Co-delivery of oncolytic virus and chemotherapeutic modality: Vincristine against prostate cancer treatment: A potent viro-chemotherapeutic approach
Abstract
Prostate cancer is a prevalent carcinoma among males, and conventional treatment options are often limited. Cytotoxic chemotherapy, despite its drawbacks, remains a mainstay. We propose a targeted co-delivery approach using nanoscale delivery units for Oncolytic measles virus (OMV) and vincristine (VC) to enhance treatment efficacy. The HA-coated OMV + VC-loaded TCs nanoformulation is designed for targeted oncolytic activity in prostate cancer. The CD44 expression analysis in prostate cancer cell lines indicates a significantly high expression in PC3 cells. The optimization of nanoformulations using Design of Expert (DOE) is performed, and the preparation and characterization of HA-coated OMV + VC-loaded TCs nanoformulations are detailed showing average particle size 397.2 ± 0.01 nm and polydispersity index 0.122 with zeta potential 19.7 + 0.01 mV. Results demonstrate successful encapsulation efficiency with 2.4 × 106 TCID50/Ml and sustained release of OMV and VC from the nanoformulation for up to 72 h. In vitro, assays reveal potent anticancer activity at 10 ± 0.71% cell viability in PC3 cells compared to 73 ± 0.66% in HPrEC and significant morphological changes at 90 µg/ml in dose and time-dependent manner. The co-formulation showed positive cell death 49.5 ± 0.02% at 50 µg PI/ml in PBS and 54.3% cell cycle arrest at the G2/M phase, 8.1% G0/G1 and 5.7% at S phase, with significant mitochondrial membrane potential (MMP) at 50 µg/ml, as assessed by flow cytometry (FACS). The surface-integrating ligand approach enhances the targeted delivery of the oncolytic virus and chemotherapeutic drug, presenting a potential alternative for prostate cancer treatment and suggested that co-administering VC and OMV in a nanoformulation could improve therapeutic outcomes while reducing chemotherapeutic drug doses.
1 INTRODUCTION
Prostate cancer (PC) is the most prevalent carcinoma among males. This disease affects several prostate cancer genetic subtypes and is typically identified by variations in membrane receptor expression. PC is over 25% of all cancers, particularly in North America.1 Gene expression analysis has shown a 56% convergence, classifying PC as a basal-like subtype.2 Patients with the last stage have a 1-year median survival time, and 45% will develop metastatic tumors in their peripheral regions.3 Compared to other types of cancers, treatment possibilities for PC are often more limited.
Contrary to the advent of revolutionary pharmacological and customized medications, cytotoxic chemotherapy remains the mainstay of PC treatment modalities.4 Reduced tumor specificity, an inaccurate toxicology pattern, a less readily accessible drug for desired clinical results, and inconsistent tumor elimination hinder the efficacy of existing conventional treatments. These drawbacks can be evaded by using targeted co-delivery of oncolytic virus (OV) with chemotherapeutic drug (vincristine) of NF (nanoformulation) based treatment strategies.5 Nanoscale (1000 nm) delivery units are well suited for the treatment modalities in the acute and chronic stages of various diseases, especially tumors.6 They exhibit accuracy towards a particular target, greater effectiveness, more solvability, and accessibility with solid action due to their compact size and prolonged release of encapsulated moieties.7 NFs of OVs and chemotherapeutic drugs still need thorough investigations and are being explored in clinical studies against numerous cancers, including PC.8 Oncolytic viruses, including Edmonston-derived strains of the OV vaccinia virus, parvovirus H1, and reovirus have demonstrated their efficacy for the treatment of different human carcinomas and are referred to as a triumph for the destruction of cancer cells in the modern era.9 The potency and pharmaceutical effectiveness of viral oncolytic activity depend upon the selection of tumor cells and more significant cytotoxicity at particular locations.10 It leads to an immune-mediated effect against tumor cells by releasing different antigenic substances and specific tumors’ inflammatory mediators. These factors eventually enhance patients’ longevity.11
Several oncolytic viruses, particularly measles virus (MV), and chemotherapeutic drugs, including VC, exhibit a robust pharmacological component for treating different tumors and demonstrate a remarkable antitumor potential. Leningrad-16, the live neonatal vaccine strain of OV, has a great range of oncolysis and zero genetic toxicity.12, 13 In the initial phases of experimental settings, data supporting its safety and efficacy demonstrate its ability to induce immunity. On the surface of lymphocytes, the wild-type MV attaches with the CD150/SLAM and epithelial nectin-4 receptors, but the vaccine strains of OMV preferentially infiltrate cells via the CD46 receptor.14
A type of variation in the binding site attaching viral hemagglutinin (H) glycoprotein in the vaccination strain causes it to have a high affinity for CD46, which leads to the production of syncytia as a characteristic cytopathic effect and subsequently to the apoptotic demise of the particular tumor cells.15, 16 As a result of an aberration in the H-protein, which is essential for the viral entrance and adhesion to cells, the vaccine strain of the measles virus is not contagious to healthy tissues. The targeted release of OMV and VC inflicts the administration's constraints. Cellular transporters are efficient for introducing OMV at the appropriate tumor site.17 Hyaluronic acid (HA) and naturally occurring environment-friendly polymeric substances after thiolation (TCs) are frequently employed for the development of specified viral and chemotherapeutic drug transmission into the host body.18 These substances are sustainable and contain multiple functional molecules necessary for surface modification. They develop a highly specified and targeted nanoformulation and release the encapsulated virus without compromising its oncolytic activity.19 Due to the covalently bonded but unrestricted nature of the thiol groups, these polymeric compounds may adhere and remain on the mucosal membrane through mechanical interactions.20 After cross-linking, negatively charged molecules such as tripolyphosphate polyanions (TPP) formed covalent or ionic bonds with each other, which improved the mechanical behavior of Chitosan (Cs). The procedure is known as “sustainable manufacturing” of virus and VC-abridged nanoparticles via the ionic gelation method, which produces biodegradable compounds with lower energy consumption and without the inclusion of any overpriced or toxic substances.21 In the ionic gelation process, molecules are bound together by electrostatic interactions among molecules with opposing charges.22 This strategy is also considered a green approach as it employs raw materials obtained from natural sources, prevents the consumption of acids made from organic materials, and produces no harmful residues.23 Hyaluronic acid (HA), a water-soluble polysaccharide, is primarily composed of successive units of N-acetylated glucose moieties and takes up the outermost layer, submucosal fluid, and connected tissues overall.19 The various tumor-commingling CD44 cell surface receptor, which is excessively expressed, especially during advanced neoplasmic phases, substantially impacts cell adhesion, cancer growth, and proliferation.20 Previous investigations have shown that CD44 regulates the glycolytic pathway, reactive oxygen species, and the development of prostate cancer cells.20 HA binds to CD44 receptors due to its comparatively higher binding affinity. According to prior studies, chitosan or chitosan-derived nanoparticle formulations exhibited more excellent durability, configurable cross-linking, sustained drug absorption profile, adherence to physiological membranes, and penetration into cells in contrast to other forms of vaccine administration.11, 24
We have previously described the oncolytic potential of HA-coated thiolated Chitosan Nanoformulations system for VC and OMV.25 The main goal of the present study was to develop an Oral measles virus, and vincristine (OMV + VC-NFs) encapsulated nanoformulation-based co-delivery system to evaluate the synergetic effect for targeted oncolytic activity in prostate cancer.
2 MATERIALS AND METHODS
2.1 Materials
Chitosan (Cs), Thioglycolic acid (TGA), and Tripolyphosphate polyanions (TPP) were taken from Dow University (Pakistan). Hydroxylamine, Sodium dihydrogen phosphate, 1-ethyl-3-3(3- dimethylamino propyl carbodiimide hydrochloride (EDC), Glacial acetic acid, Dipotassium hydrogen phosphate, Sodium hydroxide (NaOH), Potassium dihydrogen phosphate, and Calcium chloride (CaCl2) were purchased from Merck (Germany). High retention of dialysis membranes, sodium borohydride, and high molecular weight of 1500kD hyaluronic acid (HA) were bought from Scientific Worldwide traders. N-hydroxy succinimide (NHS) and Ellman's reagent (5, 5′ -dithiobis (2-nitrobenzoic acid; DTNB) were supplied by Merck Traders. In this research, only analytical-grade chemicals and reagents were employed.
2.2 CD44 expression in PC cell lines
CD44, as a homing cell adhesion molecule (HCAM), is having function in cell-cell interaction, cell adhesion, migration and various cellular functions. The expression of CD44 in different PC cell lines data was obtained from Human Protein Atlas, particularly human CD44 antibody HPA005785, specifically in PC3 cells.
2.3 Optimization using design of expert
The OMV + VC nanoformulations were optimized using Design of Expert (D.O.E.), version 8.0.6.1, which adopted the central composite structure following the factorial framework of BoxBehnken to ensure ease and less use of chemicals. The particle size of NF, zeta potential (ZP) and polydispersity index (PDI) were the dependable factors used as responses. Afterward, additional attributes were done using the optimized formulations that were chosen. By maintaining a consistent level of cross-linker solutions (TPP), the amount of the ligand (HA), polymer solution (TCs), oncolytic measles virus (OMV), and Vincristine (VC) could be adjusted, as shown in Table 1.
| Run | HA.mg | TC.mg | OMV-VC mg | Particle size nm | PDI | Zeta Potential + /-mV |
|---|---|---|---|---|---|---|
| 1 | 50.00 | 60.00 | 1.00 | 390.12 | 0.162 | 17.1 |
| 2 | 25.00 | 100.00 | 0.55 | 3191 | 1 | 10 |
| 3 | 37.50 | 100.00 | 0.10 | 1683 | 1 | 7.5 |
| 4 | 50.00 | 100.00 | 0.55 | 752.8 | 1 | 10 |
| 5 | 50.00 | 60.00 | 0.10 | 438 | 0.678 | 12 |
| 6 | 37.50 | 100.00 | 1.00 | 2133 | 1 | 6.5 |
| 7 | 37.50 | 20.00 | 1.00 | 397.2 | 0.122 | 19.7 |
| 8 | 25.00 | 60.00 | 0.10 | 416.7 | 0.584 | 12 |
| 9 | 25.00 | 20.00 | 0.55 | 492.5 | 0.433 | 28.9 |
| 10 | 37.50 | 10.00 | 0.10 | 441 | 0.42 | −22.5 |
| 11 | 25.00 | 60.00 | 1.00 | 460.7 | 0.49 | 25.6 |
| 12 | 50.00 | 20.00 | 0.55 | 1444 | 1 | −9.84 |
2.4 Oncolytic measles virus (OMV) strain
The National Institute of Health (NIH), Islamabad, Pakistan, generously supplied an undiluted solution containing 1 × 1012 Plaque forming units (PFU) of live artificial MV vaccine Edmonton variant in PBS. After 69 passes in chick embryonic fibroblast cells devoid of poultry leucosis, this was taken from the Connaught variant of MV, which originated from the same initial isolation as other vaccination isolates like Schwarz. Each time, ice was employed for thawing vials before the trial and kept at 80°C.26
2.5 In vitro prostate cancer cell line (PC3)
PC3 cancer cells derived from a 70-year-old male prostate metastasis of a grade IV tumor were used to evaluate the antitumor effects of OMV and VC-loaded NFs to the widely accessible oral vaccine strain. Prof. Dr Saeed Khan, Department of Molecular Pathology, Dow University of Health Sciences, Ojha Campus Karachi, Pakistan, graciously donated the prostate cancer cells (PC3) and normal prostate epithelial cells (HPrEc) in the culturing vessels of 80 cm2.27
2.6 Preparation of polymer and cross-linking agent solutions
The 1% TCs solution was made using deionized water and 1 mg/ml TPP following an ionic gelation approach. DV-III plus Programmable Rheometer (Brookfield Engineering Laboratories, Middleboro, MA) was used to determine consistency, an initial solution of 0.4% Cs (w/v, 4 mg/ml) with visibility of 2.5460.1 centi Poise (cP) was created after the Cs was dispersed in the deionized water. A probe sonicator operating at 30 mA for 3 min agitated the solution. The formulation's exterior layer contained 0.5 mg/ml HA, which was subsequently processed, lyophilized, and kept at 4°C for further storage.25
2.7 Preparation of OMVand VC-loaded NF
Following a minor adjustment, the OMV and VC-loaded NFs were formulated using the ionic gelation cross-linking process following sustainable manufacturing protocols. One dose of OMV, containing 500 TCID units of measles virus, 1 mg VC, 37.5 mg HA and 20 mg TCs, was added to deionized water by the syringe pump while continuously stirred at 550 rpm for 10 min. In the subsequent text, They are called nanoformulations of HA-coated OMV + VC-loaded TCs. The same procedure was used to prepare the blank NFs.
2.8 Characterization of OMV + VC-loaded NFs
The physicochemical and morphological properties of NFs of HA-coated OMV + VC-loaded TCs were assessed for potential use as a potent novel modality in cancer therapeutics. Zeta sizing was used to evaluate the particles’ size measurement, dispersity, and surface charge via PDI and zeta potential. Electron microscopic techniques were used to examine the structural and surface morphology. X-ray diffraction analysis (XRD) and Fourier-transform infrared spectroscopy (FTIR) were used to investigate the groups of functional moieties responsible for forming various therapeutic activities. The thermal endurance of the prepared nanoformulation was assessed using differential scanning calorimetry (DSC). The assays were all carried out following the indicated methods from an earlier study.26
2.9 Viral quantification from OMV + VC-loaded NFs
Based on whether the viral progeny is stimulated or non-stimulated, various quantification techniques were used to count the number of viral copies encapsulated in the NFs.28 The Plaque Forming Assay (PFA) is performed using virus isolates to generate the sequential dilutions added to an agarose-coated Petri dish of PC3 cells. When the agarose layer was removed, cells were coated with a stain to reveal Plaque on the cell surface after 72 h of infection. Plaque-forming units (PFU) were used to express the results of the assays. The Tissue Culture Infective Dose (TCID50) was calculated by measuring 50% TCID following the Reed-Muench procedure.26 The cellular morphology, such as rounding and disintegration of cells or syncytia formation in the cell divisions, was observed to determine the time and dose-dependent cytopathic effects (CPE). Following the previously mentioned protocol, OMV and VC-loaded NFs were introduced to PC3 cells at various concentrations on a 6-well plate at 37°C. The observations were recorded using inversion stage contrast microscopy (TCM-400, OEM-Optical, Labomed, U.S.A.). The six-well plates were infected with a seeding concentration of 2 × 106 cells/10 cm2 of OMV + VC-loaded NFs to determine the Multiplicity of Infection (MOI). Following established protocols, the viral vaccine strain concentrations were 0.1, 0.5, 1, 3, 5, 10, 15, and 20.26, 29
2.10 In vitro release of encapsulated moieties from NFs
The dialyzing membrane was filled with 50 ml PBS and 0.1 g nanoformulations of HA-coated OMV + VC-loaded TCs at pH 7.2. The dissolved assembly was continuously stirred at 100 rpm on a magnetized hot plate at 37°C. The 1.5 ml volume was taken from the solution and subsequently centrifuged at 10 000 g/min at the interval of, that is, 0, 0.5, 1, 2, 4, 8, 10 12, 24, 36, and 48 h. The spectrophotometer data at 595 nm examined the OMV and VC release from NFs.
2.11 In vitro protocols for OMV and VC-loaded NFs’ anticancer activity
The following in vitro experiments on prostate cancer cells (PC3) compared to normal prostate epithelial cells (HPrEc) were performed using nanoformulations of HA-coated OMV + VC-loaded TCs to determine their antitumor activity in cell culture medium.
2.11.1 Cell morphology analysis
This involves assessing changes in the appearance of cells after treatment of nanoformulations of HA-coated OMV + VC-loaded TCs at different concentrations of 90, 50, and 10 µg/ml dissolved in phosphate buffer. Morphological features of cell shrinkage, membrane blebbing, cytoplasmic and nuclear condensation, rounding and aggregation of cells are the hallmarks of apoptosis.11 A phase contrast inverted microscope was used to analyze the visual features of cells after 24 h.
2.11.2 Cytotoxicity analysis via trypan blue exclusion and MTT assay
Using Trypsin-EDTA (Gibco, New York) helps analyze the number of living cells in cellular suspension. The hemacytometer counted viable and dead cells under a phase contrast inverted microscope. Living cells appear white, while dead cells appear blue due to the porous cell membrane. The % cell viability was calculated using previous protocols.30 MTT assay is a colorimetric assay that analyses the ability of living cells to convert a water-soluble dye [3-(4,5 dimethylthiazol-2-yl)−2,5-diphenyltetrazolium bromide} into insoluble formazan. The cytotoxicity was calculated via the cell viability percentage of nanoformulations of HA-coated OMV + VC-loaded TCs using previous protocols.31, 32
2.11.3 Cell death using flow cytometry
PC3 cells were grown in flasks till confluency at 80%–90%. Media was removed, and cells were trypsinized, centrifuged and counted using a hemocytometer. Approximately 50 000 cells/wells were cultured into 24 well plates daily. The HA-coated OMV + VC-loaded TCs compared to pure OMV and pure VC were mixed in each well using unprepared media and further incubated for the next day. Some wells were left under DMSO as control. The PC3 cells were trypsinized and centrifuged. The propidium iodide (PI) dye at 50 µg PI/ml in PBS was used for staining and kept in the dark for half an hour. Flow cytometry was performed, and the number of unstained (live) cells and stained (dead) were determined using (FL2) versus cell count protocol. PI positive (cell death) was calculated compared to the control, and the experiments were performed in triplicate.33
2.11.4 Cell cycle analysis using flow cytometry
The PC3 cells were treated with an antagonist, incubated for a whole day, and centrifuged. After discarding the supernatant, the pellet was resuspended in PBS with 3% FBS. Then, the PC3 cells were treated with 50 μg/ml PI, 0.1% Triton X–100 and 3 mg/ml ribonuclease A according to the previously reported method with slight modifications. The culture plates were kept in the dark for 60 min. 10 000–20 000 events were witnessed for each sample using the BD Accuri flow cytometer, and the analysis was done using BD Accuri software.34
2.11.5 Mitochondrial membrane potential
The PC3 cells were seeded in a 24-well plate at the concentration of 1 × 104 cells in each well and incubated overnight in 5% humidified CO2 at room temperature. A 24-h treatment with DMSO (vehicle control), carbonyl cyanide m-chlorophenylhydrazone (CCCP) at 50 M (positive control), and HA-coated OMV + VC-loaded TCs compared to pure OMV and pure VC at their IC50 were observed. The PC3 cells were collected, centrifuged, washed, and treated for 20 min in the dark with 0.25 M JC-1 (5′, 5′, 6′, 6′-tetrachloro-1′, 1′, 3′, 3′-iodide) dye at room temperature.35 The cells were washed, resuspended in PBS in 200 L of 10 mM glucose solution, and transferred to a flat bottom 96-well plate. The fluorescence emission was measured for red and green signals using FLUOstar Omega Microplate Reader, BMG LABTECH GmbH, Ortenberg, Germany at 590 nm and 520 nm emission filters.36 A red/green ratio decrease indicates that the mitochondrial membrane potential (MMP) has depolarized.37
2.12 Stability parameters
The particle size and structural morphology of nanoformulations of HA-coated OMV + VC-loaded TCs were thoroughly assessed for 3 months while they were placed at 25°C.
2.13 Statistical studies
One-way analysis of variance (ANOVA) and the Student's t-test with a significant p-value of ≤0.05 was used to analyze the results obtained from the experimentation, together with the mean values of the various measurements and standard deviation (mean ± SD).
3 RESULTS AND INTERPRETATION
3.1 CD44 expression in PC cell lines
The data from the Human Protein Atlas was used to analyze the expression of CD44 in human prostate cancer cell lines (Figures S1). Immunohistochemical staining data for antibody HPA005785 was obtained from Human Protein Atlas to analyze ee human CD44 expression 5 in PC3 cells. The color code indicates the strength of CD44 expression in PC3, as red indicates a strong immunoreactivity. Orange indicates moderate to solid immunoreactivity; yellow shows weak to moderate, while blue shows negative to weak immunoreactivity (Figure S2).
3.2 Optimization of NFs using the design of expert
Design of Expert (D.O.E.) version 8.0.6.1 was used to determine the associated factors, including PS, PDI, and ZP, for optimizing HA-coated OMV + VC-loaded TCs nanoformulations. BoxBehnken factorial design was employed to show the formulation's aspects as shown in Table 1 and displayed in Figures S3–S5.
3.3 Preparation of OMV + VC-loaded NFs
According to the ionic gelation process, the cationic amino group of TCs and anionic HA substance were ionically bonded to produce HA-coated OMV + VC-loaded TCs nanoformulations for juxtaposition with the OMV oral live attenuated vaccine variant. Different structural characteristics, outlined below, then characterized these TCs nanoformulations.
3.4 Characterization of OMV + VC-loaded NFs
An extensive characterization of HA-coated OMV + VC-loaded TCs nanoformulations was performed. The results for blank and virus with drug nanoformulations after optimization are presented in Table 2, Figures 1 and 2 (Figures S6 & S7). With a ZP of 19.7 ± 0.02 mV and a PDI of 0.122, the smallest nanoparticle size after encapsulation of the virus and drug, both in the same nanoformulation, was 397.2 ± 0.01 nm. The mean nanoparticle size was a bit increased as compared to blank NFs. The zeta sizer results revealed a relatively uniform distribution of particles in formulations with positive charges at their surface.
| Features | Blank-NFs | HA-coated OMV + VC-loaded TCs | |
|---|---|---|---|
| 1 | Particle size | 390 ± 0.01 nm | 397.2 ± 0.01 nm |
| 2 | Polydispersity index | 0.162 | 0.122 |
| 3 | Zeta potential | 17.1 ± 0.03 mV | 19.7 + 0.01 mV |
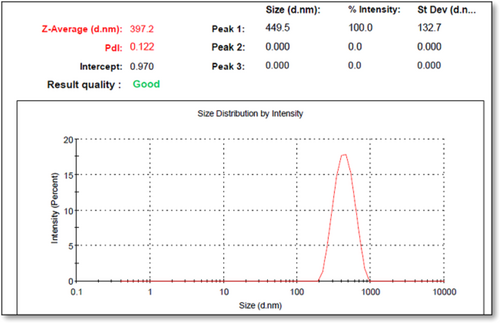
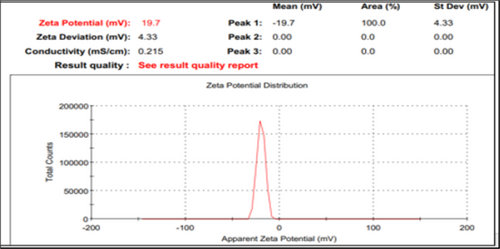
SEM observations showed a spherical surface with smooth vesicular characteristics in blank and virus with drug NFs. However, the morphology of HA-coated OMV + VC-loaded TCs nanoformulations was more compact and uniformly distributed (Figures S8 & S9).
FTIR spectrum showed the most significant peaks at 600 cm1 due to the bending vibration of the C-C-O bonds, and a constant drop was verified at roughly 1100 cm1. The spectra showed abrupt deflections at 3424–3404 cm1 due to the stretching of OH caused by the presence of bands of TCs, 2900–2880 cm1 due to the extension of CH, and 1634–1606 cm1 due to the stretching of amide C = O. All nanoformulations showed an abundance of thiol (-SH) groups, and the upward stretching peak at about 2496 cm1 confirmed the effective thiolation of chitosan (Figures S10 & S11). The XRD phenomenon was used to investigate the crystal structure of the novel formulation and the deflection showed the presence of encapsulated chemical moieties having various types of functional groups compared to blank nanoformulation (Figure S12 [blank: black & moieties: red]). The Raman analysis showed the successful encapsulation of the measles virus strain and VC in the same NFs. The amorphous nature of enclosed moieties showed brighter visual images than blank-NFs (Figure S13).
These nanoformulations were exposed to temperatures between 50 and 300°C to test the thermal stability. The thermograms created by DSC demonstrated that the nanoformulation encasing the OMV + VC was not durable at high temperatures, even after the thiolation of Cs, because the virus degrades at high temperatures and cannot retain its functional activity.
3.5 Viral quantification from OMV + VC-loaded NFs
The prepared OMV + VC-Loaded NFs were premeditated to check the effective viral progeny at 7 p.d.i in PC3 cells. The proportional distance (PD) among two dilutions determined by Reed and Munich yields an ID50 of 4.59. A benchmark for threshold activity is the dilution that would infect 50% of inoculated test units. The virus titre was measured in inverse titer units as TCID50 per unit ml. Since the viral sample was 0.1 ml, the pure MV's titer would correspond to 1/10 − 4.59/0.1 = 3.94 × 1. For HA-coated OMV + VC-loaded TCs nanoformulations, ID50 = 10−5.3 and 2.4 × 106 TCID50/ml. There was a significantly high multiplicity of measles virus when encapsulated with VC in HA-coated TCs nanoformulation at various concentrations (Figure S14).
3.6 In vitro assays for OMV and VC-loaded NFs’ anticancer activity
3.6.1 Cell morphology assay
When exposed to cytotoxic drugs, cells shrink, the membrane becomes perforated, and nuclei shrink and lose their characteristic morphology, leading to detachment, rounding and aggregation of cells. PC3 and HPrEC cells, when treated with different concentrations of pure OMV, pure VC, and HA-coated OMV + VC-loaded TCs nanoformulations, exhibited a significant change in morphology as the cells underwent apoptosis. Cells treated with HA-coated OMV + VC-loaded TCs nanoformulations showed a cytotoxic effect in a dose-dependent manner. Control cells can be compared with treated cells at time intervals of 24 h to analyze changes in morphology and detachment of cells after treatment. The highest cytotoxic effects were observed at 90 µg/ml concentration in PC3 cells treated with HA-coated OMV + VC-loaded TCs nanoformulations. The cytopathic effect was observed in the order of HA-coated OMV + VC-loaded TCs > OMV > VC for PC3 cells, while for HPrEC cells, it was pure VC> pure OMV ≈ HA-coated OMV + VC-loaded TCs nanoformulations, respectively.
3.6.2 Trypan blue exclusion assay
Trypan blue exclusion assay helped to evaluate the cytotoxic potential of treatment groups at different concentrations in PC3 and HPrEC cells. The viability of PC3 cells was 15 ± 0.09 when treated with pure OMV at 90 µg/ml, which increased to 80 ± 0.03 at 10 µg/ml. When treated with pure VC, the % cell viability of PC3 cells was 22 ± 0.62 at 90 µg/ml, which increased to 85 ± 0.12 at 10 µg/ml. Upon treatment with HA-coated OMV + VC-loaded TCs nanoformulations, the % cell viability of PC3 cells was 10 ± 0.71 at 90 µg/ml, which increased to 77 ± 0.29 at 10 µg/ml. The activity decreased dose-dependently while the cytotoxic effect was observed to be in the order of HA-coated OMV + VC-loaded TCs > pure OMV > pure VC, as shown in Table S1. For HPrEC cells, the viability was 45 ± 0.12 when treated with pure OMV at 90 µg/ml, which increased to 69 ± 0.03 at 10 µg/ml. When treated with pure VC, the % cell viability of HPrEC cells was 10 ± 0.53 at 90 µg/ml, which increased to 69 ± 0.12 at 10 µg/ml. Upon treatment with HA-coated OMV + VC-loaded TCs nanoformulations % cell viability of HPrEC was 74 ± 0.66 at 90 µg/ml, which increased to 79 ± 0.05 at 10 µg/ml. The cytotoxic effect in HPrEC cells was observed in the order of pure VC> pure OMV > HA-coated OMV + VC-loaded TCs nanoformulations.
3.6.3 MTT cytotoxicity assay
Cell viability of PC3 and HPrEC cells treated with pure OMV, pure VC and HA-coated OMV + VC-loaded TCs nanoformulations was also measured by MTT analysis. Cells were treated with 10, 50, and 90 µg/ml concentrations. The viability of PC3 cells treated was observed to be lowest when treated with HA-coated OMV + VC-loaded TCs nanoformulations compared to pure OMV and VC (HA-coated OMV + VC-loaded TCs < pure OMV < pure VC). The viability of HPrEC cells was lowest when treated with pure VC compared to pure OMV and HA-coated OMV + VC-loaded TCs nanoformulations) as shown in Figure S15.
Cell death using flow cytometry
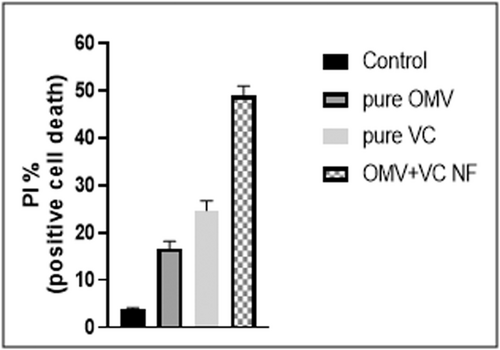
The cell death in PC3 cells was determined using a flow cytometry system (FACS). The percentage of PI-positive (cell death) cells was assessed in comparison to the control (DMSO). The HA-coated OMV + VC-loaded TCs nanoformulation showed positive cell death 49.5 ± 0.02% at 50 µg PI/ml in PBS compared to pure OMV and pure VC, 16.75 ± 0.04% and 25.76 ± 0.07%, as shown in Figure 3.
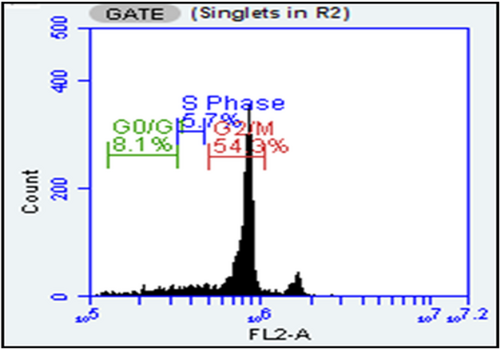
Cell cycle analysis using flow cytometry
The PC3 cells were treated with 90 µg/ml concentration of HA-coated OMV + VC-loaded TCs nanoformulation to check the cell cycle arrest at different stages following the previously reported method with slight modifications.38 The 10 000–20 000 cycles were observed and showed that NFs have 54.3% cell cycle arrest at the G2/M phase, 8.1% G0/G1 and 5.7% at S phase. Figure. So, the measles virus oncolytic activity remains intact after encapsulation into NFs, as shown in Figure 4.
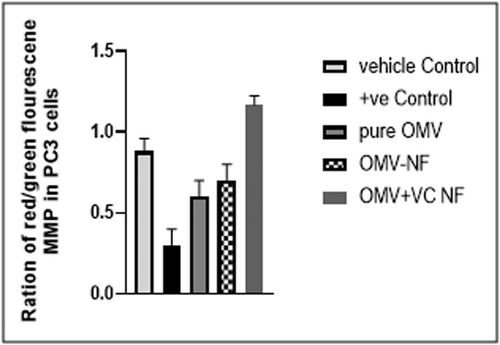
Mitochondrial membrane potential
The PC3 cells were treated with 90 µg/ml concentration HA-coated OMV + VC-loaded TCs nanoformulation compared to pure OMV and pure VC to check the Mitochondrial membrane potential (MMP) as an apoptotic indicator. The dye accumulates in the mitochondria of healthy cells as aggregates showing red fluorescence and apoptotic cells showing green fluorescence. A 24-h treatment of PC3 cells with DMSO (vehicle control) and carbonyl cyanide m-chlorophenylhydrazone (CCCP) positive control at 50 M end concentration showed red/green fluorescence ratio comparable with HA-coated OMV + VC-loaded TCs nanoformulation, pure OMV and pure VC at their IC50 as shown in Figure 5.
3.7 In vitro virus release of NFs
The dialyzing membrane was filled with 50 ml PBS and 0.1 g nanoformulations of HA-coated OMV + VC-loaded TCs at pH 6.8. The 1.5 ml volume was taken from the acidic solution and subsequently centrifuged at 10 000 g/min at intervals of, that is, 0.5, 1, 2, 3, 4, 6, 12, 24, 48, and 72 h, as indicated in Figure S16. The spectrophotometer data at 595 nm examined for these samples showed that 50% VC was released from NFs in the first 5 h, but OMV took 12 h to complete its 50% release in the same nanoformulation. The rest of the therapeutic moieties are released steadily but continuously. This release pattern showed a therapeutic effect in a sustained release manner for a long duration. In comparison, the pure VC and OMV were suddenly released in the medium in the first 4 h.
3.8 Stability parameters
The stability properties of the HA-coated OMV and VC-encapsulated TCs-nanoformulations were evaluated for changes in PS, PDI, and ZP by zeta sizing and morphological parameters by SEM. The nanoformulation in both lyophilized (powder) and reconstituted in distilled water forms was kept at 4°C. After 3 months, the lyophilized powder showed no visible change, whereas the liquid solution changed its color from light pink to yellow. The zeta sizing of liquid formulation showed APS from 397.2 ± 0.01 nm to 806 ± 0.05 nm, a change in ZP from 19.7 ± 0.02 to 27.1 ± 0.01 mV, and a change in PDI from 0.122 ± 0.02 to 0.681 ± 0.03, (Figures S17 & S18). Therefore, it is advised that virus-based nanoparticles should be kept in lyophilized form for prolonged usage and storage. The powdered formulation showed zeta sizing 522.5 ± 0.02 nm, ZP 17.3 ± 0.02 mV, and PDI 0.388 (Figures S19 & S20). These results indicated that the formulation in liquid form lost its stability and dispersity within nanoformulation, while the NF stored in lyophilized form showed very little change in characterization.
The nanoformulation in both lyophilized (powder) and reconstituted in distilled water forms was evaluated by SEM. Figure S21 shows that the lyophilized formulation showed the same spherical and amorphous structures even after 3 months of storage at 4°C, but the liquid formulation was crystalline with haphazard structural alternations Figure S22.
4 DISCUSSION
The idea to employ OMV and chemotherapeutic drug (VC) for treating tumor control first came about due to a case study linking the measles disease to tumor diminution. Following effective clinical testing and an adequate risk profile, an active, attenuated MV variant was granted authorization for vaccination in the 1960s.39 After several decades of research on cancer therapy, the usage of a variety of MV strains and other chemotherapeutic drugs has started. Preclinical research indicates substantial oncolytic activity. Hematological carcinomas were chosen as the targeted disorders at the start of the investigations. Additional studies revealed that solid tumors such as breast, lung, prostate, and blood carcinomas are susceptible to the oncolytic activity of MV and chemotherapeutic drug VC.40 However, normal cells are unaffected by this pharmacological substance because of MV's inherent lymphotropism. Significant evidence from studies that have previously been published supports the preclinical effectiveness of oncolytic virus and VC for the chemotherapy of multiple tumors with targeted delivery.11, 25 Oncolytic targeted viral and drug transmission systems include benefits such as improved immunity, no DNA damage, and superior safety criteria of vaccination strains.41 The stimulation of the body's defenses against viral diseases, the distribution of oncolytic MV and VC to specific cells, and unrestricted oncolytic immunological treatment constitute special issues related to MV. Avoiding the patient's humoral antibody reaction includes one of the main issues that emerge after the OV strain and VC have been administered. In the presence of a defense mechanism, the virus is neutralized before it can infect the target area, and viral progeny that is circulating is quickly eliminated by opsonization with immunoglobulins, various cascades, and coagulation mediators existing in the hepatocytes.42
The current study developed polymeric membrane enriched ligand-based nanoformulation (NF) using an active, weakened oral measles vaccine (OMV) variant and chemotherapeutic drug, VC. One of the intriguing methods to generate the targeted virus and drug delivery system is membrane incorporation of nanoparticles by ligands as opposed to genomic/structural modification and inactivation of the viral proteins. These ligands, such as HA, shield the oncolytic OMV and VC from degradation by antibodies. Using the cross-linker TPP, the nanoformulations of HA-enriched, OMV + VC-encapsulated TCs were modified using the ionic gelation technique. Sustainable and eco-friendly procedures prohibit the use of harmful substances or toxic solutions that could kill the virus or lessen its activity.43 The production of disulfide and covalent linkages between cysteine-rich mucosa and TCs is an additional advantageous binding contact than the electrostatic attraction of pure chitosan, which causes an upsurge in cellular absorption.25
The HA-coated OMV + VC-loaded TCs nanoformulation synthesized in this study showed that the average particle size (APS) with virus and drug was similar enough to the findings from our earlier study32 using the ionic gelation technique with less invasive chemicals. For the specified oncolytic virus vaccine strain and VC drug administration, the active weakened vaccine strain from OMV and VC were incorporated into a thiolated chitosan nanoformulation encapsulated with hyaluronic acid that was made utilizing sustainable methodology without the use of any toxic solvents or hazardous materials. Compared to normal prostate cells, prostate cancer cells were selected to test the effectiveness of the produced nanoformulation at various MOIs. The nanoformulation developed with an average particle size between 400 has a reduced positive ZP that favors MV absorption by tumour cells at low pH (acidic environment). After quantifying a substantial quantity of enclosed virus titer and active virus progeny, it demonstrated a great controlled release profile and strong stability. These nanoformulations showed effective growth suppression compared to the readily accessible OMV vaccine (p < 0.05). These characteristics increase the efficacy of OMV and VC drug co-nanoformulations and their targeted effect as highly focused immunomodulators in tumor therapies.40
The size and charge of nanoparticles (NPs) are pivotal factors influencing the efficacy of nanoformulated structures. The size dictates crucial aspects such as biodistribution, cellular uptake, and exploiting phenomena like the Enhanced Permeability and Retention (EPR) effect in targeted drug delivery. Small-sized NPs can penetrate cell membranes more efficiently, facilitating intracellular drug delivery, while optimal sizes contribute to prolonged circulation times and avoidance of rapid clearance. The charge of NPs influences cellular interactions, stability, and in vivo behavior. Positively charged NPs may enhance cellular uptake but are susceptible to opsonization, affecting their clearance by the immune system. Balancing size and charge is essential to tailor nanoformulations for specific applications, optimizing drug delivery, biocompatibility, and overall therapeutic efficacy.44
The co-encapsulated virus and drug from NF in vitro showed a potent cytotoxic profile with sustained release influenced by the particle size and charges. As they attach to the outermost layer of the cell membrane, small nanoparticles with a high surface-to-volume ratio promptly liberate the encapsulated material via cell-mediated endocytosis.45 The action of the charged antigen's neutralization, which increased the ZP of TCs nanoparticles compared to chitosan nanoparticles, may cause the drop in surface charge ratios observed in the present research after encapsulating OMV and VC with HA-coated NF. Compared to neutral nanoparticles, cationic nanoparticles exhibit greater direct absorption uptake.46
In previous studies, the innate and cell-driven immunity responses were examined against transmission of the weakened Rift Valley Fever Virus (RVFV) encapsulated into chitosan-derived nanoparticles that were generated via ion gelation. The OMV nanoparticles and VC drug were evaluated against the prostate cancer cells, which showed substantial potential as a chemotherapeutic option.47 According to a recent study, charged nanoparticles are eliminated from the body more quickly than neutral ones, and malignant cells are more likely to scavenge charged nanoparticles due to their elevated ZP. To evaluate the antimicrobial effectiveness of mucosal immunizations, OMV nanoparticles and VC were developed by employing CS in a 281.2 nm size.48
This research revealed that the entrapped virus in HA-coated OMV + VC-loaded TCs nanoformulations had a titer of 2.4 × 106 TCID50/ml units, indicating that syncytia had formed in the infected PC3 cells. Prostate cancer cells compared with normal human prostate cells showed similar results when treated with the HA-encapsulated OMV and VC-loaded NFs at MOIs of 0.1, 0.5, 1, 3, 5, 10, 15, and 20 with an IC50 of 5.1 and 3.52, respectively.26, 49 Our findings demonstrated that the OMV + VC-encapsulated TCs nanoformulations coated with HA exhibited more cytotoxic efficacy than the readily accessible OMV vaccination and VC alone. We have reported various intriguing results regarding the targeted viral and drug transmission of polymeric biodegradable substances after encasing a weakened virus variant from a synthetic vaccination and VC drug.50
5 CONCLUSION
This research aimed to employ a surface-integrating ligand to increase the activity and aimed at chemotherapeutic drugs VC and OMV. We hypothesized that lowering the dose of the chemotherapeutic drug by formulation of nanoparticles with oncolytic measles virus would be more promising. Co-administering the VC and OMV with increased on-target effectiveness could improve patient compliance and therapeutic outcomes. Moreover, additional animal model-based and molecular experimental studies will be performed to strengthen these findings.
AUTHOR CONTRIBUTIONS
Sadia Anjum: Supervision and proofreading. Faiza Naseer: conceptualization, writing—original draft, writing, and lab work. Tahir Ahmad: Supervision and proofreading. Kousain Kousar: in vitro analysis and writing. Maisa S. Abduh: Data analysis, methodology, and funding. Afrose Liaquat: cell cycle analysis and flow cytometry.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the technical and financial support provided by the Ministry of Education and King Abdulaziz University, DSR, Jeddah, Saudi Arabia. “Institutional Fund Projects funded this research work under grant no. (IFPIP:209-290- 1443). The authors gratefully acknowledge the technical and financial support provided by the Ministry of Education and King Abdulaziz University, DSR, Jeddah, Saudi Arabia.”
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request. Data will be made available on request.




