Blended BA.5 infection within 8 days after a boosted bivalent mRNA vaccination strengthens and lengthens the host immunity
Mingzhu Huang and Tingting Cui contributed equally to this study.
Abstract
The impact of SARS-CoV-2 infection shortly after vaccination on vaccine-induced immunity is unknown, which is also one of the concerns for some vaccinees during the pandemic. Here, based on a cohort of individuals who encountered BA.5 infection within 8 days after receiving the fourth dose of a bivalent mRNA vaccine, preceded by three doses of inactivated vaccines, we show that booster mRNA vaccination provided 48% protection efficacy against symptomatic infections. At Day 7 postvaccination, the level of neutralizing antibodies (Nabs) against WT and BA.5 strains in the uninfected group trended higher than those in the symptomatic infection group. Moreover, there were greater variations in Nabs levels and a significant decrease in virus-specific CD4+ T cell response observed in the symptomatic infection group. However, symptomatic BA.5 infection significantly increased Nab levels against XBB.1.9.1 and BA.5 (symptomatic > asymptomatic > uninfected group) at Day 10 and resulted in a more gradual decrease in Nabs against BA.5 compared to the uninfected group at Day 90. Our data suggest that BA.5 infection might hinder the early generation of Nabs and the recall of the CD4+ T cell response but strengthens the Nab and virus-specific T cell response in the later phase. Our data confirmed that infection can enhance host immunity regardless of the short interval between vaccination and infection and alleviate concerns about infections shortly after vaccination, which provides valuable guidance for developing future vaccine administration strategies.
1 INTRODUCTION
Pandemic preparedness through vaccination primarily occurs before the outbreak.1 During the COVID-19 pandemic over the past 3 years, many vaccines received Emergency Use Authorization (EUA) worldwide and were administered to the population when different SARS-CoV-2 variants were being circulated.2 Generally, vaccines typically require 7–14 days to generate effective protective immunity.3 Several studies have reported that hybrid immunity involving both vaccination and infection provides superior protection against subsequent COVID-19 than either vaccination or infection alone.4, 5 However, many vaccinees experienced SARS-CoV-2 infection shortly within 14 days after vaccination.6, 7 This raised a lot of concerns about whether such infection can interfere with the vaccine-indued immunity. Theoretically, Intramuscularly administered vaccines and respiratory infection could trigger two different local immune responses.8, 9 In situations with limited immune resources, it is uncertain whether two concurrent local immune responses may interact cooperatively or antagonistically.
Messenger RNA (mRNA) vaccines are known for their ability to provide robust immune protection.10 Local immunity induced by an injected mRNA vaccine is generally initiated by antigen-presenting cells, which uptake either the mRNA particles derived from the vaccine or the SARS-CoV-2 antigen produced by the muscle cells, carry them to the draining lymph node, in which present antigen or its fragments to T or B cells.11 Additionally, mRNA lipid nanoparticles (LNPs) can also be delivered to hepatocytes aligned along the liver sinusoids, stimulating systematic immunity beyond the injected side.12-14 Therefore, mRNA vaccination might utilize a significant amount of immune resources. In contrast, BA.5 infection primarily affects the upper respiratory tract and has the potential to drain into cervical and mediastinal lymph nodes, triggering robust regional pulmonary immunity.15 BA.5 infection occurring shortly after mRNA vaccine administration provides a unique perspective to study whether two comparable local immune responses can interfere with each other.
We enrolled volunteers who received the fourth dose of bivalent mRNA vaccine, with some being infected with the virus within 8 days of vaccination after vaccination, while others were not. We investigated vaccine efficacy and immune responses, including neutralizing antibodies and T cell responses, to determine whether infection shortly after the booster vaccination could interfere with stimulated adaptive immunity. Thus, this study provides a unique perspective for exploring the effect of short-term blended SARS-CoV-2 infection on vaccine-induced immunity.
2 MATERIALS AND METHODS
2.1 Study design and participants
INDA-3025 Watson's bivalent mRNA targets both Alpha/Beta and omicron (BA.2/BA.4/BA.5) spikes of SARS-CoV-2. To assess the interaction between the vaccine and breakthrough infections caused by the Omicron BA.5 mutant strain shortly after vaccination, we enrolled a total of 30 volunteers who had received three doses of inactivated vaccines (III) preceding the fourth dose of a bivalent mRNA vaccine and administered a questionnaire survey to collect data on the symptoms experienced by these volunteers. Of the 30 distributed questionnaires, 25 were successfully retrieved and included in the analysis. Among the participants in the cohort, 16 (64%) were women. This study was approved and monitored by the Ethics Committee of GMUH (No.2021-78).
2.2 iFlash-SARS-CoV-2 IgG assay
The iFlash-SARS-CoV-2 IgG assay is a chemiluminescent immunoassay that uses paramagnetic particles for the qualitative detection of anti-SARS-CoV-2 S + N IgG antibodies in human plasma. The assay was performed according to the manufacturer's instructions (Shenzhen Yhlo Biotech). Plasma samples were collected from heparinized whole blood by centrifugation at 800g for 10 min. These samples were first incubated with paramagnetic particles coated with SARS-CoV-2 antigens to allow the binding of antibodies to the coated antigens. Then, biotinylated anti-human IgG conjugate was added to form the reaction complex. After adding a triggering solution to the reaction mixture, the anti-S + N IgG titer was measured using the iFlash optical system.
2.3 SARS-CoV-2 conventional virus neutralization test
As mentioned earlier,16 a cytopathic effect (CPE)-based assay was used to assess the neutralizing activity in plasma. 25 µL of plasma samples was serially diluted in steps of 1:2. All samples were mixed with SARS-CoV-2 Wuhan-1, BA.5, and XBB.1.9.1 virus solutions, respectively, containing 100 TCID50 of the virus and incubated at 37°C with 5% CO2 for 2 h. Subsequently, 1.2 × 104 Vero E6 cells were added to the virus-plasma mixture. The plates were incubated in a humidified environment at 37°C with 5% CO2 for 4 days, and the CPE was examined using a Celigo Imaging Cytometer (Nexcelom Bioscience). The presence or absence of CPE was determined by comparing each well with a positive control (plasma samples showing high neutralizing activity against SARS-CoV-2 in infected Vero E6 cells) and a negative control (human serum samples negative for SARS-CoV-2 in enzyme-linked immunosorbent assay (ELISA) and neutralization assay, and Vero E6 cells alone). Neutralizing antibody titers below the detection limit were defined as 50% inhibitory dilutions (NT50) titers of 4.
2.4 Enzyme-linked immunosorbent assay
A direct ELISA was conducted to detect specific plasma anti-N IgG and anti-S IgG in vaccination samples collected from heparinized whole blood by centrifugation at 800 g for 10 min. These plasma samples were added to an ELISA plate coated with the SARS-CoV-2 nucleocapsid (N) protein (Sino Biological) or spike (S) protein to allow the binding of antibodies to the coated antigen. Subsequently, HRP-labeled anti-human IgG conjugates were added to form the reaction complex. A sample with a high anti-N titer was used as the standard, with an arbitrary unit (unit/mL) set at 500. Next, a 2-fold serial dilution of the standard sample was performed at each instance to evaluate the other plasma samples. A washing buffer was used as the blank control. Absorbance was measured at 450 nM using a Multiskan GO microplate spectrophotometer (Thermo Fisher Scientific). Data were analyzed using a standard curve and log-logistic model.
2.5 Peptide pool design and preparation
The design and synthesis of SARS-CoV-2 specific peptides were performed as follows.17 Each peptide was dissolved in DMSO and then pooled to form a stock solution with a concentration of 45 μM for each peptide. A total of 487 15-mer peptides specific to SARS-CoV-2, spanning the entire antigenic regions of the spike(S), membrane (M), nucleocapsid (N), and envelope (E) proteins, with an overlap of 11 amino acids, were designed using an online peptide generator (Peptide 2.0) and synthesized by GL Biochem Corporation. The purity of the synthesized peptides was >80%.
2.6 Peripheral blood mononuclear cell isolation and ex vivo stimulation
Peripheral blood mononuclear cells (PBMCs) were isolated from heparinized whole blood using Ficoll-Paque (GE Healthcare, 17-1440-02) density gradient sedimentation. PBMCs (5 × 105) were then cultured in complete RPMI (c-RPMI, RPMI 1640 medium) (Gibco) enriched with supplements, including 100 μM MEM nonessential amino acids (Gibco), 10% heat-inactivated FBS (Biological Industries), 0.1 mg/mL streptomycin (Gibco), 100 U/mL penicillin (Gibco), 55 μM 2-mercaptoethanol (Gibco), 2 mM l-glutamine (Gibco), 25 mM HEPES (Gibco), and 1 mM sodium pyruvate (Gibco). The PBMCs were treated with a peptide library containing 487 15-mer peptides (250 nM/peptide) and cultured for 12 h at 37°C and 5% CO2 in the presence of 10 U/mL rIL-2. Stimulation of PBMCs with the peptide library was performed using a validated method described by Chevalier et al.18
2.7 Flow cytometry
After 12 h of stimulation, 1 μM GolgiPlug (BD Biosciences) and a chemokine-related antibody, anti-CCR7-APC (BioLegend, clone G043H7, Cat #353214), were added to the cells. After 4 h of stimulation, the cells were harvested from the culture. The cells were washed and incubated on ice for 15 min with Live/Dead FVS440 (Thermo Fisher Scientific, Cat #L34957). Subsequently, the cells were washed again and surface-stained on ice for 30 min using the following antibodies: anti-CD3-BUV395 (BD Pharmingen™, clone SK7, Cat #564001), anti-CD4-PerCP/Cyanine5.5 (BioLegend, clone RPA-T4, Cat #300530), anti-CD8-BV605 (BD Pharmingen™, clone SK1, Cat #564116), and anti-CD45RA-APC-Cy7 (BioLegend, clone HI100, Cat #304128). After fixation and permeabilization on ice for 15 min using Cytofix and Perm (BD Bioscience, cat #554714), intracellular staining was performed on ice for 30 min using anti-TNF-AF700 (BD, clone Mab11, cat #557996), anti-IFNγ-BV785 (BioLegend, clone4S.B3, Cat #502542), and anti-IL2-PE (BioLegend, clone MQ1-17H12, Cat #500307). Finally, after washing, the cells were resuspended in 200 μL of FACS buffer. Data acquisition was performed using the FACSFortessa instrument (BD Bioscience), and analysis was conducted using FlowJo software (Treestar).
2.8 Statistical analyses
All statistical analyses were performed using the GraphPad Prism software. Statistical significance was set at p < 0.05 (*p < 0.05, **p < 0.01, and ***p < 0.001). A Student's t test was used to analyze the differences in mean values between groups. The Mann‒Whitney U test was employed to compare the central tendencies of the two groups (mean or median). Antibody responses were reported as GMTs with a 95% confidence interval (CI). The Wilcoxon rank-sum test was used to compare the paired continuous variables that were not normally distributed. Cutoff values were assigned to evaluate the significance of the p value according to the different statistical analysis methods indicated in each figure legend.
3 RESULTS
3.1 Boosted mRNA vaccination provides 48% protection efficacy against symptomatic infections
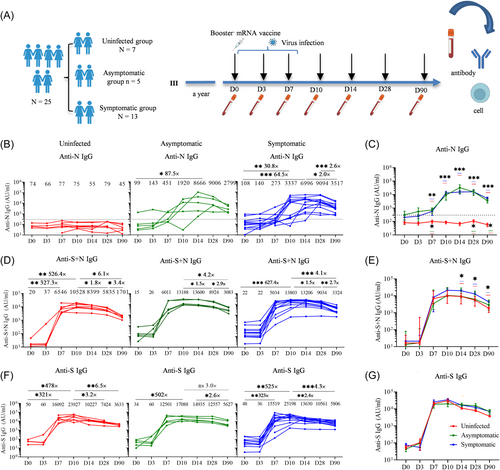
Among the 25 respondents, 12 individuals reported no infection symptoms, while the remaining 13 individuals reported various symptoms such as fever (>37.4°C), nasal congestion, sore throat, and muscle soreness (Table 1). The effectiveness of the bivalent booster against symptomatic SARS-CoV-2 infection was determined to be 48% (1–13/25) within 3 months. To further confirm whether the participants were infected with the BA.5 strain after receiving mRNA vaccines, we observed that the anti-N antibody levels remained at baseline in seven individuals, indicating that they were uninfected and categorized as the uninfected group. The efficacy of the vaccine against the infection in this group was 28%. However, compared to baseline levels, anti-N IgG antibody levels increased in five individuals without symptoms (8666, 95% CI, 592–126 985, p = 0.062), defined as the asymptomatic group, and in 13 individuals with the symptomatic infections (6996, 95% CI, 2607– 18 774, p < 0.0001), defined as the symptomatic group (Figure 1B). Statistically significant differences were observed between the uninfected and asymptomatic groups, as well as between the uninfected and symptomatic groups (Figure 1C).
| Cardinal symptoms (duration) | Breakthrough infection time after vaccination | SARS-Cov-2-positive test results | Gender (n, %) | Age (mean ± SD) | |
|---|---|---|---|---|---|
| Symptomatic | |||||
| p1 | Fever (38.7°C) | Day 8 | Yes | Female (7, 54%) | 47.15 ± 9.73 |
| p2 | Nasal congestion, cough | Day 8 | Yes | ||
| p3 | Fever (38°C), hypogeusia | Day 3 | Yes | ||
| p4 | Sore throat, nasal congestion | Day 6 | Yes | ||
| p5 | Fever (39°C), sore throat | Day 6 | Yes | ||
| p6 | Muscle soreness, sore throat | Day 3 | Yes | ||
| p7 | Fever (39.8°C), hypogeusia | Day 4 | Yes | ||
| p8 | Fever (37.9°C, 2 days), muscle soreness | Day 1 | NA | ||
| p9 | Muscle soreness (4 days), hypogeusia | Day 3 | No | ||
| p10 | Fever (39.4°C, 3 days), tiredness (a week) | Day 0 | Yes | ||
| p11 | Sore throat | Day 6 | Yes | ||
| p12 | Sore throat, nasal congestion | Day 5 | Yes | ||
| p13 | Sore throat (a week) | Day 3 | No | ||
| Asymptomatic | |||||
| n = 5 | None | None | No | Female (3, 60%) | 48.20 ± 12.99 |
| Uninfected | |||||
| n = 7 | None | None | No | Female (6, 86%) | 41.00 ± 10.10 |
- Abbreviation: NA, not applicable.
3.2 BA.5 infection within 8 days after vaccination generates higher and more persistent levels of binding antibodies
To assess the interaction between the vaccine and breakthrough viral infection caused by the Omicron BA.5 within 8 days of mRNA booster vaccination, we compared the kinetics of anti-S + N IgG and anti-S IgG antibodies at specified time points (D0, D3, D7, D10, D14, D28, and D90) following the mRNA vaccination. The levels of anti-S + N IgG antibody in the uninfected (6546, 95% CI, 3671–11 674, p = 0.0061) and symptomatic infection groups (5034, 95% CI, 1934–13 102, p < 0.0001) showed significant increases on D7, peaked on D10, and declined thereafter (Figure 1D). Notably, the anti-S + N IgG antibody titers in the symptomatic infection group were significantly higher than those in the uninfected group at D14, D28, and D90 (Figure 1E). The anti-S + N IgG antibody titers showed a significant decrease at 3 months compared to D10 postvaccination in all three groups. However, the decline was more gradual in the infected group compared to that in the uninfected group. Specifically, in the uninfected group, the anti-S + N IgG antibody titer was 6.1-fold lower, whereas 4.2-fold and 4.1-fold lower anti-S + N IgG antibody titer was observed in the asymptomatic and symptomatic infection groups, respectively (Figure 1D). Additionally, the anti-S IgG antibody titers exhibited similar kinetic patterns to the anti-S + N IgG, with peak antibody levels observed on day 10 after vaccination and declined thereafter. Furthermore, anti-S IgG antibodies exhibited a slower decrease in the infected group compared to the uninfected group. This is reflected by the ratio between anti-S IgG titer at D10 and at D90, which decreased by 6.5-fold for the uninfected group, whereas only by 3.0-fold and 4.3-fold for the asymptomatic and symptomatic infection groups, respectively (Figure 1F,G).
3.3 Stronger, more durable, and broader neutralizing antibodies are induced in the symptomatic group than in the uninfected group
To study the impact of short-term BA.5 infections and vaccine on the generation of neutralizing antibodies (Nabs) and their breadth against different variants, especially against the circulating strain and the coming potential pandemic strains, we conducted an analysis using an authentic SACS-CoV-2 neutralization assay at multiple time points against BA.5. Our findings showed that the level of Nabs against WT and BA.5 strains in the uninfected group trended higher than those in the infection groups at Day 7 postvaccination (uninfected > symptomatic infection > asymptomatic infection; WT: 1965 > 1793 > 1383; BA5: 336 > 284 > 142) (Figure 2A,C), although these differences were not statistically significant. However, the Nabs titers against WT in the symptomatic group were significantly higher at D28 postvaccination compared to those in the uninfected group (3296, 95% CI, 1692–6421 vs. 1367, 95% CI, 674–2777, p = 0.039) (Figure 2B). Similarly, the symptomatic group exhibited higher levels of Nabs against BA.5 than the uninfected groups on both D10 (1348, 95% CI, 538–3375 vs 619, 95% CI, 393–976, p = 0.049) and D14 (1135, 95% CI, 608–2116 vs. 474, 95% CI, 313–717, p = 0.014) (Figure 2D). Furthermore, compared to that on D10, the uninfected group, asymptomatic infection group, and symptomatic infection group respectively induced 10-fold, 3.2-fold, and 4.7-fold lower Nab titers against BA.5 on D90, indicating that the decline was more gradual in the infected group than in the uninfected group. Importantly, our findings indicated that the coefficient of variation (CV) of BA.5 Nabs in the symptomatic (129%) and asymptomatic (125%) groups was higher than that in the uninfected group (61%) on D7 (Figure 2C).
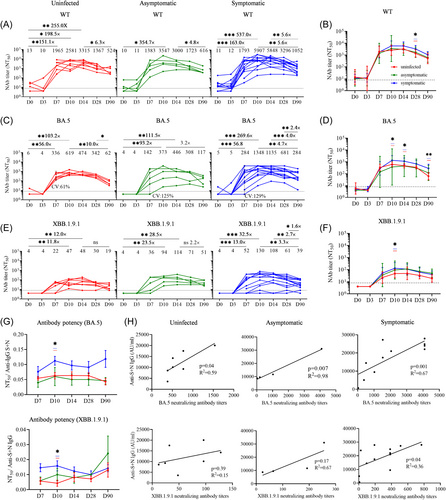
XBB.1.9.1 variant infections surged rapidly, initially among individuals who had not previously experienced BA.5 infection, accounting for approximately 75% of cases. The increase in XBB.1.9.1 reinfections indicated that the protective immunity derived from vaccine infection is weakening. How is the protective efficiency of the fourth vaccination-BA.5 infection against new variant XBB.1.9.1 is of interest to investigate. Therefore, the level of XBB.1.9.1 Nabs was measured in the three groups using samples collected at the indicated time points. The results showed that in the uninfected group, the titer against XBB peaked on D14 (48, 95% CI, 15–160), exhibiting a 12-fold increase compared to D0. Similarly, in the asymptomatic group, the titer peaked on D14 (114, 95% CI, 35–377), which was 28-fold higher than that on D0. Surprisingly, in the symptomatic group, the titer peaked earlier on D10 (130, 95% CI, 43–390), showing a 32-fold increase compared to that on D0 (Figure 2E). Notably, compared with the uninfected group, the symptomatic group exhibited a significantly higher XBB Nab titer on D10 (p = 0.029) (Figure 2F).
To further investigate the quality of the antibodies produced, we used the neutralizing antibody/binding antibody ratio to assess the antibody potency.19 The results showed that the antibody potency against BA.5 and XBB in the symptomatic group was higher than that in the uninfected and asymptomatic groups. Moreover, antibody potency in the symptomatic infection group (BA.5: p = 0.029; XBB: p = 0.026) at D10 was significantly higher than that in the uninfected group (Figure 2G). Notably, a significant correlation existed between neutralizing antibodies against BA.5 and anti-S + N IgG antibodies across all three groups on D10. Additionally, neutralizing antibodies against XBB showed a significant correlation with anti-S + N IgG antibodies in the symptomatic infection group (Figure 2H). These results indicate that symptomatic infection, perhaps due to increased antigen exposure, facilitates the host immune response to generate more antibodies, in the face of recent boost vaccination.
3.4 Blended infection impairs host virus-specific CD4+ T cell response at early time points
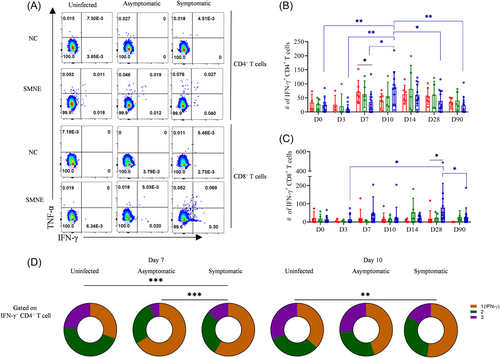
Because blended infection generates more antigens, longer antigen exposure, and higher levels of inflammation than a single dose of mRNA booster, which was evident from the antibody response, we investigated whether the blended infection could also lead to higher T cell responses. Our results showed a significant decrease in the population of SMNE-specific CD4+ T cells in the symptomatic infection group (34/million PBMCs, 95% CI, 12–57, p = 0.029) compared to that in the uninfected group (71/million PBMCs, 95% CI, 33–109) on D7 after vaccination (Figure 3A,B). However, no significant difference was observed in the proportion of SMNE-specific CD4+ T cells among the three groups at other indicated time points (Supporting Information: Figure S1A,B). These results indicate that infection occurring within 8 days postvaccination may impair the generation of virus-specific CD4+ T cell responses at the early time points. The uninfected group did not show an improvement in the virus-specific CD4+ T cell response across different time points, whereas symptomatic infections showed a higher CD4+ T cell response on D10 than that on D0 (86 vs. 25, 3.4-fold, p = 0.0047) (Figure 3B), indicating that the blended infection led to increased virus-specific CD4+ T cells. Similarly, the absolute number of SMNE-specific CD8+ T cells was significantly higher in the infected group than in the uninfected group on D28 (79 vs. 18, 4.4-fold, p = 0.042), and the number and proportion of SMNE-specific CD8+ T cells in the symptomatic infection group on D28 were significantly higher than those observed on D3 (79 vs. 14, 5.6-fold, p = 0.022) (Figure 3C and Supporting Information: Figure S1B). Further, functionality analysis with the co-expression of IFN-γ, TNF-α, and IL-2 by CD4+ T cells after activation revealed that the uninfected group exhibited a higher proportion of multifunctional CD4+ T cells compared to the symptomatic group on D7 (p = 0.00034) and D10 (p = 0.0083) (Figure 3D).
3.5 Infection over vaccination does not facilitate T cell differentiation when compared to vaccination alone
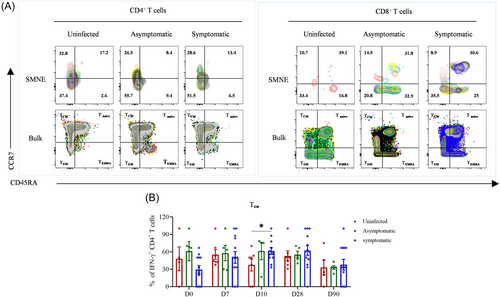
To investigate whether infection can facilitate the differentiation of virus-specific CD4+ T cells from central memory (TCM) to effector memory (TEM) cells or from effector T cells into the memory phase, we characterized the differentiation states of antigen-specific T cells at the indicated time points using CD45RA and CCR7 markers. Our results revealed that the percentages of the four IFN-γ-producing CD4+ and CD8+ T cell subsets (TEM, TCM, Tnaïve, and TEMRA) did not show significant differences among the three groups (Supporting Information: Figure S2A,B), except for CD4+ TEM cells, which were higher in the symptomatic group than those in the uninfected group on D10 (Figure 4A,B). These results indicate that blended infection did not promote the differentiation of virus-specific CD4+ and CD8+ T cells toward the effector memory phenotype.
4 DISCUSSION
Compared to the uninfected group, our data demonstrated that symptomatic infection led to a trend of decreased BA.5 neutralizing antibodies and dampened virus-specific CD4+ T cells on D7, but generated more Nabs at later time points, indicating that mRNA-induced immunity was interfered by BA.5 infection at early time points. It is possible that some of the peripheral antigen-specific lymphocytes migrated into the respiratory mucosa due to BA.5 infection and some to the axillary lymph nodes due to mRNA vaccine.20 This competition may subsequently impact the expansion of stromal cells and lymph node remodeling in the draining lymph nodes within a short period of time, thus suppressing B-cell responses, sequestering lymphocytes in the distal LN, and constraining early time point immunity in the draining lymph nodes.21 Interestingly, 10 days after vaccination, the symptomatic group exhibited higher levels of anti-S + N IgG-binding antibodies and broader Nabs titers against WT and BA.5 variants than those in the asymptomatic infection and uninfected groups. Our results indicated that the blended vaccination strengthened immunity in the later phase. This robust response could be attributed not only to the increased exposure time and antigen concentration of SARS-CoV-2 but also to the number and quality of virus-specific memory B cells.22 However, further investigations are needed to understand the transition from immune suppression to immune strengthening in the context of mRNA vaccination and BA.5 infection.
We observed that the group with symptomatic breakthrough infections exhibited higher levels of neutralizing antibodies against the XBB.1.9.1 variant compared to the asymptomatic infection group and the uninfected group. It is possible that individuals with symptomatic breakthrough infections produce more antigens due to the co-infection, which in turn induces a stronger serological response.23 Interestingly, the neutralizing antibody titers against the XBB variant were similar to those observed in individuals with breakthrough BA.5-XBB reinfections.24 This robust response can be attributed not only to the higher antigen concentration of SARS-CoV-2 but also to the cooperative interaction between immune responses induced by intramuscularly administered vaccines and respiratory infections, leading to a greater peak antibody response.25 Further studies are required to investigate the underlying reasons for the induction of high levels of cross-neutralizing antibodies following mRNA vaccination-BA.5 infection.
Viral gene expression within dendritic cells or other antigen-presenting cells is considered the most efficient mechanism for presenting antigens to MHC-I, which is essential for initiating CD8+ T-cell responses.26, 27 Even if the virus does not directly infect dendritic cells, persistent infection with other cells can provide a continuous supply of viral proteins for uptake and cross-presentation.28 In contrast, inactivated viral vaccines lacking replication capacity face challenges in inducing robust CD8+ T-cell responses.29 Our study revealed that effective induction of CD8+ T cell responses in the symptomatic infection group occurred on D28 postvaccination, which was 14 days after the viral infection. This indicates that virus-specific CD8+ T cells constituted the primary immune response. Conversely, the activation of virus-specific CD4+ T cells in the symptomatic infection group was observed on D10 postvaccination, implying the generation of a strong memory CD4+ T cell immune response. However, the inability of the fourth dose of the mRNA vaccine to elicit a robust virus-specific CD4+ T-cell response may be attributed to the fact that the mRNA vaccine is based on a single antigen (spike protein), whereas SARS-CoV-2 consists of 29 different viral proteins.30 Thus, the breadth of epitopes recognized by the CD4+ and CD8+ T cells was more limited in the vaccine-only group than that in the vaccine-plus viral infection group.31
Although mRNA vaccines typically require 7–14 days to generate effective protective immunity, this small-scale study indicated that the fourth dose of the bivalent mRNA vaccine enhanced immunity against symptomatic BA.5 infection with an effectiveness of approximately 48% (12/25). We considered infections occurring within 8 days of vaccination, which is comparable to the real-world efficacy of Pfizer's bivalent mRNA booster immunization (52%), where infections within 14 days were not accounted for.32 Our data clearly demonstrate that the fourth dose of Watson's bivalent mRNA vaccine continues to provide protection against BA.5 infection within 8 days after administration, showing an efficacy similar to that of Pfizer's bivalent mRNA vaccine.32 Furthermore, rapid BA.5 infection enhances the host immune response, including stronger neutralizing antibodies and higher virus-specific T-cell responses. Our study has several limitations, particularly the small sample size. The trial unexpectedly encountered the BA.5 pandemic rapidly after a small group of individuals were enrolled for the fourth dose of mRNA vaccination. However, this unexpected BA.5 pandemic led to a small sample size, which offered a unique opportunity to investigate the impact of short-term SARS-CoV-2 co-infection on vaccine-induced immunity. As a limited sample size may restrict the generalizability of the findings, further studies should incorporate large sample sizes to provide more accurate and comprehensive insights. Despite the limited sample size in our study, our findings suggest that mixed immunity (vaccine-infection) can boost immune responses, regardless of the short interval between vaccination and infection, and alleviate concerns about infections shortly after vaccination. This study provides valuable guidance for developing future vaccine administration strategies.
AUTHOR CONTRIBUTIONS
Zhongfang Wang and Zhuxiang Zhao designed this study. Zhongfang Wang and Mingzhu Huang interpreted data and drafted the manuscript. Mingzhu Huang, Tingting Cui, Siyi Liu, Xiaoling Su, Yuan Wang, Junxiang Wang, Jiaying Zhong, Jinpeng Cao, Xinyue Mei, Kaiyi Li, Qi Luo, Xi Sun, Li Cheng, and Rui Wei performed neutralizing assay and flow cytometry assay. Mingzhu Huang, Tingting Cui, Siyi Liu, and Jinpeng Cao collected samples. Tingting Cui and Yuan Wang helped to conduct the statistical analysis. Zhongfang Wang and Zhuxiang Zhao made critical revision of the manuscript. all authors read and approved the final manuscript.
ACKNOWLEDGMENTS
This work was supported by the R&D Program of Guangzhou National Laboratory (SRPG23-005), the National Key Research and Development Program of China (2023YFC2306400, 2022YFC2604104 and 2019YFC0810900), the S&T Program of Guangzhou Laboratory (SRPG22-006), and NSFC (81971485, 82271801, and 81970038), Zhongnanshan Medical Foundation of Guangdong Province (ZNSA-2020013), Guangdong Basic and Applied Basic Research Foundation (2022B1111070002, 2019B1515120068, and 2020B1111330001), and Emergency Key Program of Guangzhou Laboratory (No. EKPG21-30), Major Project of Guangzhou National Laboratory (GZNL2023A01009).
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author.




