by the fact that these vaccinated patients, presumed to be at high risk of exposure, may have experienced Mpox disease before the injection of vaccine without reporting it, or they may had an asymptomatic infection. These results underscore the importance of broad serosurveillance to capture unexpected immune profiles.
We also detected the Mpox virus in urine, genital, anal and oral swabs commonly used for STI testing, including samples from asymptomatic individuals. This capability may be critical in understanding MPXV transmission dynamics in sexually active populations and supports the utility of Mpox screening in STI clinics, especially since the same type of specimen could be used to detect bacterial or viral concomitant presence. The simultaneous performance of several diagnostic tests would make it possible to study the dynamics of these co-infections. Additionally, we detected the presence of Mpox virus in Montpellier as early as May 13, 2022, predating, to our knowledge, the first confirmed case at the Montpellier University Hospital (CHU) on June 21, the first recognized case in France on May 19 [49] and following the first European case imported in United Kingdom on May 7 [50]. This earlier detection highlights the possibility of undetected community transmission and underscores the importance of proactive testing and early identification protocols, particularly in outbreak-prone regions. Additional studies are needed to examine the impact of virus transmission by asymptomatic patients.
These findings emphasize the potential benefits of integrating Mpox screening into existing public health measures, especially in clinics managing other sexually transmitted infections. Detection of the virus in samples routinely collected for STI screening facilitates widespread screening, and could mean that paucisymptomatic or atypical cases are less likely to be missed. Seroprevalence surveys may also have a role to play in identifying Mpox cases. Further studies with longitudinal designs are needed to clarify the durability of immune response, assess the protective efficacy of natural and vaccine-induced antibodies over time, and select the appropriate antigens for these tests.
Author Contributions
Edouard Tuaillon and Vincent Foulongne designed the study. Ahidjo Ayouba, Martine Peeters, Edouard Tuaillon and Vincent Foulongne supervised the study. Léo-Pol Rio and Carmen Alcocer Cordellat collected samples. Steven Henry, Marie Bistoquet and Sylvain Godreuil collected data. Steven Henry and Maeliss Champagne conducted experiments. Steven Henry and Léo-Pol Rio analyzed the data. Steven Henry wrote the manuscript. Edouard Tuaillon and Vincent Foulongne reviewed the manuscript.
Ethics Statement
This study received approval by the Institutional Review Board of the Montpellier University Hospital (approval number IRB-MTP_2023_09_202301466).
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




