Novel diarylpyrimidine subtypes as HIV-1 nonnucleoside reverse transcriptase inhibitors with improved resistance profile
The virally encoded reverse transcriptase (RT) is the hallmark of human immunodeficiency virus (HIV) and other retroviruses. RT uses two distinct catalytic active sites (Figure 1), the polymerase (pol, D110, D185, and D186) and the RNase H (D443, E478, D498, and D549), to convert the single-stranded genomic viral RNA to double-stranded viral DNA. Structurally, RT is a heterodimer with a catalytic p66 subunit containing both pol and RNase H domains, and a noncatalytic p51 subunit lacking the RNase H domain (Figure 1).1 RT pol has the classic right-hand structure comprising four domains, and the active site is housed in the palm domain. Importantly, RT pol is the target of two well-known classes of FDA-approved HIV drugs: the active site targeting nucleos(t)ide RT inhibitors (NRTIs) and the allosteric site targeting nonnucleoside RT inhibitors (NNRTIs). In contrast, RT RNase H has not been targeted by any drugs approved or in the development pipeline, despite medicinal chemistry efforts.2 While NRTIs were the first HIV drug class with eight drugs approved, they all require cellular kinases for intracellular activation to triphosphates (TP). Mechanistically, the TPs compete against natural dNTPs for incorporation, and once incorporated, act as obligate chain terminators by virtue of lacking the 3′-OH. As such, NRTIs can cause toxicity by inhibiting human mitochondrial DNA polymerase (Pol γ), leading to depletion of mtDNA and mitochondrial dysfunction.
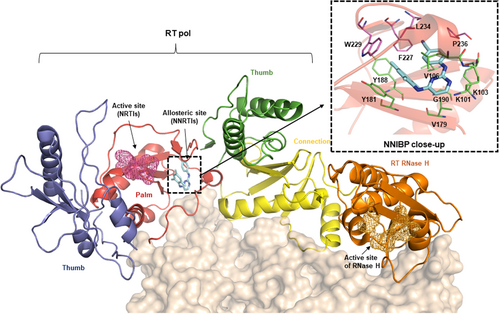
A conceptually distinct class of RT-targeting drugs is the NNRTIs which allosterically target RT by binding to a largely hydrophobic pocket ~10 Å away from the active site (Figure 1, palm domain). The well-defined NNRTI binding pocket (NNIBP, Figure 1, upper right) is lined by p66 residues in the palm domain, including the easily mutable residues forming the floor: L100, K101, K103, V106, V179, Y181, Y188, G190 (shown in green sticks); and the relatively conserved residues at the ceiling extending into the primer grip region: F227, W229, L234, P236 (shown in purple sticks), and Y318 (thumb domain, not shown). In addition, the NNIBP also entails p51 E138 (not shown). Importantly, the NNIBP is absent in HIV-1 RT apo structures and is created upon NNRTI-binding via significant structural rearrangements.1 These changes inhibit RT3, 4 via (a) allosterically altering the polymerase active site; (b) distorting the positioning of the primer grip to affect its alignment with the polymerase active site. NNRTIs are specifically active against HIV-1, as HIV-2 are intrinsically resistant presumably due to a different RT binding site.
Since the report of HEPT and TIBO as the first two NNRTI chemotypes, many structurally diverse chemical classes have been discovered as NNRTIs.5 These efforts culminated in the FDA-approval of the first-generation NNRTIs (Figure 2) nevirapine (NVP, 1996), delavirdine (DLV, 1997, discontinued) and efavirenz (EFV, 1998). These early NNRTI drugs share a common butterfly shape and a similar binding mode to the NNIBP.6 Since NNRTIs do not interfere with cellular kinases or human mitochondrial DNA polymerase, they are generally better tolerated than NRTIs. However, they typically exhibit low generic barrier to resistance as mutations in the NNIBP do not significantly impact RT functions or viral fitness. Further efforts geared toward better NNRTIs with improved resistance profile led to the development of the second-generation NNRTIs (Figure 2), including FDA-approved etravirine (ETR, 2008), rilpivirine (RPV, 2011), and doravirine7 (DOR, 2018), as well as dapivirine8 (DPV, TMC-120, Figures 1 and 2) recently approved in Africa as a vaginal ring (VR) for pre-exposure prophylaxis (PrEP).
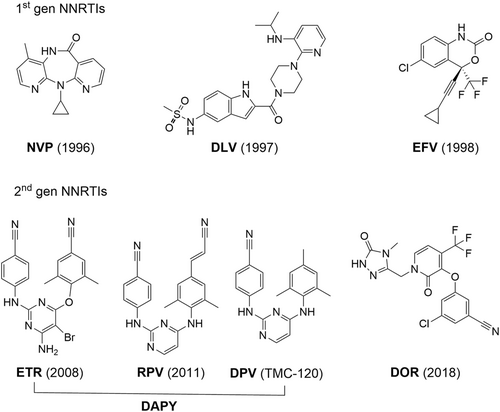
Importantly, the second-generation NNRTIs share a flexible three ring pharmacophore: a central ring connected to two flanking rings via one-atom flexible linkers (Figure 2). The increased flexibility, although supposedly disfavoring binding free energy, is believed to be critical to the vastly improved resistance profile, as it confers better adaptability in binding to the mutant NNIBP and allows evenly distributed binding interactions to reduce the binding dependence on certain easily mutable residues. In addition, all second-generation NNRTIs feature a largely linear CN group, as well as other groups on the flanking aryl rings to enable effective interactions with the less mutable prime grip region (Figure 1, upper right). Notably, of the four approved second-generation NNRTIs (Figure 2), three (ETR, RPV, and DPV) belong to the diarylpyrimidine (DAPY)9 chemotype, which is by far the most important NNRTI chemotype. Despite the success of the second-generation NNRTIs, further antiviral research in designing chemically distinct DAPY subtypes is warranted due to their unique clinical benefits10 in HAART and the selection of mutations conferring clinically significant resistance to ETR and RPV, including L100, K101, Y181, and Y188 (HIV drug resistance database11).
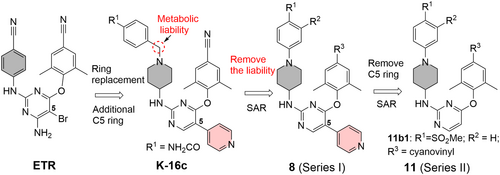
In line with the need to develop better DAPY NNRTIs, a recently published paper in the Journal of Medical Virology (JMV), entitled “Structure-based design of DAPYs and triarylpyrimidines as potent HIV-1 NNRTIs with improved metabolic stability and drug resistance profiles” has been selected as an editor's choice paper.12 Previously these authors have identified K-16c, a 5-substituted DAPY analog, as a potent NNRTI lead (Figure 3),13 albeit with low metabolic stability. Structurally, the pyridine ring (shadowed in salmon) at the C5 position of the central pyrimidine ring and the piperidine ring (shadowed in gray) in place of a phenyl ring represent deviations from the DAPY prototype. In the current work, the benzylic methylene (circled) right next to the piperidine N was identified as a metabolic liability which may account for the low metabolic stability of K-16c. Designing out the benzylic methylene and the subsequent structure–activity relationship (SAR) led to the synthesis of subtype 8 (Series I, Figure 3). Further design involved the removal of the C5 pyridine ring to yield subtype 11 (Series II, Figure 3) which conforms better to the DAPY pharmacophore. Structure-guided SAR allowed the authors to identify analog 11b1 as the best overall compound. Notably, 11b1 bears a methanesulfone group at R1 and a cyanovinyl (CV) moiety at R3 (Figure 3), both enabling critical H-bonding interactions. The long CV moiety is also featured in RPV (Figure 2).
The analogs of both subtype 8 (Series I) and subtype 11 (Series II) were first tested for antiviral activity in an MTT cytoprotection assay in MT-4 cell lines infected with HIV-1 wild-type (WT) strain IIIB and a NNRTI-resistant strain RES056 containing the K103N + Y181C double mutation. Major SAR observations included (a) a methanesulfonamide (for subtype 8) or methanesulfone (for subtype 11) group as R1 or R2 was optimal for potency; and (b) for R3, the longer CV moiety conferred substantially better potency than the CN group against both WT strain IIIB and the resistant strain RES056, particularly the latter. When tested against single mutants L100I, K103N, Y181C, Y188L, and E138K, and the F227L + V106A double mutant, the best compounds from both subtypes displayed low nM potency against all mutants. Remarkably, compound 11b1 strongly inhibited all mutants with EC50 values of 5.8 nM (L100I), 2.4 nM (K103N), 10.7 nM (Y181C), and 12.4 nM (E138K), superior to those of ETR in the same assay. Against the highly resistant double-mutant F227L + V106, 11b1 showed significantly better potency (EC50 = 10.1 nM) than ETR (EC50 = 22.4 nM), RPV (EC50 = 81.6 nM), and DOR (EC50 = 67849 nM).
To confirm the target engagement, selected analogs were tested in a biochemical assay against recombinant WT HIV-1 RT. In this assay, 11b1 inhibited RT (IC50 = 40 nM) with a potency comparable to that of ETR (IC50 = 13 nM), RPV (IC50 = 15 nM), and DOR (IC50 = 19 nM). The target binding of 11b1 was further studied computationally via molecular docking and molecular dynamic simulation. These studies confirmed that 11b1 can accommodate binding into the both WT and mutant NNIBPs, with favorable binding free energies.
To further characterize 11b1 as a drug lead, in vivo toxicity and pharmacokinetic (PK) were investigated. The safety profile was first assessed in an acute toxicity assay where 11b1 was administered orally to a cohort of 10 Kunming mice at a single dose of 2000 mg/kg. After 7 days, no difference was observed between the treatment group and the control group in body weight change. Based on this, 11b1 was further evaluated for subacute toxicity over 2 weeks with intragastric administration dosed at 50 mg/kg every other day. From these studies, behavioral or histological signs of toxicity were not observed. The PK was evaluated in SD rats using both IV and PO routes to allow the calculation of the oral bioavailability (F). Although the overall F was very poor (4%), the half-life of 11b1 (T1/2 = 2.23 h) was moderately improved over that of K-16c (T1/2 = 1.74 h), suggesting that removing the methylene group may partially mitigate the metabolic vulnerability. Scientifically, this could have been better explored by directly measuring the microsomal stability, as other contributing factors to drug half-life may exist. The poor oral bioavailability also confounds the interpretation of the acute toxicity results where animals were dosed orally. Nevertheless, the lack of any tangible toxicity at a very high dose aligns with 11b1 being a safe drug lead.
In summary, this JMV editor's choice report presented an appealing antiviral drug discovery story highlighting structure-based rational design, comprehensive SAR, rigorous antiviral testing and resistance profiling, and in vivo tox and PK assessments. While continued efforts are warranted in further improving the oral PK, preferably guided by the in vitro ADME (absorption, distribution, metabolism, and excretion) assays, exceptional potency and resistance profile of 11b1 strongly validate the new DAPY subtype as an important chemical platform for developing improved DAPY NNRTIs.
CONFLICT OF INTEREST STATEMENT
The author declares no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
No primary data are included in this article.




