Exploring the incidence of peripheral arterial occlusive disease following COVID-19 infection: A retrospective cohort study
Abstract
Peripheral arterial occlusive disease (PAOD) is a clinical manifestation of systemic atherosclerosis and is always associated with cerebrovascular disease and various complications. The aim of our study is to evaluate the relationship between the coronavirus disease 2019 (COVID-19) infection and the subsequent PAOD development. A retrospective cohort study was conducted and individuals with COVID-19 infection were identified from the TriNetX analytics platform. A total of 2 206 065 patients with COVID-19 infection and 2 206 065 patients without COVID-19 infection were recruited after exclusion and matching. The primary outcome was the development of PAOD after the COVID-19 infection. The Cox proportional hazard regression was adopted to yield the hazard ratio (HR) and 95% confidence interval (CI) of PAOD between groups. After the whole follow-up period, the incidence of PAOD was significantly higher in the COVID-19 group at both the 3-month follow-up (HR: 1.27, 95% CI: 1.24–1.30) and the 12-month follow-up (HR: 1.33, 95% CI: 1.31–1.35) The Kaplan-Meier analysis with the log-rank test demonstrated a higher cumulative probability of PAOD in the COVID-19 group compared to the non-COVID-19 group (p < 0.001). In stratified analysis using 65 years as the threshold, both age groups in the COVID-19 group exhibited a higher risk of PAOD. Similarly, in the sex and race stratified analysis, the COVID-19 group performed a higher risk of PAOD in both subgroups. In conclusion, the COVID-19 infections are strongly associated with an increment of PAOD incidence.
1 INTRODUCTION
Coronavirus disease 2019 (COVID-19) has been swept globally for more than 3 years. The outbreak of COVID-19 was first reported as a new coronavirus in Wuhan, Hubei province, China, since December 2019.1 WHO renamed the virus, severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2) based on phylogenetic analysis to differentiate it from the first outbreak caused by SARS-CoV in 2003.2 Due to its rapid spread through international travel of people and serious respiratory symptoms in the human population, the WHO declared Covid-19 a public health emergency of international concern in 2019 (Huang et al., 2020) (Wang et al., 2021).3 After massive application of the vaccine campaign,4 antiviral drug treatment, and policy to mandatory mask wear, the COVID-19 pandemic finally declined at the beginning of 2023. According to the WHO report on July 19, 2023, there are 768 233 788 confirmed cases and nearly 6 951 677 people died from COVID-19 infection throughout the world.
People infected with COVID-19 usually caused mild to serious respiratory disease.5 In addition to people who died from COVID-19, the common symptoms of COVID-19 are similar to influenza, including fever, sore throat, fatigue, and cough. However, some people would have more serious clinical symptoms such as dyspnea, confusion, and chest pain. Especially people would lose taste and smell after infection in many cases. Many people who recovered from COVID-19 appeared post-acute sequelae of SARS-CoV-2, also known as post-COVID-19 syndrome or “long COVID-19.”6 To date, more than 200 symptoms have been identified with impacts on different organ systems in about 10% of patients who had been recovered from COVID-19 infection and even caused multi-organs disorders.7, 8 Among these post-COVID-19 syndromes, some of them were associated with blood circulatory system and rendered different types of cerebrovascular diseases (CVDs) or increased the risks of CVDs such as cerebrovascular disorders, dysrhythmias, ischemic and nonischemic heart disease, pericarditis, myocarditis, heart failure and thromboembolic disease.9, 10
Peripheral arterial occlusive diseases (PAOD) are clinical manifestations of systemic atherosclerosis and are always associated with brain vascular diseases and various complications.11, 12 The main cause of PAOD is thrombosis and embolism in the lower extremities and can lead to serious vascular atherosclerosis stenosis, vessel obstruction, and even tissue necrosis in the extremities.13, 11 Although many post-COVID syndromes that associated with blood circulatory system in the literatures, few reported them with PAOD.
In this study, we enrolled patients who have previously diagnosed COVID-19 and who have never diagnosed COVID-19 before using the TriNetX analytics platform and analyzed their risk of PAOD.
2 METHOD
2.1 Data sources
This retrospective cohort study employed the TriNetX analytics platform, which is a web-based database comprising deidentified electronic health records of more than 100 million patients from multiple countries. The database contains a broad spectrum of information, including essential demographics, diagnoses (coded using ICD-10-CM codes), medications (coded using RxNorm or Anatomical Therapeutic Chemical codes), procedures (coded using ICD-10-PCS or Current Procedural Terminology), and laboratory measurements (coded using Logical Observation Identifiers Names and codes, LOINC). The utilization of deidentified data in this retrospective analysis was exempted from Institutional Review Board approval. The study conducted a retrospective cohort analysis using electronic health records from the USA collaborative network within the TriNetX database, which included approximately 93 million patients. This study was approved by the Institutional Review Board for Ethics of Chung Shan Medical University Hospital (IRB number: CS2-23180) and performed in accordance with the Declaration of Helsinki.
2.2 Study participants
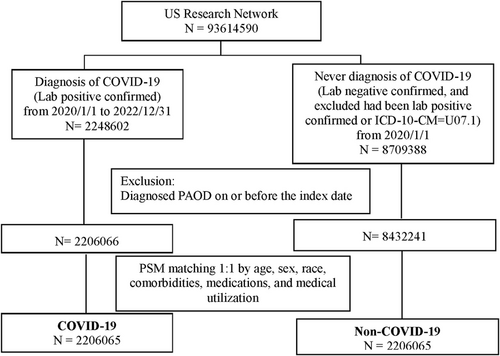
The cohort construction flow chart is depicted in Figure 1. The exposure group consisted of subjects diagnosed with SARS-CoV-2 infection (COVID-19) between January 1, 2020, and December 31, 2022. This was determined by a positive result from a COVID-19-specific polymerase chain reaction (PCR) test or a positive immunoassay for immunoglobulin in serum or plasma (Supporting Information: Table 1). The non-COVID-19 group comprised individuals who did not have SARS-CoV-2 infection from January 1, 2020. Individuals were excluded from the cohort if they tested positive for PCR or COVID-19-related laboratory tests or received a diagnosis of COVID-19 (ICD-10-CM = U07.1). The index date was defined as the date of the first COVID-19 test. A total of 2 248 602 COVID-19 patients from 2020 to 2022 were included in the study from the US research network database. In the comparison group, 8 709 388 individuals without a COVID-19 diagnosis were identified. After excluding individuals diagnosed with PAOD on or before the index date, there were 2 206 066 subjects in the COVID-19 group and 8 432 241 subjects in the non-COVID-19 group. Following a 1:1 matching process based on age, sex, race, comorbidities, medications, and medical utilization, there remained 2 206 065 subjects in both the COVID-19 and non-COVID-19 groups (Figure 1).
2.3 Collection of clinical characters
Baseline characteristics were obtained from records spanning 1 year before the index date up until 1 day before the index date. Demographic factors of interest included age, sex, race, and body mass index. Relevant baseline comorbidities comprised hypertension (ICD-10-CM = I10), diabetes mellitus (ICD-10-CM = E08–E13), ischemic heart diseases (ICD-10-CM = I20–I25), hyperlipidemia (ICD-10-CM = E78), heart failure (ICD-10-CM = I50), cerebral infarction (ICD-10-CM = I63), chronic kidney disease (ICD-10-CM = N18), other venous embolism and thrombosis (ICD-10-CM = I82), and varicose veins of lower extremities (ICD-10-CM = I83). Medication use encompassed aspirin (RxNorm: 1191), clopidogrel (RxNorm: 32 968), ticagrelor (RxNorm: 1 116 632), prasugrel (RxNorm: 613 391), cilostazol (RxNorm: 21 107), warfarin (RxNorm: 11 289), enoxaparin (RxNorm: 67108), rivaroxaban (RxNorm: 1 114 195), apixaban (RxNorm: 1 364 430), edoxaban (RxNorm: 1 599 538), and dabigatran etexilate (RxNorm: 1 037 042). Medical utilization data included ambulatory, emergency, and inpatient encounters. Propensity score matching by using a nearest neighbor greedy matching algorithm with a caliper of 0.1 pooled standard deviations was performed to address baseline characteristic differences between the two groups, reducing the impact of confounding factors. The matching process was conducted at a 1:1 ratio using the built-in function in TriNetX, considering age, sex, race, comorbidities, medications, and medical utilization.
2.4 Primary outcome
The primary outcome of interest in this study was the diagnosis of PAOD, identified using ICD-10-CM codes (I73.81, I73.89, I73.9, I74.01, I74.09, I74.11, I74.2, I74.3, I74.4, I74.5, I74.8, I74.9, I79.1, and I79.8) occurring after the index date. To ensure the accuracy of the diagnosis, both the exposure and non-exposure groups excluded individuals who had been diagnosed with PAOD before the index date.
2.5 Statistical analysis
All statistical analyses were conducted within the TriNetX platform. The balance of baseline characteristics between the matched cohorts was assessed using standardized mean differences (SMD). Variables with an SMD less than 0.1 were considered well-matched. Cox proportional hazards regression analysis was employed to compare the matched cohorts, providing hazard ratios (HR) along with 95% confidence intervals (CI). Incidence rates for PAOD were calculated using the Kaplan-Meier method, and the log-rank test was utilized. Subgroup analyses based on age, sex, race, and comorbidities were also conducted. Statistical significance was defined as a two-sided p value of less than 0.05.
3 RESULTS
3.1 Baseline characteristics
The baseline characteristics, including demographics, comorbidities, medications, and medical utilization, of the COVID-19 and comparison groups before and after propensity score matching, are presented in Table 1. After matching, the mean age of participants in the COVID-19 group was 40.6 years (standard deviation: 22.4 years). In the COVID-19 group, 55.8% of the participants were female, and the majority (62%) were white. The two groups exhibited well-matched distributions in terms of demographics, comorbidities, medications, and medical utilization, with SMD of less than 0.1. The built-in density of propensity score before and after matching was presented as Supporting Information: Figure S1.
| Characteristics | Before PSM matching | After PSM matching | ||||
|---|---|---|---|---|---|---|
COVID-19 N = 2 206 066 |
Non-COVID-19 N = 8 432 241 |
SMD | COVID-19 N = 2 206 065 |
Non-COVID-19 N = 2 206 065 |
SMD | |
| Age, Mean ± SD | 40.6 ± 22.4 | 40.5 ± 24.4 | 0.007 | 40.6 ± 22.4 | 40.6 ± 22.4 | <0.001 |
| Sex | ||||||
| Female | 1 231 164 (55.8) | 4 587 405 (54.4) | 0.028 | 1 231 163 (55.8) | 1 231 180 (55.8) | <0.001 |
| Male | 973 636 (44.1) | 383 6252 (45.5) | 0.027 | 973 636 (44.1) | 972 902 (44.1) | 0.001 |
| Race | ||||||
| White | 1 368 500 (62.0) | 5 238 742 (62.1) | 0.002 | 1 368 500 (62.0) | 1 368 537 (62.0) | <0.001 |
| Black or African American | 430 415 (19.5) | 1 486 070 (17.6) | 0.049 | 430 414 (19.5) | 430 525 (19.5) | <0.001 |
| Asian | 50 522 (2.3) | 218 638 (2.6) | 0.020 | 50 522 (2.3) | 54 136 (2.5) | 0.011 |
| BMI | ||||||
| <18.5 | 71 769 (3.3) | 32 7269 (3.9) | 0.034 | 71 769 (3.3) | 72 272 (3.3) | 0.001 |
| 18.5-24.9 | 167845 (7.6) | 640 601 (7.6) | <0.001 | 167 845 (7.6) | 178 913 (8.1) | 0.019 |
| 25-29.9 | 187174 (8.5) | 629 753 (7.5) | 0.038 | 187 173 (8.5) | 181 971 (8.2) | 0.009 |
| ≥30 | 243909 (11.1) | 704 587 (8.4) | 0.091 | 243 908 (11.1) | 215 179 (9.8) | 0.043 |
| Mean ± SD | 28.3 ± 7.7 | 27.0 ± 7.6 | 0.168 | 28.3 ± 7.7 | 27.7 ± 7.6 | 0.075 |
| Comorbidities | ||||||
| Hypertension | 345 693 (15.7) | 1 112 337 (13.2) | 0.071 | 345 692 (15.7) | 345 642 (15.7) | <0.001 |
| Diabetes mellitus | 183 584 (8.3) | 520 126 (6.2) | 0.083 | 183 584 (8.3) | 153 651 (7.0) | 0.051 |
| Ischemic heart diseases | 102 111 (4.6) | 317 632 (3.8) | 0.043 | 102 110 (4.6) | 91 325 (4.1) | 0.024 |
| Hyperlipidemia | 287 106 (13.0) | 885 604 (10.5) | 0.078 | 287 105 (13.0) | 249 944 (11.3) | 0.052 |
| Heart failure | 62 572 (2.8) | 175 477 (2.1) | 0.049 | 62 571 (2.8) | 50 620 (2.3) | 0.034 |
| Cerebral infarction | 32 009 (1.5) | 87 749 (1.0) | 0.037 | 32 009 (1.5) | 26 947 (1.2) | 0.02 |
| Chronic kidney disease | 84 781 (3.8) | 229 537 (2.7) | 0.063 | 84 780 (3.8) | 65 710 (3.0) | 0.048 |
| Other venous embolism and thrombosis | 29 013 (1.3) | 65 496 (0.8) | 0.053 | 29 013 (1.3) | 20 079 (0.9) | 0.039 |
| Varicose veins of lower extremities | 12 789 (0.6) | 30 419 (0.4) | 0.032 | 12 789 (0.6) | 8808 (0.4) | 0.026 |
| Medications | ||||||
| Aspirin | 135 118 (6.1) | 421 201 (5.0) | 0.049 | 135 117 (6.1) | 135 082 (6.1) | <0.001 |
| Clopidogrel | 26 642 (1.2) | 75 876 (0.9) | 0.030 | 26 641 (1.2) | 22 179 (1.0) | 0.019 |
| Ticagrelor | 3804 (0.2) | 10 390 (0.1) | 0.013 | 3804 (0.2) | 3266 (0.1) | 0.006 |
| Prasugrel | 1129 (0.1) | 2792 (0.0) | 0.009 | 1129 (0.1) | 846 (0.0) | 0.006 |
| Cilostazol | 762 (0.0) | 1437 (0.0) | 0.011 | 762 (0.0) | 398 (0.0) | 0.010 |
| Warfarin | 16 795 (0.8) | 47 751 (0.6) | 0.024 | 16794 (0.8) | 16 681 (0.8) | 0.001 |
| Enoxaparin | 82 681 (3.7) | 198 394 (2.4) | 0.081 | 82 680 (3.7) | 59 546 (2.7) | 0.059 |
| Rivaroxaban | 13 130 (0.6) | 44 155 (0.5) | 0.010 | 13 130 (0.6) | 11 917 (0.5) | 0.007 |
| Apixaban | 33 698 (1.5) | 103 436 (1.2) | 0.026 | 33 698 (1.5) | 27 845 (1.3) | 0.023 |
| Edoxaban | 42 (0.0) | 174 (0.0) | <0.001 | 42 (0.0) | 44 (0.0) | <0.001 |
| Dabigatran etexilate | 972 (0.0) | 3891 (0.0) | 0.001 | 972 (0.0) | 1049 (0.0) | 0.002 |
| Medical utilization | ||||||
| Ambulatory | 1 337 359 (60.6) | 4 757 527 (56.4) | 0.085 | 1 337 358 (60.6) | 1 337 452 (60.6) | <0.001 |
| Emergency | 437 836 (19.8) | 1 311 788 (15.6) | 0.113 | 437 835 (19.8) | 366 626 (16.6) | 0.084 |
| Inpatient Encounter | 216 068 (9.8) | 640 900 (7.6) | 0.078 | 216 067 (9.8) | 185 408 (8.4) | 0.048 |
- Abbreviation: SMD, standardized mean difference.
3.2 Risk of PAOD based on different follow-up durations
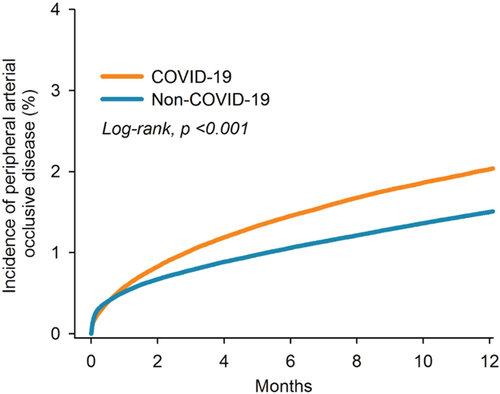
We evaluated the risk of PAOD between the COVID-19 group and the non-COVID-19 comparison group at various follow-up durations: before propensity score matching (12 months), 3 months, 6 months, 9 months, and 12 months (Table 2). Before propensity score matching, the COVID-19 group showed a higher risk of PAOD compared to the non-COVID-19 group, with a HR of 1.37 (95% CI = 1.35–1.39). After propensity score matching, the HR was 1.27 (95% CI = 1.24–1.30) at the 3-month follow-up and 1.33 (95% CI = 1.31–1.35) at the 12-month follow-up. Kaplan-Meier analysis with the log-rank test (p < 0.001) demonstrated a higher cumulative probability of PAOD in the COVID-19 group compared to the non-COVID-19 group (Figure 2).
| Follow-up duration | No. of event | HR (95% CI) | |
|---|---|---|---|
COVID-19 N = 2 206 065 |
Non-COVID-19 N = 2 206 065 |
||
| Before PSM (12 months)a | 29 ,294 | 82 038 | 1.37 (1.35–1.39) |
| After PSM | |||
| 3 months | 16 917 | 13 676 | 1.27 (1.24–1.30) |
| 6 months | 22 777 | 17 455 | 1.33 (1.30–1.36) |
| 9 months | 26 648 | 20 265 | 1.34 (1.32–1.37) |
| 12 months | 29 294 | 22,642 | 1.33 (1.31–1.35) |
- a COVID-19, N = 22;06 066; Non-COVID-19, N = 8 432 239.
3.3 Subgroup analysis stratified with different covariates
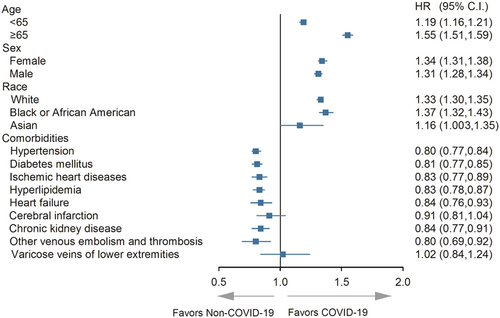
In stratified analysis using 65 years as the threshold, both age groups in the COVID-19 group exhibited a higher risk of PAOD. Similarly, in the sex and race stratified analysis, the COVID-19 group performed a higher risk of PAOD in both subgroups (all the lower limits of 95% CI higher than 1). However, in terms of comorbidities such as hypertension, diabetes mellitus, ischemic heart diseases, hyperlipidemia, heart failure, chronic kidney disease, and other venous embolism and thrombosis, the COVID-19 group exhibited a lower risk of PAOD compared to the non-COVID-19 group (all the upper limits of 95% CI lower than 1) (Figure 3).
4 DISCUSSION
In this study, the patients diagnosed with COVID-19 presented a higher incidence of PAOD compared to the non-COVID-19 group in each checkpoint. Moreover, the cumulative probability of PAOD was significantly higher in the COVID-19 group than the non-COVID-19 group. In addition, the COVID-19 patients with different age, sex, and race demonstrated a higher possibility of developing PAOD than the non-COVID-19 population.
The present of COVID-19 infection correlates to several disorders of the vascular system in previous publications.10, 14, 15 The COVID-19 virus can damage the vascular endothelium and thus impaired the vasculature,15 and the elevation of oxidative stress and inflammation response during the COVID-19 infection would further influence the health of circulation system.15 In previous study, the COVID-19 infection is associated with the development of coronary heart disease, and the risk of arrhythmia related disorders is also higher in the patients with COVID-19 infection.9 Other than the cardiac disease, the patients with COVID-19 infection demonstrated a significant association to the following CVD events.16 Besides, the inflammation of vascular system, including the vasculitis and myocarditis, showed higher incidences in the patients with COVID-19 infection than the non-COVID-19 infection counterparts.10, 14 And the rate of thromboembolic disease like the deep vein thrombosis was also elevated after the infection of COVID-19 virus.10 On the other side, the PAOD is a vascular disorder that relates to atherosclerosis and several co-morbidities.17-19 The aging is a prominent risk factor for the PAOD development,20 and the systemic disease such as hypertension, diabetes, hyperlipidemia, obesity and hyperhomocysteinemia are also risk factors for PAOD that have been reported in the past decades.21 Furthermore, the systemic vasculitis like the polyarteritis nodosa, hypersensitivity vasculitis, Wegener's granulomatosis, and giant cells arthritis were recently reported to own a strong association to PAOD progression and lower extremity amputation in the research using the Taiwan's populations.22 Because the COVID-19 virus can affect the vascular structure to a large extent and the formation of PAOD is correlated to the vascular damage,23, 24 we speculate that the presence of COVID-19 infection may associates with higher incidence of subsequent PAOD. The hypothesis was supported by the results of our study at least to some degrees.
The results of our study illustrated a higher incidence of PAOD in the individuals that experienced COVID-19 infection than the non-COVID-19 group. In previous study, the COVID-19 infection was associated with various vascular disorders including stroke, arrhythmia related disorders, myocarditis, ischemic heart disease,9 while rare studies have mentioned either COVID-19 as a risk factor for the development of PAOD. To our knowledge, this may be a preliminary experience to demonstrate the significant correlation between the COVID-19 infection and the following PAOD development. Besides, we excluded the patients with PAOD before the first COVID-19 test thus the time sequence between COVID-19 infection and PAOD could be established. In addition, several confounders for PAOD like the age, hypertension and metabolic syndromes were included in the statistical analysis and their effects on PAOD development were adjusted.20, 21 Consequently, the COVID-19 infection may be an independent risk factor for the PAOD development. The damage of COVID-19 to the vascular endothelium in COVID-19 infection could be the main reason for the significant correlation between COVID-19 infection and following PAOD events which we discussed in previous paragraph.15 On the other hand, the incidence of PAOD in the COVID-19 group was significantly higher than the non-COVID-19 group in all time points and the cumulative probability of PAOD episode increased significantly in the COVID-19 group than the non-COVID-19 group. The prolonged COVID-19 infection could increase the inflammatory response and damage to vasculature in the human body.15, 25 In previous studies, the incidence of coronary heart disease and CVDs also elevated even months after the COVID-19 infection.10, 26 The cumulative probability analysis of our study further supported the findings of the previous publication.10, 26 Of note, the cumulative probability of PAOD after COVID-19 infection keep increasing 1 year after the COVID-19 infection in our study, which may imply an adequate follow up interval in cardiovascular department is needed.
In the subgroup analysis of our study, COVID-19 patients in all age, sex, and ethnicity showed a higher risk of developing PAOD compared to the non-COVID-19 individuals. In preceding study, the COVID-19 can affect the patients with all age, sex and ethnicity,2 and the incidence of cardiovascular complications was prominently higher in the elderly population in another research.27 Still, there was scant evidence to evaluate the relationship of COVID-19 infection and subsequent PAOD development in populations with difference demography or clinical characters. About the age-based subgroup analysis, the COVID-19 patients aged 65 years old or older presented with a numerically higher incidence of PAOD compared to those COVID-19 patients aged younger than 65 years old, and the 95% CI of the two groups did not overlap which indiate the difference of PAOD incidence between the two age subgroups might be prominent. Since the elderly population was also a high-risk group for the development of PAOD,20 it is reasonable for the higher incidence of PAOD of elderly population with COVID-19 infection in our study. On the other hand, the HR of PAOD between male and female sex populations with COVID-19 infection did not showed prominent difference. The possibility of development PAOD is equal in the male and female populations according to previosu study.21 We speculate the influence of COVID-19 infection on vasculature is also similar between different sexes thus the incidence of PAOD did not showed significant difference. Besides, the Asian population with COVID-19 infection presented a numerically lower rate of PAOD than other ethnicity, which is conflicting to the finding of previous publicaiton.28 This discordance may due to the few numbers of patients and possible statistical analysis. Interestingly, the patients with systemic co-morbidities including hypertension and other vascular morbidities demonstrated a lower incidnece of PAOD in the COVID-19 group than the non-COVID-19 group. A possible explanation is that the patients with COVID-19 infection also had more systemic diseases than the non-COVID-19 individuals in our study, and the multiple diseases diluted the effect of COVID-19 on PAOD development. Further study is warranted to investigate this hypothesis.
There were several strengths in our study compared with previous studies. First, we used TriNetX analytics platform, which included large enough information from multiple countries. Since the COVID-19 infection can cause prominent symptoms on all ethnicity,29 the multi-ethnicity design of TriNetX analytics platform can increase the external validity of our study. Second, we focus on the association between COVID-19 infection and PAOD with the application of Cox proportional hazards regression analysis, which can adjust the effect of other potential covariates in the analysis model and the relationship between COVID-19 infection and PAOD development could be clearer. Finally, our study also examined the impact of the COVID-19 infection on the subsequent PAOD of patients with different sex, age and disease subgroups, which can provide useful and customized information for the patients with different clinical characteristics.
This study, however, has several limitations. First, the information recorded in TriNetX analytics platform is only the diagnosis/exam/procedure codes, thus some important information including the titer of COVID-19 virus, the results of physical examination in COVID-19 patients, the results of image and laboratory exams in COVID-19 patients, the detailed disease course of other diseases, the treatment outcome of COVID-19 infection, the site of PAOD, the image results of PAOD cases, the laboratory results of PAOD cases, the prognosis of PAOD patients, and the limb function of patients with PAOD cannot be evaluated. Second, the retrospective nature of this study may decrease the homogeneity of the study population despite the application of PSM. Besides, because the data of TriNetX analytics platform were obtained from the multiple participating healthcare organizations, there could be certain disparities of diagnoses and treatments of PAOD and other co-morbidities in each region. Thirdly, the study does not rely on a population-wide database. Variations in patients' search for healthcare across different institutions could potentially lead to an underestimation of both COVID-19 prevalence and the incidence of PAOD. Finally, the COVID-19 pandemic, which erupted over the past 3 years, necessitates an extended follow-up period to validate the association between COVID-19 and PAOD. In conclusion, our study showed that COVID-19 infection is strongly associated with an increment of following PAOD development after adjusting multiple demographic and systemic diseases factors. Furthermore, the results become more robust in the patients with COVID-19 infection that own a longer disease period. Consequently, the periodically peripheral vascular examination with an interval of at least 1 year could be suggested for those diagnosed with COVID-19 infection. Further large-scale prospective study to evaluate whether the severity of COVID-19 infection will influence the severity of subsequent PAOD is mandatory.
AUTHOR CONTRIBUTIONS
Liang-Tsai Yeh: This author designed the study, search the literature, responsible for data interpretation, prepare the manuscript draft and was approved the final version of the manuscript. Chi-Ho Chan: This author designed the study, was responsible for data analysis, data interpretation, refines the manuscript and approved the final version of the manuscript. Yu-Hsun Wang: This author was responsible for data collection, data analysis, performed the statistical analyses and approved the final version of the manuscript. Chia-Yi Lee: This author designed the study, was responsible for data collection, data analysis, and approved the final version of the manuscript. Shun-Fa Yang: This author designed the study and approved the final version of the manuscript. Chao-Bin Yeh: This author designed the study, was responsible for data interpretation; prepare the manuscript and approved the final version of the manuscript.
ACKNOWLEDGMENTS
This research was funded by grants from the Chung Shan Medical University Hospital (CSH-2023-C-042).
CONFLICT OF INTEREST STATEMENT
All authors declare no conflict of interest
Open Research
DATA AVAILABILITY STATEMENT
The findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.




