TRIM6 facilitates SARS-CoV-2 proliferation by catalyzing the K29-typed ubiquitination of NP to enhance the ability to bind viral genomes
Jian Zhou and Yuzheng Zhou are contributed equally to this study.
Abstract
The Nucleocapsid Protein (NP) of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is not only the core structural protein required for viral packaging, but also participates in the regulation of viral replication, and its post-translational modifications such as phosphorylation have been shown to be an important strategy for regulating virus proliferation. Our previous work identified NP could be ubiquitinated, as confirmed by two independent studies. But the function of NP ubiquitination is currently unknown. In this study, we first pinpointed TRIM6 as the E3 ubiquitin ligase responsible for NP ubiquitination, binding to NP's CTD via its RING and B-box-CCD domains. TRIM6 promotes the K29-typed polyubiquitination of NP at K102, K347, and K361 residues, increasing its binding to viral genomic RNA. Consistently, functional experiments such as the use of the reverse genetic tool trVLP model and gene knockout of TRIM6 further confirmed that blocking the ubiquitination of NP by TRIM6 significantly inhibited the proliferation of SARS-CoV-2. Notably, the NP of coronavirus is relatively conserved, and the NP of SARS-CoV can also be ubiquitinated by TRIM6, indicating that NP could be a broad-spectrum anti-coronavirus target. These findings shed light on the intricate interaction between SARS-CoV-2 and the host, potentially opening new opportunities for COVID-19 therapeutic development.
1 INTRODUCTION
Since its outbreak in 2019, COVID-19 has caused nearly seven million deaths and immeasurable losses to the human society, motivating scientists to continue investigating severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) on a comprehensive scale. SARS-CoV-2 belongs to the β coronavirus genus,1, 2 harbors a single-stranded positive-sense RNA that encodes 29 viral proteins including 4 structural proteins, 16 nonstructural proteins, and 9 accessory proteins. The structural proteins include the Spike (S), Membrane (M), Envelope (E) and Nucleocapsid Protein (NP),3 which assemble into a proteinaceous coat that encapsulates the viral genomic RNA.4 NP is a crucial structural protein that wraps the viral genomic RNA into a ribonucleocapsid protein complex and facilitates the assembly of viral particles via interactions with the M.5 Structurally, the NP primarily comprises the C-terminal domain (CTD), N-terminal domain (NTD), and three intrinsically disordered regions that separate the CTD from the NTD.6
Post-translational modifications (PTMs) of the protein constitute a vital foundation for the functionality of NPs. The multifunctionality of NP requires the engagement of various PTMs, including phosphorylation, methylation, succinylation, and acetylation.7 Thirty-two phosphorylation sites primarily located within the serine-rich (SR) region of NP has been reported.8 Phosphorylation of NP regulates the formation of dynamic liquid-like condensates, which in turn affects viral replication, and inhibition of phosphorylation of NP inhibits viral replication.9, 10 The methylation of NP regulates its ability binding to viral genomic RNA,11 and the succinylation of NP exerts a dual impact on viral replication by modulating the assembly and replication process.12 Additionally, acetylation of NP disrupts the positive charge of the NTD, involving in the interaction with the viral genomic RNA.13 Our previous study and other omics studies have also identified numerous ubiquitination sites on the NP,14, 15 but its corresponding function remains unknown.
The TRIM family contains more than 70 members that play significant roles in immune response, cancer pathogenesis, and drug resistance as E3 ligases.16-18 TRIM6 plays a crucial role in viral infection and inflammatory response, like regulating IκB kinase-ε (IKK-ε) activation,19 STAT1 phosphorylation,20 DHX16 ubiquitination.21 TRIM6 functions differently in different viral infections, for example, Nipah virus M protein inhibits IKK activation by degrading TRIM6 to escape host immunity.21, 22 Ebola virus hijacked TRIM6 promoting the ubiquitination of VP35, enhancing virus replication.23 Our previous study has shown that TRIM6 positively regulates SARS-CoV-2 infection.14 In this study, we found that TRIM6 catalyzed the K29-polyubiquitination at three sites of NP, which enhanced the ability of NP to bind to viral genomic RNA, thus promoting SARS-CoV-2 proliferation. These findings increased our understanding on the complex interplay between SARS-CoV-2 and host and may offer new therapeutic targets against COVID-19.
2 RESULTS
2.1 The E3 ligase TRIM6 promotes SARS-CoV-2 replication
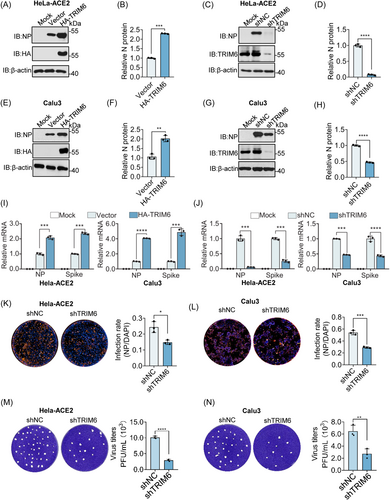
TRIM6 was screened as a host factor that promotes SARS-CoV-2 infection in our previous study.14 To validate this phenotype, we initially established stable HeLa-ACE2 cell lines with TRIM6 overexpression or knockdown and assessed the impact on viral infection (Figure S1A-D). Consistently, overexpression of TRIM6 enhances viral replication, and knockdown has the opposite effect (Figure 1A−D). To enhance the reliability of our findings, we conducted identical experiments in Calu3 cells and obtained consistent data, confirming TRIM6 as a potential enhancer of SARS-CoV-2 replication (Figure 1E−H and Figure S1E−H). In addition to the translational aspect, we also observed that TRIM6 influences viral replication at the transcriptional level. Overexpression and knockdown of TRIM6 in the two cell lines depicted in the figure resulted in differential effects on the mRNA levels of viral Spike and NP (Figure 1I−J). To further assess the cumulative effect of TRIM6 on viral replication, we varied the infection duration and found that TRIM6 overexpression increased the NP accumulation rate, whereas its knockdown elicited the opposite effect (Figure S1I−L). In addition, we also examined the impact of TRIM6 knockdown on the generation of progeny virus by detecting supernatant viral titers. Knocking-down TRIM6 reduced viral titers in the supernatant to varying degrees in both cell lines through immunofluorescence and Plaque-Forming Unit (PFU) experiments (Figure 1K−N). These findings suggest that the E3 ligase TRIM6 plays a pivotal role in facilitating SARS-CoV-2 replication.
2.2 Viral NP was the downstream target of TRIM6
To elucidate the mechanism of TRIM6 promoting SARS-CoV-2 infection, affinity mass spectrometry was used to identify the downstream target of TRIM6. Notedly, SARS-CoV-2 NP possessed the highest interaction score with TRIM6 (Figure 2A,B). To confirm the reliability of the interaction between TRIM6 and NP, we investigated the interaction between the viral proteins and TRIM6 protein through an exogenous immunoprecipitation (IP) experiment. Only the NP was strongly interacted with TRIM6 in a specific manner (Figure 2C, Figure S2A,B]. Despite our findings suggesting a weak interaction between TRIM6 and viral NSP15 and ORF6, it does not impact their ubiquitination levels (Figure S2C−F). Besides, endogenous TRIM6 has also been found to interact with NP during SARS-CoV-2 infection (Figure 2D). Co-incubation of TRIM6 and NP after purification also confirmed that TRIM6 and NP interacted directly (Figure 2E). The colocalization experiment demonstrated that TRIM6 and NP had the same spatiotemporal expression characteristics (Figure 2F). These results suggest that TRIM6 plays a crucial role in regulating SARS-CoV-2 infection by interacting with viral NP.
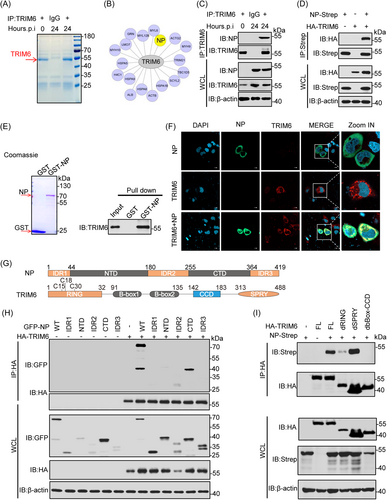
To identify the key domains of TRIM6 and NP binding the truncated and deletion mutants of NP and TRIM6 were constructed (Figure 2G). Co-IP experiments revealed that the NP's CTD is responsible for its independent interaction with TRIM6 (Figure 2H). Notably, NP lacking the CTD failed to interact with TRIM6 (Figure S2G). Additionally, the interaction experiment between the differential structural domains of TRIM6 and NP suggest that the RING and B-box-CCD domains are necessary for interaction with the viral NP (Figure 2I). These results show that the NP of SARS-CoV-2 was the downstream target of TRIM6.
2.3 TRIM6 is a specific E3 ubiquitin ligase for NP ubiquitination
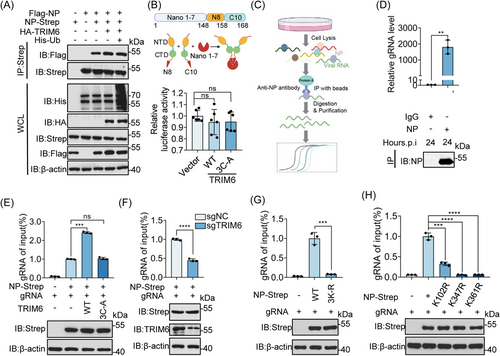
To investigate whether TRIM6 mediated the ubiquitination of NP. TRIM6, NP and Ub were co-overexpressed into HEK293T cells, and the level of ubiquitination was detected after enrichment of NP. Expectedly,the polyubiquitination of the NP was significantly increased in the presence of TRIM6 (Figure 3A). Conversely, TRIM6-knockdown significantly reduced this effect (Figure 3B). While the absent and inactivated mutated TRIM6 could not promote NP ubiquitination (Figure 3C−E), an unsurprising discovery showed that only the RING domain of TRIM6 was necessary for the ubiquitination of the NP, and the effect of the B-box-CCD domain on ubiquitination of the NP may be caused by the absence of an interaction between TRIM6 and the NP, these may indicate that TRIM6 is a specific E3 ubiquitin ligase for NP. In order to simulate the ubiquitination of NP by TRIM6 in vitro, we induced the expression of GST-NP in BL21 E. coli by IPTG (0.5 mM) and purified the TRIM6 from HEK293T cells. Coomassie staining was used to detected these proteins and in vitro ubiquitination assay further confirmed that TRIM6 ubiquitinated the viral NP directly (Figure 3F,G). Moreover, mutation of the TRIM6 active site led to the loss of its promoting effect on viral replication at protein and mRNA level (Figure 3H,I). As well as IFA and PFU experiments (Figure 3J,K). This suggests that TRIM6 plays a virus-promoting role by modifying NP ubiquitination.
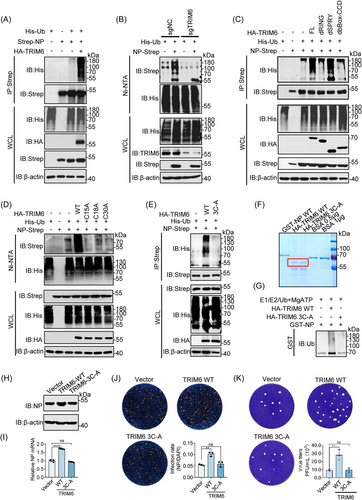
2.4 TRIM6 promotes K29-typed ubiquitination of NP and specifically targets K102, K347, and K361
Since the NP was ubiquitinated by TRIM6, NP stability was evaluated in its natural state by adding cycloheximide (CHX) and found that the NP has a long half-life, strong stability, and was not easily degraded (Figure S3A,B). These data indicated that TRIM6 ubiquitinated NP but did not affect its protein level. It is of great significance to identify the types of ubiquitin chain of NP modified by TRIM6 and resolve the functional effects. Therefore, we allowed NP to react with a series of ubiquitin mutants containing only one lysine residue each (K6, K11, K27, K29, K33, K48, and K63). We found that only the K29 ubiquitin mutant induced NP ubiquitination in response to TRIM6 (Figure 4A). Additionally, the mutation of lysine at site 29 of ubiquitin prevents it from being added to NP by TRIM6 unlike that in the wild-type (Figure 4B). A series of potential ubiquitination sites on the SARS-CoV-2 NP were identified in our previously study14 (Figure S3C). Point mutation screening showed that the E3 ubiquitin ligase TRIM6 specifically modified the ubiquitin lysine chain at sites 102, 347, and 361 of NP (Figure S3D-H). To further assess the precise ubiquitination type at target sites, we cotransfected E3 ligase, mutated NP, and K29 Ub to evaluate the ubiquitination type. The results indicate that TRIM6 mediates ubiquitin chain at NP's 102, 347, and 361 sites through K29-typed (Figure 4C). Moreover, simultaneous mutation of the three lysine residues significantly weakened the K29-typed ubiquitination by TRIM6 (Figure 4D). Furthermore, co-incubation of the purified proteins E1 (UBE1), E2 (UBE2K), E3 (TRIM6), MgATP, and K29-Ub in vitro showed that TRIM6 ubiquitinates wild-type NP in vitro,19 whereas ubiquitination was greatly reduced following the mutation of the target sites (Figure 4E). Notably, the ubiquitination sites were located on NTD (K102) and CTD (K347 and K361) of NP, respectively (Figure 4F). To validate the role of NP ubiquitination in SARS-CoV-2 infection, we used a cell culture system to produce SARS-CoV-2 trVLPs.24 The results showed that the mutation of lysine to arginine at positions 102, 347, and 361 of the NP significantly inhibited trVLP infectivity of SARS-CoV-2 (Figure 4G, and Figure S3I), suggesting that NP ubiquitination is essential for viral survival.
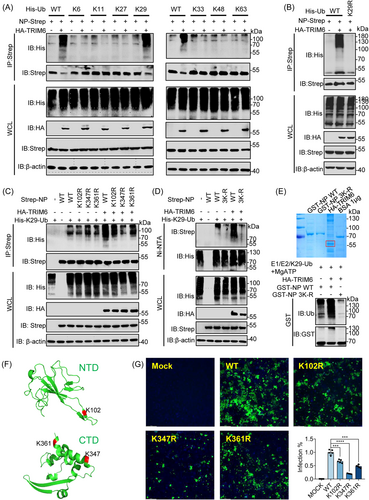
2.5 TRIM6 promotes the binding of NP to genomic RNA through ubiquitination
NP, as an important structural protein of virus, promotes viral replication by intertwine with viral genomic RNA and promotes viral assembly by forming dimerization of CTD domain.25 The investigation into the impact of TRIM6 on NP oligomerization. We first assessed the impact of TRIM6 catalysis on the interaction between NPs. Initially, IP experiment and bioluminescence resonance energy transfer (BRET) assays were utilized to observe the influence of ubiquitination on NP interaction, revealing that there is no significant impact of TRIM6 on NP interaction (Figure 5A,B). Subsequently, we evaluated the influence of TRIM6 on NP oligomer formation through experiments on NP oligomerization. The results indicated that TRIM6 ubiquitination of NP similarly does not affect the formation of NP oligomers (Figure S3J). This provides a clearer understanding of the impact of TRIM6 on the interaction and dimerization of NP. Next, TRIM6 affects the binding of NP to genomic RNA to promote viral replication, RNA IP (RIP) was performed (Figure 5C). We tested the binding of the viral NP to genomic RNA under natural infection conditions to validate the feasibility of our research system (Figure 5D). In addition, using an enzyme inactivated mutation of TRIM6 showed that WT TRIM6 catalyzed ubiquitination of the NP significantly enhanced its ability to bind to genomic RNA (Figure 5E). When TRIM6 was knocked down, the interaction between the NP and genomic RNA significantly decreased (Figure 5F). These results suggest that TRIM6 plays a crucial role in regulating NP during its binding to genomic RNA. Further study of the changes in the binding ability of NP to genomic RNA after lysine mutations at key sites showed that lysine mutations at sites 102, 347, and 361 on NP significantly reduced its binding ability to genomic RNA. And simultaneous mutation of the three sites also obtained consistent conclusions (Figure 5G,H).
2.6 Virus infection may hijack TRIM6 to support its lifecycle
Previous RNA-seq data showed that TRIM6 was upregulated after viral infection.26, 27 We also conducted an analysis of the transcriptional changes in TRIM6 levels in the peripheral blood of patients with different symptoms from data set GSE213313 and observed a gradual increase in TRIM6 expression as disease progressed (Figure 6A). A significant increase in TRIM6 transcription was observed following airway epithelial organoid infection (Figure 6B). Virus-infected Calu3 cells displayed an increase of TRIM6 at the protein level as MOI and infection time increased (Figure 6C,D), which can be attributed to the increase in TRIM6 transcription levels caused by the infection (Figure 6E). Additionally, public data analysis and live virus infection experiments showed that SARS-CoV, MERS-CoV, HCoV-229E, and HCoV-OC43 promoted TRIM6 expression at different transcription levels after infecting host cells (Figure 6F,G).26, 27 These findings indicate that viral infection causes a systemic inflammatory response in the host, which in turn activates the transcription of TRIM6 and promotes its protein expression.28, 29 The high expression of TRIM6 in turn modifies the NP of SARS-CoV and SARS-CoV-2 through ubiquitination, promoting the binding of NP and viral genomic RNA, thereby promoting viral replication.
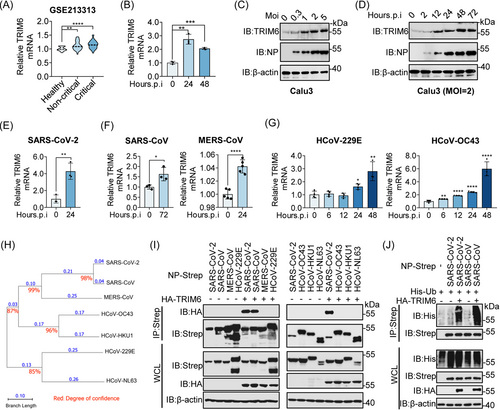
Comparing the evolutionary lineages of several coronavirus NPs to determine whether TRIM6 exerted a broad-spectrum functional regulation of the SASR-CoV-2 NP showed that the SARS-CoV NP has the highest homology with SARS-CoV-2 (Figure 6H). Furthermore, immunocoprecipitation experiments showed that TRIM6 specifically interacted with the NPs of SASR-CoV-2 and SARS-CoV (Figure 6I). The results of the ubiquitination experiments indicated that TRIM6 modified the SARS-CoV NP similar to its ubiquitination of the SARS-CoV-2 NP (Figure 6J). We performed sequence alignment and confirmed that the lysine residues at positions 102, 347, and 361 of the SARS-CoV-2 NP were highly conserved in the SARS-CoV NP (Figure S4A). This may indicate a specific role for TRIM6 in SARS-CoV and provide a strategy for the clinical development of related drugs.
3 DISCUSSION
This century has seen multiple coronavirus epidemics, especially the ongoing COVID-19 pandemic is exerting an unprecedented devastating effect on public health. To mitigate the public health disaster caused by coronavirus infection, understanding virus-host interactions and identifying potential broad-spectrum antiviral targets are critical. Based on the previous discovery of SARS-CoV-2 NP ubiquitination and the screening of E3 ubiquitin ligase TRIM6 to promote viral infection, this study deeply analyzed the molecular mechanism of TRIM6 regulation of SARS-CoV-2 infection, and preliminarily explored the potential of TRIM6 broad-spectrum anti-coronavirus. TRIM6 can catalyze NP ubiquitination, promote the modified NP binding to viral genomic RNA, thus increasing SARS-CoV-2 replication (Figure 7). Future studies may be needed to explore the possibility of targeting TRIM6 for COVID-19 treatment by developing inhibitors that inhibit TRIM6 activity or destroy TRIM6 binding to NP.
The NP undergoes PTMs, and studying these modifications is of significant importance for the development of potential medical applications.30 Firstly, phosphorylation plays a crucial role in regulating RNA binding and altering the physicochemical properties of the NP.31 During the early stages of infection, the SR region of the NP is rapidly phosphorylated at multiple sites by intracellular kinases,9, 32, 33 leading to its association with the RNA helicase DDX1, thereby facilitating the structural RNA changes required for the transcription of long subgenomic RNAs in the RTC.34 In the late stages of infection, the formation of the nuclear envelope and virus assembly no longer seem to rely on phosphorylation of the NP, as the phosphorylation levels of NP significantly decrease.9 This phosphorylation status can also influence interactions between the NP and viral NSP3 protein as well as host proteins, including 14-3-3 protein.35 Phosphorylated NP binds to 14-3-3 protein in the host cytoplasm, regulating the shuttling of NP between the nucleus and cytoplasm.35, 36 Second, the NP can also undergo methylation modifications. Cai et al. confirmed that PRMT1 methylates the R95 and R177 residues of the RGG/RG motif of NP, thereby regulating its binding to genomic RNA.11 Methylation of R95 modulates the NP by inhibiting the formation of stress granules (SGs). Arginine methylation impacts nearby phosphorylation sites, which often function in an antagonistic manner.37 Given that NP residues S176, S180, S183, and S184 are phosphorylated by cell cycle-dependent kinases SRPK1 and GSK3, there may be potential crosstalk between phosphorylation and methylation, especially in the vicinity of R177, in regulating its binding to the 5'UTR of viral RNA.11, 34, 38 Lastly, the NP can also undergo glycosylation and acetylation. Key glycosylation sites for the NP are at positions 48 and 270.39 Lys375 is acetylated by host acetyltransferases, and mutations at this acetylation site often result in acetyl-like modifications that adversely affect the liquid-liquid phase separation of NP and RNA.40 Furthermore, PTMs of NP are also linked to its production pathways. Commercially purified NP contains extensive N- and O-linked glycosylation as well as O-phosphorylation at the Thr393 site. In contrast, the natural NP has O-phosphorylation at Ser176 but lacks glycosylation.30
Our previous work and another important research found that viral NP can undergo ubiquitination by mass spectrometry,14 but how ubiquitination of NP affects its function is unclear. Our study shows that the K29-typed ubiquitination of NP affects its binding to viral genomic RNA for the first time. Currently, the known ubiquitination types mainly include K6, K11, K27, K29, K33, K48, and K63. Each type serves distinct functional roles, for example, K48 predominantly facilitates the proteasome degradation of target proteins, while K63 primarily engages in signal transduction and autophagy processes.41 Other ubiquitination types remain less commonly reported and are mainly associated with functions such as the cell cycle, DNA damage repair, histone modification, and protein transport.41 Indeed, some studies have demonstrated that K29-typed ubiquitination exerts a broad-spectrum of effects on target proteins, influencing substrate protein activity, degradation, and signal transduction.42-44
According to studies, the NTD of NP is primarily implicated in binding to viral genomic RNA. And there is a β-hairpin structure rich in basic amino acids, and at the junction with the core structure, there exists a positively charged pocket that is considered an RNA binding site.25, 45 Additionally, studies by Dinesh et al. have demonstrated through the construction of atomic models of protein-RNA complexes that both double-stranded RNA (dsRNA) and single-stranded RNA (ssRNA) bind to this positively charged pocket in a similar manner. Key arginine residues, namely R92, R107, and R149, are located within this structure and directly engage with the RNA.25, 45, 46 In addition, the CTD domain is primarily associated with dimer formation and viral packaging.25, 47, 48 It has been noted in studies that the NP dimers of SARS-CoV exhibit structurally positive grooves, suggesting the potential presence of RNA binding sites.25, 45, 49-52 In the case of the SARS-CoV-2 CTD, residues identified as RNA binding sites include Arg319, Thr334, and Ala336.52, 53 Here, we identify three ubiquitination sites on NP catalyzed by TRIM6, located in the NTD (K102) and CTD (K347 and K361) domains, which have the potential influence on NP function. The residues at positions K102, K347, and K361 are all located in proximity to the RNA binding pockets within both the NTD and the CTD. Therefore, we hypothesize that ubiquitination at these sites is likely to impact the spatial structure of these pockets, thereby influencing the binding of NP to viral RNA. Further functional experiments revealed that ubiquitination of NP by TRIM6 significantly enhanced the ability of NP binding to genomic RNA. It is noteworthy that the ability to directly resolve the structure of ubiquitinated NP or the structure of NP-virus RNA complex is crucial to verify the function of the three sites we found.
In our previous studies, we identified 15 lysine sites existing on NP through ubiquiomics, and in this study, we found three sites were catalyzed by TRIM6, and preliminarily explored the functions of the three sites after ubiquitination. It is worth noting that the function of the other 12 sites and the corresponding E3 ligase are still worthy of further exploration. At present, relevant studies have also reported that E3 ligases interacting with NP, such as TRIM21, promotes the degradation of viral NP through ubiquitination at K375.54 And TRIM26, TRIM37, and USP9X were also identified to interact with NP through mass spectrometry.14, 15 These potential E3 ubiquitin ligases that interact with NP may modify other sites of NP, thereby regulating other functions of NP. As an important structural protein, NP still needs to be further explored for its protein function, so as to contribute to the development of better antiviral drugs.
As a classic E3 ubiquitin ligase within the TRIM family, TRIM6 plays a pivotal role in various physiological contexts. Notably, it positively regulates IKKε activity, thereby enhancing the induction of interferon type I (IFN-I) and signaling for optimal induction of interferon-stimulated genes.14, 19 Furthermore, TRIM6 has been found to interact with Myc and is implicated in the maintenance of pluripotency in mouse embryonic stem cells.55 Additionally, TRIM6 plays a role in driving the progression of colorectal cancer through the SOCS2-STAT3 signaling axis.56 Moreover, TRIM6 contributes to the in vitro assembly process of the HIV-1 capsid-nuclear envelope complex.56 Therefore, the targeting of TRIM6 for therapeutic purposes requires careful consideration due to potential side effects, such as compromising the overall immune system function. Notably, to date, only a limited number of studies have developed clinically evaluated small molecule inhibitors that target single-protein and multi-subunit RING E3 ubiquitin ligases.57, 58 Given TRIM6's substantial involvement in tumor progression and viral infection, its development should prioritize minimizing adverse effects while effectively addressing the needs of patients. In summary, our findings underscore the significance of understanding the regulatory role of ubiquitination in NP function and emphasize the pivotal role of TRIM6 in this process. This knowledge has critical implications for the development of therapeutic strategies against SARS-CoV-2 and related viruses.
4 MATERIALS AND METHODS
4.1 Cell lines
HEK293T, HeLa, Vero E6 and Huh7 cells were cultured in Dulbecco's Modified Eagle Medium (DMEM, Gibco, 11965118) supplemented with 10% fetal bovine serum (FBS, Gibco, 10099141) and antibiotics (100 U/mL penicillin and 100 μg/mL streptomycin, Gibco, 15140-122). The cells were maintained in a humidified atmosphere containing 5% CO2 at 37°C. Similarly, Calu3 and Caco2 cells were cultured in Minimum Essential Medium (MEM, Gibco, 11095098) supplemented with 20% FBS and antibiotics (100 U/mL penicillin and 100 μg/mL streptomycin, Gibco, 15140-122) and maintained in a humidified atmosphere containing 5% CO2 at 37°C.
4.2 Virus
The SARS-CoV-2 strain is a WuHan strain, which was provided by the Biosafety Level III Laboratory of the Third People's Hospital of Shenzhen. All live virus-related experiments were performed in the Biosafety Lab 3 platform.
4.3 Plasmids and antibodies
The plasmids of NP of SARS-CoV-2 were fused with a GFP, Strep II or Flag tag at the C-terminus. Different Mutants and truncations of NP was tagged with GFP or Strep II, the human TRIM6 plasmids and different mutations and truncations of TRIM6 were fused with an HA tag at the N-terminus. Additionally, the human Ubiquitin and different mutation ubiquitin were fused with a 6×His tag at the N-terminus, all of the genes were subcloned into pET expression vector. Lentiviral shRNA clones targeting human TRIM6 and the nagetive control plasmid were purchased from SUNV Bio (http://www.sunvbio.com/). The following antibodies were used in immunoblotting: TRIM6 polyclonal antibody (pAb) (proteintech, Cat No. 11953-1-AP), SARS-CoV-2 Nucleocapsid antibody (Sino Biological, Cat No. 40143-T62), anti-HA pAb, (Proteintech, Cat No. 51064-2-AP), anti-His mAb (TransGen Biotech, Cat No. HT501), anti-Flag mAb (TransGen Biotech, Cat No. HT201), anti-GFP mAb (TransGen Biotech, Cat No. HT801), anti-β-Actin mAb (TransGen Biotech, Cat No. HC201), anti-Strep II mAb (Abbkine, Cat No. A02230). For confocal microscopy, Alexa Fluor® 488 Endoplasmic Reticulum Labeling Kit (Invitrogen, Catalog No. S34200), Goat anti-Mouse IgG (H + L) Highly Cross-Adsorbed Secondary Antibody (TransGen Biotech, China, Cat No. HS201), Goat anti-Rabbit IgG (H + L) Highly Cross-Adsorbed Secondary Antibody (TransGen Biotech, Cat No. HS101), Alexa Fluor™ Plus 555 (Invitrogen, Cat No. A32732).
4.4 Real-time quantitative PCR
Total RNA was isolated from the cells using TRNzol (TianGen, DP424), and 1 μg RNA was reverse-transcribed to cDNA using the FastKing gDNA dispelling RT SuperMix (TianGen, KR118-02). Quantitative reverse transcription-PCR (qRT-PCR) was performed using SYBR green master mix (TianGen, FP217), and the threshold cycle (CT) values of the samples were measured with a LightCycler 96 (Roche). The relative level of target gene was calculated as two power values of △CT (CT of target cDNA −CT of housekeeping gene GAPDH). All the primers used are listed in Table S1.
4.5 IP
Total proteins were extracted with cell lysis buffer (10×Tris-HCl [pH=7.0], 50 mM NaCl, 1% NP-40, 1 mM NaF, 6% glycerol) supplemented with protease inhibitor cocktail (Thermo, Prod #78440). The whole-cell lysates were transferred to a new tube and incubated with anti-strep or HA agarose beads at 4°C overnight after centrifugation at 12,000 rpm for 20 min. Unbound protein was washed by lysis buffer for three times and 5 min each time. Samples were eluted by boiling at 100°C for 10 min with protein loading buffer (0.01% bromophenol blue, 0.1 M dithiothreitol [DTT], 6% glycerol, 2% SDS, 0.25 M Tris-HCl [pH = 6.8]). The lysates and IPs were detected by the indicated primary antibodies.
4.6 GST-pulldown assay
For GST-pulldown with SARS-CoV-2 NP, BL21 (DE3) cells containing pGEX-GST-NP plasmid were induced with 0.5 mM IPTG (isopropyl-β-d-thio -galactopyranoside) at 16°C overlight and lysed in PBS with 100 mg/mL lysozyme, 1% Triton X-100, 0.1% sarcosyl, 1 mM DTT, and protease inhibitor cocktail. The lysate was centrifuged, passed through a 0.45 μm-pore filter, and incubated with glutathione S-transferase (GST) agarose (AS044, Abclonal) at 4°C for 4 h. The beads were then washed with PBS containing 1% Triton X-100% and 0.1% sarcosyl. HEK293T cells transfect with HA-TRIM6,then cell were lysed in binding buffer (50 mM Tris-HCl [pH = 7.6], 100 mM NaCl, 10% glycerol, and 0.5% NP-40) with protease inhibitor cocktail. Cell lysates were centrifuged, and the supernatant was incubated with agarose beads containing HA at 4°C for overnight. The beads were washed with binding buffer for three times and resolved with protein loading buffer. The target proteins were detected by immunoblotting.
4.7 Confocal microscopy
To localize NP or TRIM6, or co-localize NP with TRIM6 in HeLa cells, the following experimental protocol was employed: HeLa cells were transfected with either GFP-NP or HA-TRIM6 alone or together, and cultured for 24 h. The cells were then fixed with 4% paraformaldehyde at room temperature for 20 min, washed with PBS, permeabilized with 0.5% Triton X-100 for 10 min, washed with PBS three times, blocked with 2% BSA for 30 min, and incubated at room temperature for 2 h with mouse anti-HA antibody at a dilution of 1:25. Following a wash, cells were incubated at room temperature with the corresponding highly cross-adsorbed secondary antibody at a dilution of 1:800 for 1 h. After being washed with cold PBS, the cells were analyzed using a laser scanning confocal microscope. (FV 3000, Olympus).
4.8 Stable cell line generation
HEK293T cells were transfected with the lentivirus packaging plasmids VSV-G and PSPAX2 and the lentiviral shRNA plasmids. 48 h after transfection, the supernatant was harvested and filtered. HeLa-ACE2 cells were infected with the lentivirus in the presence of Polybrene (5 μg/mL). Cells were selected 72 h postinfection with puromycin (1 to ~2 μg/mL). The knockdown efficiency of TRIM6 was detected by immunoblotting with anti-TRIM6 antibody. The sequences of shRNA were as follows: shTRIM6 No.1, 5′-GGGTTGACGTGACCCTGAATC−3′,
shTRIM6 No.2, 5′- GCTAAAGTATCTGGACCTTCC−3′,
shTRIM6 No.3, 5′-GGTGATTGGGTTACAGCATAA−3′.
4.9 In vivo ubiquitination assay of NP
HEK293T cells were transfected with NP and ubiquitin-expressing plasmids. Cells were lysed 24 h after transfect. The cell lysis buffer supplemented with 0.1% SDS and 10 mM DTT. The lysates were centrifuged to remove the pellets and incubated with anti-strep agarose beads at 4°C for 2 h. Then the beads were washed three times with cell lysis buffer and 5 min each time. The immunoprecipitated proteins were boiled in protein loading buffer and then released from the beads and analyzed by Western blot analysis.
4.10 In vitro NP ubiquitination assay
The SARS-CoV-2 NP was cloned and expressed as GST fusion proteins. The NP plasmids was expressed in BL21 bacterial cells and purified with glutathione Sepharose beads. In addition, the HA-TRIM6 and HA-TRIM6 3C-A were purified from HEK293T cells. The beads with NP protein were incubated with TRIM6-WT or 3C-A mutant, together with 200 ng of E1 (UBE1, abcam), 400 ng of E2 (UBE2K, abcam), and 3 μg of recombinant WT Ub and K29 Ub (abcam) in reaction buffer (50 mM Tris [PH = 7.6], 2 mM MgATP [Beyotime]) at 37°C for 1 h. The supernatant was removed, and the reaction was terminated by adding 2 × SDS loading buffer. The ubiquitin chain was detected by immunoblotting.
4.11 Mass spectrometry
Calu3 were infected with SARS-CoV-2 virus for 24 h, then treated 1% NP-40 lysis buffer and shaker at 4°C horizontal was used for 30 min to lysis. TRIM6 antibody was used to enrich TRIM6 protein complex. And the sample was loaded onto a sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), the target band was excised and subjected to affinity mass spectrometry detection (Affinity mass spectrometry services are provided by PTM Biolabs).
4.12 RIP
After co-transfect the plasmid of NP and gRNA of SARS-CoV-2 into HEK293T cell with culture for 24 h. Cells were resuspended in pre-cooled PBS, centrifuged at 1000 r/min for 5 min, and the cell pellet was collected. The cells were then lysed with RIP lysis buffer (Bersin-Bio). The lysates were incubated with the specific antibody against the target protein overnight at 4°C, and then incubated with protein A/G beads at 4°C with shaking for 1 h. The immunoprecipitated RNAs were obtained using the elution buffer from the kit and were quantified by qRT-PCR using specific genomic RNA primers.
4.13 Nonreducing PAGE
Plasmids transfected HEK293T cells were lysed with radioimmunoprecipitation assay (RIPA) buffer. Lysates were clarified by centrifugation, and the supernatants were mixed with Laemmli buffer without both SDS and β-mercaptoethanol. Samples were subjected to sodium dodecyl sulfate-10% polyacrylamide gel electrophoresis, followed by blotting onto polyvinylidene difluoride filters. Filters were blocked with phosphate-buffered saline with Tween 20 (PBST)-milk and incubated with anti-Flag antibody. Filters were incubated with HRP conjugate Anti-Mouse IgG antibodies.
4.14 Virus infection and detection
An indirect immunofluorescence assay (IFA) was used to detect NP expression in SARS-CoV-2 infected HeLa-ACE2 cells. Huh7 cells infected with the HCoV-229E or HCoV-OC43 (MOI = 0.005 and MOI = 0.01, respectively) were fixed using 4% paraformaldehyde (PFA) in PBS, and then the cells were permeabilized with 0.5% Triton X-100 in PBS. Cells were incubated with rabbit anti-SARS-CoV-2 NP pAb (Sino Biologicals) at room temperature for 1 h and then stained with Fluor 594-conjugated goat anti-rabbit antibody (Thermo) (1:500 diluted in PBS containing 1% BSA), The nucleus was stained with DAPI. After being washed three times with PBS, the coverslips were mounted on glass slides. Cells were examined by confocal microscopy (Zeiss). An experiment on wild-type plaque formation of SARS-CoV-2 induced by changes in TRIM6 protein levels. Vero E6 was seeded to 12-well cell culture plates at a density of 1 × 104 cells per well and the original viral supernatant was diluted 200 times as the initial concentration, then conducted serially diluted with DMEM containing 1% FBS. After incubation at 37°C for 1 h, virus-infected cells were replaced with agarose medium (1 × DMEM, containing 10% FBS, and 1% agarose). After 48 h of culture, cells were fixed with 4% paraformaldehyde for 30 min, followed by staining with 0.1% crystal violet solution containing 1% paraformaldehyde, and each set was subjected to three technical replicates.
4.15 BRET assay
Based on the structure of NanoLuciferase (Nano), it was divided into three distinct domains, including a larger domain (Nano 1−7) and two smaller domains (Nano 8 and Nano 10). These two smaller domains were separately recombined at the N-terminal (N8) and C-terminal (C10) regions of the NP protein. In HeLa cells, co-transfections were performed with Nano 1−7 and Nano-NP (N8-NP) or NP-Nano (NP-C10), along with various TRIM6 and its mutants. After 24 h of incubation, cell lysis was initiated by the addition of a lysis buffer for a 10 min period. Subsequently, fluorescence readings were recorded. all data expressed as mean ± standard deviations (SDs). A Student's t test was utilized to make statistical comparisons between the two groups. Significant differences are denoted as * means p < 0.05, ** means p < 0.01, and *** means p < 0.001, while p Values less than 0.05 are considered statistically significant. Additionally, “ns” represents no significance.
4.16 Statistical analysis
All the figure legends have been provided a comprehensive statistical analysis, with all data expressed as mean ± SDs. A Student's t test was utilized to make statistical comparisons between the two groups. In the figures, significant differences are denoted as * means p < 0.05, ** means p < 0.01, and *** means p < 0.001, while p Values less than 0.05 are considered statistically significant. Additionally, “ns” represents no significance.
AUTHOR CONTRIBUTIONS
Jian Zhou carried out most of the biochemical experiments and cell experiments and wrote the paper. With help from Yuzheng Zhou conducted the confocal microscopy experiments. With help from Xia-fei Wei conducted the BRET assay. Lujie Fan, Yunfei Li, and Yezi Wu performed partially plasmids constructed. Xiang Gao assisted in completing virus infection experiments. Wei Feng and XiaoTong Shen contributed some key reagents. Zheng Zhang, Gang Xu, Yuzheng Zhou, and Lei Liu directed the project, Yuzheng Zhou also provided some experimental technical support and revised the manuscript.
ACKNOWLEDGMENTS
We thank the professor Qiang Ding of Tsinghua University in China for providing trVLP model. This work was supported by grants from National Key Research and Development Program of China (Grant No. 2021YFA0910900, No. 2021YFC2300100), the National Science Fund for Distinguished Young Scholars (Grant No. 82025022), the National Natural Science Foundation of China (Grant No. 82151212, 92369109), the China Postdoctoral Science Foundation (2023M731520), Guangdong Science and Technology Plan Project, Construction of high-level biosafety laboratories (2021B1212030010), Shenzhen Science and Technology Program (Grant No. JCYJ20220818103017036) and Natural Science Research Project of Anhui Educational Committee (2022AH030080).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
All data, materials, and methods are included in the article.




