PCR testing for herpesviruses in aqueous humor samples from patients with and without clinical corneal endothelial graft rejection
Abstract
To compare prevalence of positive PCR tests for herpesviruses between patients with and without a history of clinical corneal endothelial allograft rejection (AGR). Retrospective cross-sectional study with two-group comparison. A total of 307 aqueous humor (AH) samples from 235 Patients and 244 eyes who underwent penetrating keratoplasty or Descemet membrane endothelial keratoplasty or had a diagnostic AH aspiration due to clinical AGR between 2019 and 2023 were tested for DNA of herpes simplex virus (HSV), varicella-zoster virus (VZV), cytomegalovirus (CMV), and Epstein-Barr virus (EBV). PCR test results were compared between the two groups (with/without AGR). Another sub-analysis examined the results of patients without a history of herpetic keratitis. A total of 8% of eyes with clinical AGR (9/108) had a positive PCR result for one of the herpesviruses (HSV:3, CMV:3, EBV:2, VZV:1). All patients in the group without AGR had negative PCR results for all previous viruses (0/136). The difference was statistically significant (p < 0.001). The sub-analysis of eyes without a history of herpetic keratitis also revealed significantly more positive herpes PCR results (7/87) in eyes with AGR than in eyes without AGR (0/42, p = 0.005). Clinical AGR after keratoplasty shows a significant correlation to viral replication. Herpetic infection and AGR could occur simultaneously and act synergistically. Timely differentiation between active herpetic infection and/or AGR is pivotal for proper treatment and graft preservation.
1 INTRODUCTION
Corneal transplantation is the oldest and most successful transplantation in humans.1 In the absence of risk factors, the graft survival rate is estimated to be as high as 90%.1 Corneal allograft rejection occurs when the recipient's immune response is directed against antigens of the donor graft.2 Corneal epithelium, stroma, and endothelium can all be targets for immune system.3 When endothelial cells of the donor graft are targeted, an endothelial allograft rejection occurs. It is a major cause of corneal graft failure and is seen in 5%–18% of cases after penetrating keratoplasty (PKP).4 Clinical manifestations include corneal edema, retrocorneal precipitates on the graft and cells, and/or a Tyndall effect in the anterior chamber.4 However, these manifestations are similar to those of viral endothelial keratitis, an infection of the corneal endothelium caused by a viral pathogen.5-11 The involved viral agents include herpes simplex virus (HSV) 1, HSV 2, varicella-zoster virus (VZV), cytomegalovirus (CMV) and, possibly, Epstein-Barr virus (EBV).5-12 Viral endothelial keratitis can also occur in patients who have undergone keratoplasty,6-8, 11-16 also leading to persistent corneal inflammation, corneal edema, possibly inflammation in the anterior chamber and graft failure. Therefore, distinguishing between viral infection and corneal allograft rejection (AGR) can pose a considerable difficulty17 (Figure 1). In such cases, timely and accurate diagnosis along with appropriate treatment, including the use of antiviral therapy, is critical for effective treatment of the viral infection and graft preservation.12, 13
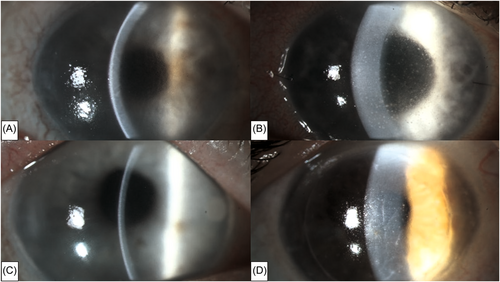
Viral keratitis after PKP is usually the result of reactivation of latent viruses.8, 18 However, it may also occur in patients with no clinical history of viral keratitis,8, 19-21 possibly through donor-to-recipient transmission.14, 22, 23 This can occur both after Descemet membrane endothelial keratoplasty (DMEK)14 as well as after PKP.22, 23
The prevalence of HSV in corneal buttons from patients who have undergone PKP depends on the clinical history of HSV infection and ranges from 1.5% to 4% in patients with a negative HSV history8, 18 and 36%–82% in patients with a positive HSV history.8, 18, 24 However, a positive polymerase chain reaction (PCR) test for HSV in a corneal button is not necessarily indicative of active viral infection.25, 26 In addition, a corneal button can be obtained only in cases of permanent graft failure requiring PKP, and thus does not provide evidence for the treatment of acute graft failure. In contrast, aqueous humor (AH), can be rapidly obtained and used to differentiate between allograft rejection (AGR) and viral endothelial keratitis, providing a viable diagnostic option in such cases. AH is not a known source of viral latency.25 Thus, the detection of viral deoxyribonucleic acid (DNA) in AH is indicative of active and deep stromal or endothelial corneal disease or of active viral uveitis.25, 27
Therefore, the aim of this study is to investigate the prevalence of herpesviral DNA, such as HSV-, CMV-, VZV- and EBV-specific DNA, in the AH of patients with clinical signs of endothelial AGR.
2 MATERIALS AND METHODS
This is a retrospective cross-sectional study comparing the prevalence of herpesviruses (HSV, VZV, EBV, and CMV) in AH between two groups of patients who underwent PKP or DMEK. The study was performed in accordance with the Declaration of Helsinki and approved by the Ethics Committee of the Medical Association of Saarland, Germany (Nr. 146/23). Written informed consent was waived due to the retrospective nature of the study. All data were collected as part of the regular clinical examination and treatment process.
The medical records of the Department of Ophthalmology at the Saarland University Medical Center, Saarland, Germany were reviewed for patients who underwent diagnostic anterior chamber aspiration immediately before keratoplasty or due to AGR immediately before flushing the anterior chamber with Fortecortin (dexamethasondihydrogenphosphat-dinatrium 4 mg/mL) between 2019 and 2023.
All patients in whom at least one diagnostic AH sample was obtained in the setting of PKP or of DMEK or later in the course of endothelial graft rejection after PKP or DMEK were included. AGR was defined as the presence of partial or total corneal edema, retrocorneal precipitates on the graft or Descemet's folds and a Tyndall effect and/or cells in the anterior chamber.
Patients in whom the PCR examination of AH was not possible for technical reasons or in whom AH sampling was performed in the context of deep anterior lamellar keratoplasty (DALK) were excluded from the study.
Demographic data, type of keratoplasty, number of AH samples, time from sampling of AH to the occurrence of AGR, underlying disease, the history of herpes keratitis (HK) (HSV, VZV, CMV, or EBV) with prophylactic use of antiviral therapy at the time of sample collection, the history of AGR episodes, and the results of PCR testing of AH were collected.
Data from 244 eyes from 235 patients was analyzed. We divided the patients into two groups, one with at least one AGR episode (n = 108) and one without an AGR episode (n = 136), and compared the prevalence of PCR tests positive for at least one herpesvirus type in AH (Table 1).
| Graft rejection group (n = 108) | No graft rejection group (n = 136) | p-value | |
|---|---|---|---|
| Age (years; mean ± SDa) | 62 ± 17 | 65 ± 15 | 0.23b |
| Sex (male:female) | 54:54 | 78:58 | 0.25c |
| History of herpes keratitis (yes:no) | 21:87 | 42:94 | 0.04c |
| Aqueous humor samples | |||
| Total number of AHd samplese | 163 | 144 | |
| AHd samples during DMEK | 29 | 39 | |
| AHd samples during PKP | 60 | 105 | |
| AHd samples during AGR | 74 | 0 | |
| Patients with repeat keratoplasty (yes:no) | 90:18 | 96:40 | 0.02c |
| Baseline disease | |||
| Infectious keratitis | 22 | 31 | |
| Ectatic corneal diseases | 23 | 37 | |
| Endothelial corneal diseases | 48 | 48 | |
| Traumatic corneal injuries | 6 | 5 | |
| Other corneal diseases | 9 | 15 |
- Abbreviations: AH, aqueous humor; DMEK, Descemet membrane endothelial keratoplasty; EBV, Epstein-Barr virus; HSV, herpes simplex virus; VZV, varicella-zoster virus.
- a SD: Standard deviation.
- b Student t-test.
- c Chi-square test.
- d AH: Aqueous humor.
- e In some patients, more than one AH sample was acquired.
Our standard operating procedures for keratoplasty include the following: In case of a positive history of herpes keratitis (HK+) (Table 2), patients received 5× ganciclovir gel 1.5 mg/g daily and 5 × 400 mg of aciclovir systemically for 2–4 weeks preoperatively and 6 weeks postoperatively and then 2 × 400 mg of aciclovir for at least 12 months after keratoplasty as standard treatment. All patients who underwent a keratoplasty received prednisolone acetate eye drops 5× daily postoperatively, slowly reduced over 6–8 months, and then used at least 1× daily for at least 2 years after keratoplasty in case of pseudophakia.8
| History of herpetic keratitis | Positive DNA for herpesviruses | Negative DNA for herpesviruses | p-value | |
|---|---|---|---|---|
| Patients with AGR* (n = 108) | Yes (n = 21) | 2a | 19a | |
| No (n = 87) | 7a, b | 80a, b | 0.8a | |
| Total number | 108 | 9c | 99c | |
| Patients without AGR* (n = 136) | Yes (n = 42) | 0 | 42 | |
| No (n = 94) | 0b | 94b | 0.005b | |
| Total number | 136 | 0c | 136c | >0.001c |
- Note: *AGR—positive history of allograft rejection. Comparisons of the frequency of patients with and without positive DNA test for herpesviruses between various patient groups using Chi-square tests. Values included in a certain Chi-square test as well as its p-value have been labeled as follows:
- a The test comparing patients with AGR and a history for herpetic keratitis to those with AGR and without a history for herpetic keratitis (p = 0.8).
- b The test comparing patients with AGR and without a history for herpetic keratitis to those without AGR and without a history for herpetic keratitis (p = 0.005).
- c The test comparing patients with AGR compared to those without AGR (p < 0.001).
- Abbreviations: AGR, allograft rejection; AH, aqueous humor; CMV, cytomegalovirus; DMEK, Descemet membrane endothelial keratoplasty; EBV, Epstein-Barr virus; HSV, herpes simplex virus; VZV, varicella-zoster virus.
AH samples were collected in a sterile collection tube and sent to the laboratory for PCR testing. DNA was extracted from AH using the easyMAG or eMAG platforms (bioMérieux) according to the manufacturer's instructions. The sample input of the AH was 200 µL or, if less material was available, the maximum possible volume, to ensure sufficient material for extraction and subsequent real-time PCR. A total of 70 µL were eluted and 8 µL eluate were used for each PCR. When possible, the remaining material was kept as a reserve sample for possible repeat clinical examination. Real-time PCR was performed for the detection of HSV, VZV, EBV, and CMV DNA according to the protocols in File S1. Primers were used at a final concentration of 0.5 µM, probes at 0.25 µM.
Statistical analysis was performed using SPSS Version 25. Continuous data were described as mean and standard deviation. Categorical variables were described as percentages. When continuous variables were normally distributed, they were compared using the Student t-test. Categorical variables were compared using the Chi-Square test. A p-value of less than 0.05 was considered a statistically significant result.
3 RESULTS
A total of 307 AH samples from 244 eyes of 235 patients were eligible for analysis. The demographic data, clinical history, and context of AH sample collection for both groups are summarized in Table 1. Of the 244 eyes, 63 eyes were HK+, 21 of them (33%) had at least one AGR.
The PCR test was positive in 9 of the 108 patients (8%) with AGR and in none of the patients without AGR (p < 0.001). A total of 2 of the 21 patients with HK+ and AGR had a positive PCR test (10%). The difference between HK+ patients with AGR and HK− patients with AGR in positive PCR tests was not statistically significant (p = 0.8). To further investigate the influence of HK+ on the results, we compared the HK− group with AGR to the HK− group without AGR. The difference was still statistically significant between both groups (p = 0.005) (Table 2). Of the nine eyes with positive PCR test, 3 had HSV, 3 CMV, 2 EBV and one had VZV (Figure 2).
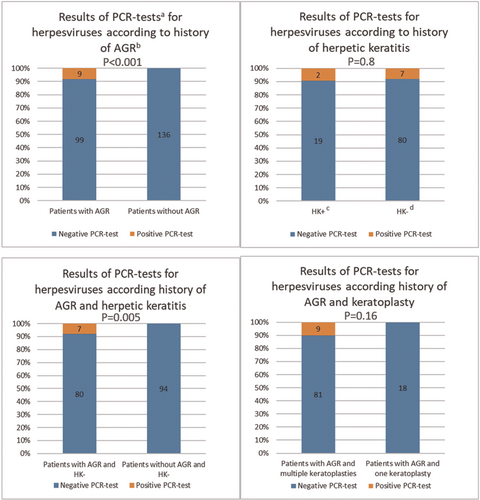
Out of the 108 patients with AGR, 90 had at least one repeat keratoplasty and 18 had only one keratoplasty. Interestingly, all nine patients with a positive PCR test were in the repeat keratoplasty group (p = 0.16) (Table 3).
| Patients with repeat keratoplasty | Positive DNA for herpesviruses | Negative DNA for herpesviruses | p-value | |
|---|---|---|---|---|
| Patients with AGRa (n = 108) | Yes (n = 90) | 9b | 81b | |
| No (n = 18) | 0b | 18b | 0.16b | |
| Total number | 108 | 9 | 99 | |
| Patients without AGRa (n = 136) | Yes (n = 42) | 0 | 42 | |
| No (n = 94) | 0 | 94 | ||
| Total number | 136 | 0 | 136 |
- Abbreviations: AGR, allograft rejection; CMV, cytomegalovirus; EBV, Epstein-Barr virus; HSV, herpes simplex virus; VZV, varicella-zoster virus.
- a AGR: positive history of allograft rejection.
- b Comparison of the frequency of patients with and without positive DNA test for herpesviruses using chi-square test between the group of patients with AGR and repeat keratoplasty and those with AGR and a single keratoplasty (p = 0.16). Numbers included in the Chi-square test as well as its p-value have been listed in the table.
The time between onset of AGR and AH sampling was known for 122 of the samples in eyes with a history of AGR. The missing data concerned patients in whom AGR had been documented and treated in other departments before they were transplanted in our department.
All AH samples collected during the administration of Fortecortin into the anterior chamber were taken during an active AGR (time to onset of AGR = 0 days). For the 48 samples collected during keratoplasty, the median was 93 days (range: [0–455], 25% quantile: 44 days, 75% quantile: 153).
PCR tests were positive in 5/74 samples during active AGR and in 5/48 samples during keratoplasty. One eye had two positive PCR tests, one during active AGR and one during keratoplasty. Thus, all 10 positive PCR tests were preceded by AGR. In eyes with positive PCR tests after AH sampling during keratoplasty, the time from onset of AGR to keratoplasty was 0 days in two of these eyes and 20, 151, and 152 days in the other three eyes. The different number of positive PCR tests between samples after keratoplasty (5/48) and samples during active AGR (5/74) was not statistically significant (p = 0.5).
4 DISCUSSION
This study provides evidence for the possible role of different herpesviruses in clinical AGR. It estimates the prevalence of herpesviruses in AH in patients with a clinical AGR using specific PCRs and examines the influence of previous herpetic keratitis on these results, as well as the association between a positive PCR test and repeat keratoplasty.
4.1 Evidence for and prevalence of herpetic endothelial keratitis in patients with clinical AGR
Several case reports demonstrated an association between herpesviruses and AGR leading to graft failure.12-15, 22, 28 The role of herpesviruses in graft failure after keratoplasty has also been suggested in some larger studies.7, 8, 29 We had previously examined the prevalence of HSV in corneal buttons of patients with graft failure after AGR and found that it was positive in 7 of 95 patients.8 Van Gelderen et al.7 reached a similar conclusion by testing the corneal buttons and AH of 28 patients with allograft failure who underwent PKP for HSV and VZV using a combination of PCR tests of corneal tissue and of AH as well as testing for antibodies in AH. PCR testing for HSV was positive in 10 of 28 patients, two of whom also tested positive for VZV, suggesting a prevalence up to 35%. AH testing was simultaneously performed in 17 of the previous 28 patients. A test with a Goldmann-Witmer coefficient value >3 was considered positive.30 The antibodies were positive in 7 of these patients (45%). They concluded that HSV could, therefore, be involved in corneal infection leading to allograft failure. Interestingly, PCR testing for HSV and VZV in corneal tissue was negative in 5 of the previous 7 patients with positive antibody production in AH. The simultaneous PCR test of AH of the previous 17 patients was also negative, probably due to the small sample size and the fact that the PKPs were performed in the quiescent phase of viral keratitis.7
While the previous findings confirm the role of herpesviruses in allograft failures, they also show that investigation of viral infection of corneal tissue versus AH directly via nucleic acid amplification techniques (NAT), such as PCR, may give rise to different results on the estimation of the prevalence of herpetic endothelial keratitis in corneal grafts compared to antibody testing of AH.
PCR testing of herpesviruses in AH has the advantage of good sensitivity in the early phase of intraocular infection with a very high specificity.30 The test was truly negative in 140 of 140 in AH in patients who underwent cataract surgery,27, 30-32 although many of them had a positive serological test for these viruses. Thus, a positive PCR test result would reliably identify patients with active intraocular viral infection,27, 31-33 strongly suggesting a causative role of the detected viruses in the observed endothelial keratitis in our patients. However, due to the rapid clearance of viral DNA in AH compared with the slow clearance in avascular corneal tissue, it would likely not identify patients, in whom infection has resolved. AH could theoretically be contaminated with host blood, either during surgery or by spontaneous bleeding in the anterior chamber of the eye. This would potentially lead to a false-positive PCR test, especially in the case of CMV and EBV viremia without concomitant eye infection. However, such a contamination is very unlikely in practice. Circulating blood cells cannot access the anterior chamber of the eye under normal circumstances due to the immune privilege of the eye.34 Additionally, the aspiration of the anterior chamber of the eye is done under sterile surgical conditions in the operation room, using a very fine (30 Gauge) needle that goes through the non-vascularized cornea. The aspiration is done before the corneal transplantation begins, hence minimizing the risk of contamination with blood through the following surgical manipulation. The negative PCR tests in the control group in our study as well as the high true negative values in the above-mentioned studies provide good evidence for the extremely low contamination rate of AH aspirates in clinical practice.
In contrast, testing for intraocular herpesvirus infection using antibodies in AH reaches good sensitivity with lower specificity no earlier than 5 days after onset of infection.30 Antibodies also continue to be produced after the resolution of the infection, which makes it more suitable for identifying patients with a recently cleared or long-lasting herpesvirus infection.
Negative PCR in AH does not exclude a latent or recently resolved herpesvirus infection.7 Therefore, the true prevalence of herpetic keratitis masked as AGR is likely to be even higher than estimated in this study. On the other hand, antibodies in AH cannot reliably detect a herpetic infection in the early phase, and the DNA in corneal tissue persist long after active infection has resolved,25, 26, 30 limiting the usefulness of PCR test in corneal buttons.18, 35 An accurate estimation of prevalence would, therefore, likely require simultaneous testing with PCR and antibodies in AH, as well as PCR in corneal buttons, as these would complement each other and together provide a better understanding of the evolution of the disease over time.
4.2 Influence of a history of herpetic keratitis on the incidence of herpetic endothelial keratitis after keratoplasty
When comparing patients with AGR and HK+ to patients with AGR and HK−, there was no statistically significant difference between the two groups in our study (p = 0.8). The difference between patients with and without AGR was still statistically significant even after excluding patients with HK+ from the analysis. These results suggest that a history of herpetic keratitis does not increase the risk of herpetic endothelial keratitis after keratoplasty, when prolonged topical and systemic prophylactic therapy with antivirals is initiated.
In the study of Van Gelderen et al.7 A total of 8 of the 17 patients in whom AH was tested were HK+ and 9 were HK−. Antibodies were positive in 7 of these patients, 4 of whom were HK+ and 3 HK−. They concluded that HSV could, therefore, be involved in corneal infection during allograft failure, even in HK− corneas. These conclusions agree with those of the current study.
4.3 Association between a positive PCR test and repeat keratoplasty
In our study, 4 of the 9 patients with a positive test for herpesviruses underwent repeat keratoplasty, 3 underwent 2 repeat keratoplasties, and 2 underwent 3 repeat keratoplasties. After herpesvirus detection using PCR in AH and initiation of prophylactic treatment, 4 of the patients did not require further keratoplasties, 4 of them required another keratoplasty, and one required three keratoplasties and again had a positive PCR test for HSV in AH at the last surgery. This indicates that rapid initiation of therapy could prevent failure of the current graft and, if the current graft is already damaged, failure of subsequent grafts.
It was interesting to see that all patients with positive PCR tests had more than one keratoplasty, which could indicate that viral infection/reactivation is a major cause for graft failure and consequent repeat keratoplasty. Further studies with a larger cohort are required to explore this possibility.
4.4 Origin and risk factors for viral endotheliitis after keratoplasty
The relatively high estimated prevalence of viral activity of various herpesviruses after keratoplasty raises several questions about the origin and risk factors for these infections.
Infections with herpesviruses are very common in the general population. Such infections are usually mild and are controlled in most immunocompetent patients, but establish lifelong latency.36 After primary infection, HSV and VZV remain latent in neural cells,36 whereas CMV remains latent in polymorphonuclear leukocytes, monocytes, and hematopoietic progenitor cells.37 EBV also establishes a lifelong latency in infected lymphocytes after an acute infection.38 The prevalence of HSV-1 in the world population under 50 years of age is estimated at 67%.39 More than 90% of the world's population is infected with EBV.40 A total of 99% of the German population over the age of 40 are infected with VZV.39 Furthermore, the seroprevalence of CMV infection was estimated to be 66%–90% worldwide.41
The high prevalence of positive serostatus in the population and the emergence of PCR tests have reduced the value of serologic tests in the diagnosis of herptic corneal infections. In the Japanese corneal endothelial study, 106 patients with corneal endotheliitis and positive PCR tests for CMV in AH samples were examined. The study showed that serum CMV immunoglobulin G (IgG) was positive in 100% of tested cases and that CMV IgM was negative in 100% of tested cases.42 Additionally, Serum CMV antigen is not typically elevated in patients with CMV endotheliitis.43 However, recent studies on CMV infections after solid organ transplantation have shown that recipients with negative CMV serostatus from donors with positive CMV serostatus have a high risk of CMV infection after such transplantation.44 To the best of our knowledge, there are no studies that have investigated the influence of donor/recipient CMV serostatus, nor of other herpesviruses, on the risk of viral keratitis after corneal transplantation. Therefore, exploring the influence of herpesvirus serostatus of recipients and donors as a risk factor for viral keratitis after corneal transplantation is warranted.42
Herpesviruses could be reactivated under several circumstances leading to an infection of the corneal transplant. The high prevalence of latent herpesviruses makes reactivation of these viruses a likely explanation for the observed endothelial keratitis,8, 18 especially in patients with a previous history of viral keratitis. However, this may also occur in patients with no such history,8, 19-21 possibly through donor-to-recipient transmission.14, 22, 23
Many of the herpesviruses’ reactivation risk factors are inherent to the corneal transplantation procedure. Risk factors for HSV and VZV reactivation include emotional stress, surgical trauma, postoperative tear dysfunction, use of corticosteroids and occasionally use of other immunospressants,45 and neural stress response induced through the operation itself.36 The Japanese corneal endotheliitis study showed that 96.3% of patients with CMV endotheliitis were using topical corticosteroids and 25.7% of patients had a history of corneal transplantation, underscoring the importance of these factors in CMV infection/reactivation.42 Immunosuppression is also a known risk factor for EBV reactivation.46
Additionally, a history of herpetic keratitis is a known risk factor for infection, even after a successful corneal transplantation. This risk can be effectively reduced by the postoperative prophylactic use of antiviral therapy for at least 1 year.39, 47-50
On the other hand, a positive PCR test for herpesvirus in the donor cornea does not appear to increase the risk of herpes keratitis after successful corneal transplantation. Morris et al.35 tested 80 donor corneas for HSV PCR and with viral culture before transplantation. Although 3 out of 80 transplants were positive for HSV DNA, viral culture was negative in all three, and the follow-up after transplantation showed no sign of viral keratitis. Another study from Horstmann et al.39 showed similar results. They tested donor corneas for HSV, VZV, and CMV PCR and found a prevalence of 8.2% (7/85) positive PCR tests. However, none of the patients developed viral keratitis after transplantation.
Various systematic factors are known to increase the risk of viral reactivation, including older age, diabetes mellitus, compromised immune system, autoimmune diseases, ocular rosacea, long-term immunosuppression, corticosteroid use and history of organ transplantation.
It is important to keep in mind that a viral infection could undermine the immune privilege of the anterior chamber and the cornea leading to exposure of transplant antigens to the host immune system and to a secondary AGR after corneal transplantation.34 Additionally, AGR as well as other co-infections of the cornea could lead to differentiation of corneal dendritic cells and/or to activation of B-lymphocytes, thus triggering the reactivation of CMV51 and EBV,38 respectively.
Endotheliitis following keratoplasty with accompanying viral replication is, therefore, more of an interplay between viral infection and immune response, whereby the two diagnoses are not mutually exclusive but can also occur simultaneously and act synergistically. Therefore, in the case of viral endotheliitis after keratoplasty, immunosuppressive therapy should always be administered in addition to appropriate antiviral therapy. In the case of significant or persistent AGR under corticosteroids, viral activity should also be excluded by means of AH aspiration.
4.5 Regular screening and prophylactic therapy for herpesviruses in patients with allograft rejection
Based on our findings, the number of patients needed to be screened (NNS) is estimated to be 12 when the AH of patients with current or recent clinical AGR is tested for herpesvirus DNA. Although the NNS is relatively high, the consequences of overlooking a viral infection, such as permanent graft failure and the need of repeat keratoplasty with a higher risk of viral keratitis recurrence after transplantation, warrant regular testing for herpesviruses in AH in patients with AGR and would possibly justify empirical use of antiviral therapy until test results are available. We hence recommend testing AH for herpesviruses in patients with AGR. Empirical antiviral therapy has also been proposed by van Gelderen et al.7 Further studies exploring other possible risk factors would help to further reduce NNS, and thus make screening more efficient. AH testing for herpesviruses with a combination of PCR testing and antibody testing with formation of the Goldmann-Witmer coefficient30 could potentially increase the detection of herpes infections and overcome the shortcomings of both methods, leading to a more accurate estimate of the prevalence of actual as well as previous herpesvirus infection.30 Further studies in this field are required to answer this question.
The present study also highlights the importance of testing not only for HSV but also for other herpesviruses including VZV, CMV, and EBV. Further studies with a larger number of positive cases would allow differentiation between the clinical manifestations and prognosis of infections with different herpesviruses.
This study is limited due to its retrospective nature and the fact that predominantly patients with moderate to severe acute graft rejection were included, as patients with mild AGR are usually treated as outpatients, selection bias cannot be excluded. However, the results strongly suggest that viral infection is a frequent cause of what is usually clinically perceived as endothelial allograft rejection.
Although a positive PCR test result in AH could also indicate an anterior uveitis, keratouveitis, or a deep stromal or endothelial viral keratitis,17 this distinction has no consequence for subsequent therapies with antiviral drugs and steroids and is, therefore, not of relevance in the clinical setting of acute graft rejection.
It is important to keep in mind that corneal transparency is extremely reduced after graft failure. Therefore, due to the limited visibility and the resulting difficulty in assessing the inflammatory status of the anterior chamber (cells/Tyndall), it is hard to say whether a new AGR has occurred after the first one. We, therefore, think that the actual time between AGR and keratoplasty might be overestimated in our study.
The antiviral preoperative treatment of patients with a history of herpes keratitis could reduce the sensitivity of a viral PCR test afterwards. Nevertheless, it is the only ethically acceptable approach and thus cannot be avoided in future studies.
5 CONCLUSION
Clinical AGR after keratoplasty shows a significant correlation to viral replication. Herpetic infection and AGR could occur simultaneously and act synergistically. A rapid test of AH helps to distinguish between active herpes infection and allograft rejection and is critical for proper treatment and graft preservation. We recommend examining AH for herpesviruses using a combination of PCR and antibody testing to increase the accuracy of diagnosis and reduce graft failure. A short course of empirical antiviral therapy until the results are available may also be advisable. Further studies examining AH using the above-mentioned combination and exploring other risk factors may also provide a better estimate of the prevalence of viral infections in these patients and make the screening process more effective.
AUTHOR CONTRIBUTIONS
Yaser Abu Dail: conceptualization, methodology, formal analysis, investigation, visualization, writing. Loay Daas: conceptualization, methodology, writing. Elias Flockerzi: methodology, writing. Cristian Munteanu: formal analysis, writing. Julian Kahlert: methodology, writing. Sigrun Smola: methodology, investigation, writing. Berthold Seitz: conceptualization, methodology, writing, supervision. All authors attest that they meet the current ICMJE criteria for Authorship.
ACKNOWLEDGMENTS
The authors thank Luisa Scheidhauer, Jonas Klinz, Christine Karrenbauer, Iris Kaffenberger, Ellen Zott, Angelina Glahn and Constanze Clemens for excellent technical support. Open Access funding enabled and organized by Projekt DEAL.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.




