Biosensor-based multiple cross displacement amplification platform for visual and rapid identification of hepatitis C virus
Xu Chen and Shilei Dong contributed equally to this work.
Abstract
Hepatitis C remains a global health problem, especially in poverty-stricken areas. A rapid and sensitive point-of-care (POC) diagnostic tool is critical for the early detection and timely treatment of hepatitis C virus (HCV) infection. Here, for the first time, we reported a novel molecular diagnostic assay, termed reverse transcription multiple cross displacement amplification integrated with a gold-nanoparticle-based lateral flow biosensor (RT-MCDA-AuNPs-LFB), which was developed for rapid, sensitive, specific, and visual identification of HCV. HCV-RT-MCDA induced rapid isothermal amplification through a specific primer set targeting the 5′untranslated region gene from the major HCV genotypes 1b, 2a, 3b, 6a, and 3a that are prevalent in China. The optimal reaction temperature and time for RT-MCDA-AuNPs-LFB were 68°C and 25 min, respectively. The limit of detection of the assay was 10 copies per test, and the specificity was 100% for the experimental strains. The whole detection procedure, including crude nucleic acid isolation (~5 min), RT-MCDA (68°C, 25 min), and visual AuNPs-LFB result confirmation (less than 2 min), was performed within 35 min. The preliminary results indicated that the HCV-RT-MCDA-AuNPs-LFB assay could be a valuable tool for sensitive, specific, visual, cost-saving, and rapid detection of HCV and has potential as a POC diagnostic platform for field screening and early clinical detection of HCV infection.
1 INTRODUCTION
Hepatitis C virus (HCV) is a single-stranded positive-sense RNA virus that causes acute and chronic hepatitis, as the progression of liver damage resulting in liver cirrhosis, liver failure, and hepatocellular carcinoma.1, 2 It was estimated that approximately 58 million people were living with chronic HCV infection worldwide and 300 000 HCV-related deaths occur each year.1, 3 HCV typically spreads through direct percutaneous exposure to blood, blood transfusion, sexual transmission, injecting drug use, and mother-to-infant transmission.4, 5 The development of highly effective, oral direct-acting antivirals with few side effects in 2011 has provided an exceptional opportunity to treat hepatitis C, but less than 20% of patients know they have the infection.4 Currently, a major challenge is to engage and screen as many people as possible in need of treatment, especially in less-developed regions.1, 6 Hence, developing affordable HCV point-of-care (POC) diagnostics for screening of HCV is critical to achieve 2030 global elimination targets.1-3
Currently, enzyme immunoassay and polymerase chain reaction (PCR)-related techniques are the most reliable approaches for the diagnosis of hepatitis C in clinical settings.7, 8 Immunoassays are based on serological response that targets HCV antibody and core antigen, which can be detected within 4–10 weeks after infection.7 In addition, immunosuppressed infected individuals can have false-negative outcomes.7 In recent decades, nucleic-acid-based assays, including reverse transcription PCR (RT-PCR) and real-time quantitative RT-PCR, have been widely used for detecting HCV because of their high sensitivity and specificity.9, 10 However, these methods require expensive thermocycling equipment, and well-trained technicians, which limits their application in many resource-constrained areas. Besides, these approaches are time-consuming (more than 2 h).
Multiple cross displacement amplification (MCDA) is a novel and isothermal single enzyme-promoting nucleic acid amplification technology that can robustly amplify trace amounts of target genes with high specificity and sensitivity and in less time than for PCR-related techniques.11, 12 MCDA could form the basis of POC testing because it is easy to use, rapid, and cost-saving, and it has been used to identify several important human pathogens, such as monkeypox virus, SARS-CoV-2, and Mycobacterium tuberculosis.13-15 Gold-nanoparticles-based lateral flow biosensor (AuNPs-LFB) is a paper-based platform that has potential as an advanced POC diagnostic approach owing to its ease of operation, high sensitivity, low cost, and rapid detection.16, 17 This approach has become widespread in biomedical, agricultural, food safety, and environmental fields within a short period.18, 19
In the current study, the reverse transcription MCDA was integrated with a gold-nanoparticles-based lateral flow biosensor (RT-MCDA-AuNPs-LFB) to develop a novel assay for rapid and visual identification of HCV targeting the gene encoding the 5′untranslated region (5'-UTR),20 which showed has no homology to other microbes in BLAST searches of the GenBank database. The feasibility of our diagnostic system was verified with clinical samples from patients with suspected HCV infection.
2 MATERIALS AND METHODS
2.1 Target gene and clinical samples preparation
The 5′-UTR target gene sequences of five HCV dominant subtypes in China (1b, 2a, 3b, 6a, and 3a) were obtained from GenBank (Accession Nos. EU781827.1, HQ639944.1, JQ065709.1, AY859526.1, and D17763.1).21, 22 Each sequence was synthesized and cloned into pUC57 plasmids, and the concentration of each plasmid was 108 copies/mL. HCV subtype 1b plasmid was used as a positive control.
Eighty-three clinical serum samples were collected from patients with suspected HCV infection treated at the Second Affiliated Hospital, Guizhou University of Traditional Chinese Medicine from January 2022 to August 2023. Nucleic acids were rapidly isolated through Nucleic Acid Releasing Agent (GenDx Biotech Co. Ltd.). In brief, the nucleic acid releasing agents RNA LB1 (120 μL) and RNA BB2 (500 μL) were added to 50 μL serum samples for 20 s, and the nucleic acids were enriched in a centrifugal absorption column. Finally, the nucleic acids were eluted into 30 μL nuclease-free water. The genomic RNA was stored at −80°C before use.
2.2 HCV-MCDA primer design and synthesis
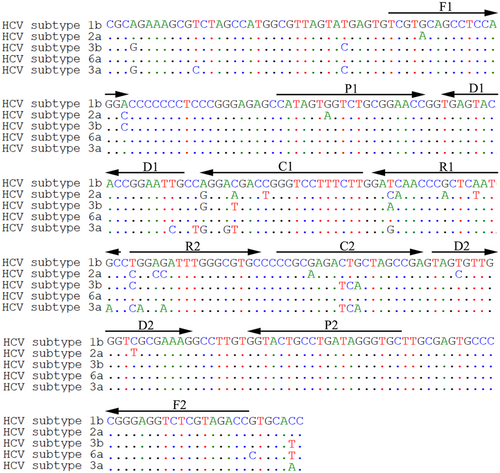
The 5′UTR sequences of HCV subtypes 1b, 2a, 3b, 6a, and 3a were aligned by DNASTAR software, and the conserved sequence was used for MCDA primer design with Primer Explorer version 5 (http://primerexplorer.jp/e/) and Primer Premier version 5.0. The MCDA primer set contained two displacement primers, F1 and F2; two cross primers CP1 and CP2; and six amplification primers, C1, C2, D1, D2, R1, and R2. For AuNPs-LFB detection, FAM and biotin was labeled at the 5′ ends of the D1 and C1 primers, respectively. The primer locations, sequences, and modifications are shown in Figure 1 and Table 1. All primers were synthesized by TsingKe Biotech Co. Ltd at an HPLC purification grade.
| Primer name | Sequence and modifications | Length | Gene |
|---|---|---|---|
| F1 | 5ʹ-TCGT(G/A)CAGCCTCCAGG(A/C)-3ʹ | 17 nt | 5ʹUTR |
| F2 | 5ʹ-GGTCTACGAGACCTCCCG-3ʹ | 18 nt | |
| CP1 | 5ʹ-AAGAAAGGACCC(G/A)GTC(G/T/A)(T/C)CC(T/C)-CATAGT(G/A)GTCTGCGGAACC-3ʹ | 40 mer | |
| CP2 | 5ʹ-CCGC(G/A)AGA(C/T)(T/C)(G/A)CTAGCCGA-GCACCCTATCAGGCAGTACC-3ʹ | 39 mer | |
| C1 | 5ʹ-AAGAAAGGACCC(G/A)GTC(G/T/A)(T/C)CC(T/C)-3ʹ | 21 nt | |
| C1* | 5ʹ-Biotin- AAGAAAGGACCC(G/A)GTC(G/T/A)(T/C)CC(T/C)-3ʹ | 21 nt | |
| C2 | 5ʹ-CCGC(G/A)AGA(C/T)(T/C)(G/A)CTAGCCGA-3ʹ | 19 nt | |
| D1 | 5ʹ-C(A/G)ATTCCGGTGTACTCA-3ʹ | 17 nt | |
| D1* | 5ʹ-FAM-C(A/G)ATTCCGGTGTACTCA-3ʹ | 17 nt | |
| D2 | 5ʹ-TAG(T/C)GTTGGGT(C/T)GCGAAAG-3ʹ | 19 nt | |
| R1 | 5ʹ-G(C/T)AT(T/A)GAG(C/T)GGGTT(G/T)(A/T/C/G)TC-3ʹ | 18 nt | |
| R2 | 5ʹ-(T/C)(G/A)G(A/C)(G/C/A)ATTTGGGCGTGC-3ʹ | 15 nt |
- Note: C1*, 5ʹ-labeled with biotin, D1*, 5ʹ-labeled with FAM, when used for the AuNPs-LFB assay.
- Abbreviations: FAM, 6-carboxy-fluorescein; HCV, hepatitis C virus; MCDA, multiple cross displacement amplification; mer, monomeric unit; nt, nucleotide; UTR, untranslated region.
2.3 Schematic mechanism of the AuNPs-LFB
The AuNPs-LFB (60 × 4 mm) comprised four components: sample pad, conjugate pad, nitrocellulose (NC) membrane (detection pad), and absorbent pad. Crimson dye streptavidin (SA) gold nanoparticles (SA-AuNPs) were assembled onto the conjugate pad. Anti-FAM and biotin-BSA were sprayed onto the NC membrane for the test line and control line, respectively. The four components were laminated on a plastic adhesive backing card. The AuNPs-LFB used in this study were manufactured by Tian-Jin HuiDeXin Biotech Co. Ltd according to our design (Figure 2A).

For AuNPs-LFB detection, 2.0 μL aliquots of the MCDA amplicons and 100 μL running buffer were dripped simultaneously onto the sample pad (Figure 2A-1). Due to capillary action, the running buffer containing MCDA amplicons moved forward onto the conjugate pad and NC membrane. The SA-AuNPs were rehydrated and conjugated with FAM/biotin-labeled MCDA products (Figure 2A-2). The FAM/biotin-labeled MCDA amplicons were captured by anti-FAM at the test line, and SA-AuNPs were captured by biotin-BSA at the control line (Figure 2A-3). For a positive outcome, both the control and test lines of the biosensor turned red. If only the control line turned red, it indicated a negative result (Figure 2A-4).
2.4 Standard HCV-RT-MCDA assay
The HCV-RT-MCDA primer set targeting the 5′-UTR gene was verified through a standard MCDA assay. The 25 μL MCDA reaction mixtures contained 2.5 μL isothermal amplification buffer (10×) (Mg2+ free); 1 μL Bst 4.2 DNA/RNA polymerase (8 U); 3 μL dNTP Mixture (10 mM); 1.5 μL 100 mM Mg2+; 0.4 mM each F1 and F2; 1.6 mM each CP1 and CP2; 0.8 mM each C1 or C1* (for AuNPs-LFB only), C2, D1, or D1* (for AuNPs-LFB only), D2, R1, and R2; 1.5 μL leuco-hydroxynaphthol blue (L-HNB, for colorimetry only); 1 μL standard plasmid template (or 5 μL clinical sample template); and double-distilled water (DW) up to 25 μL. The reaction process was performed at 65°C for 1 h and was terminated at 85°C for 5 min.
The MCDA products were analyzed by agarose gel electrophoresis, visual indicator (L-HNB), real-time turbidity, and AuNPs-LFB. For agarose gel electrophoresis, the agarose gel showed ladder-like bands suggested of a positive result, and there were no bands for negative outcomes. For visual indicator, a positive result was indicated when the MCDA mixture turned from deep violet to light green. However, the color of the mixture was always deep violet for a negative reaction. For real-time turbidity measurement, turbidity >0.1 indicated a positive MCDA outcome. For AuNPs-LFB identification, a positive outcome was indicated when both the control and test lines of the biosensor turned red. For negative and blank controls, only the control line turned red.
2.5 Optimization of reaction temperature and time for HCV-RT-MCDA AuNPs-LFB assay
To obtain an optimal reaction temperature for HCV-RT-MCDA, a range of 63–70°C with 1°C intervals was tested under the standard HCV-RT-MCDA condition with 5′-UTR plasmids (5.0 × 103 copies). The results were monitored using real-time turbidity (LA-500; Eiken Chemical Co. Ltd.). The optimal reaction time was verified from 20 to 35 min (at 5 min intervals) under the optimal amplification temperature. The results were analyzed simultaneously through visual indicators L-HNB and AuNPs-LFB. Each assay was conducted in triplicate.
2.6 Sensitivity and specificity of the HCV-RT-MCDA-AuNPs-LFB assay
The 5′UTR-plasmid templates were 10-fold serial diluted from 104 to 10−2 copies/test to verify the limit of detection (LoD) of the HCV-RT-MCDA-AuNPs-LFB assay. The assays were performed under optimal reaction conditions, and results were analyzed with visual indicators L-HNB and AuNPs-LFB. Each test was repeated three times. The specificity of the assay was tested using five HCV 5′-UTR-plasmid templates (HCV subtypes 1b, 2a, 3b, 6a, and 3a), HCV-positive clinical samples, and other microbial nucleic acid templates at ≥104 copies (Table 2). The assays were performed under optimal reaction conditions, and all of the amplicons were analyzed through AuNPs-LFB. DW was used as a blank control, and each test was performed at least three times.
| No | Pathogen | Source of pathogensa | No of strains | HCV-RT-MCDA-AuNPs-LFB resultb |
|---|---|---|---|---|
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 |
HCV 1b 5′UTR-plasmid HCV 2a 5′UTR-plasmid HCV 3b 5′UTR-plasmid HCV 6a 5′UTR -plasmid HCV 3a 5′UTR -plasmid HCV clinical samples Hepatitis B virus Human immunodeficiency virus Human papillomavirus Influenza A virus Influenza B virus Human enterovirus EV71 Coxsackie virus CAV16 Epstein-Barr virus Escherichia coli Staphylococcus aureus Pseudomonas aeruginosa Klebsiella pneumoniae Candida albicans Haemophilus influenzae Chlamydia trachomatis Listeria monocytogenes Neisseria gonorrhoeae Streptococcus pneumoniae Shigella flexneri Shigella sonnei |
Constructed by Tsingke Biotech (Beijing, China) Constructed by Tsingke Biotech (Beijing, China) Constructed by Tsingke Biotech (Beijing, China) Constructed by Tsingke Biotech (Beijing, China) Constructed by Tsingke Biotech (Beijing, China) 2nd GZUTCM 2nd GZUTCM GZCDC GZCDC GZCDC GZCDC GZCDC GZCDC 2nd GZUTCM ATCC8739 ATCC25923 ATCC27853 ATCC700603 ATCC14053 ATCC49247 Hangzhou Women's Hospital Hangzhou Women's Hospital Hangzhou Women's Hospital Zhejiang Hospital Zhejiang Hospital Zhejiang Hospital |
1 1 1 1 1 6 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 |
P P P P P P N N N N N N N N N N N N N N N N N N N N |
- Abbreviations: HCV, hepatitis C virus; MCDA, multiple cross displacement amplification; UTR, untranslated region.
- Note: aATCC, American Type Culture Collection; GZCDC, Guizhou Provincial Center for Disease Control and Prevention; 2nd GZUTCM, the Second Affiliated Hospital, Guizhou University of Traditional Chinese Medicine.
- b P, positive; N, negative.
2.7 Feasibility of HCV-RT-MCDA-AuNPs-LFB for clinical samples
The feasibility of the assay was validated using clinical serum samples from patients with suspected HCV infection. The genomic RNA was rapidly isolated using nucleic-acid-releasing agents (GenDx Biotech) as previously described, and the HCV-RT-MCDA-AuNPs-LFB assays were performed according to the optimized reaction conditions. All the samples were also tested by using HCV-RT-qPCR assays. HCV-RT-qPCR was performed using HCV Nucleic Acid Assay Kits (Xi'an Tianlong Technology Co. Ltd) on an Applied Biosystems 7500 Real-Time PCR System (Life Technologies). HCV > 50 IU/mL (~45 copies/mL) was deemed as a positive outcome. The HCV-positive samples were also identified with Sanger sequencing (Dian Medical Laboratory Center Co. Ltd.). Online statistical software MedCalc (http://www.medcalc.org/calc/diagnostic_test.php) was used to analyze the conventional HCV-RT-qPCR and HCV-RT-LAMP-AuNPs-LFB methods.23
3 RESULTS
3.1 Schematic mechanism of the HCV-RT-MCDA-AuNPs-LFB assay
The principle of AuNPs-LFB detection of HCV-RT-MCDA products is outlined in Figure 2A. Aliquots of 2 μL MCDA amplicons and 100 μL running buffer were dripped simultaneously into the sample pad of AuNPs-LFB (Figure 2A-1). The HCV-RT-MCDA amplicon-containing running buffer was moved along the biosensor platform via capillary action. SA-AuNPs were rehydrated with running buffer, and FAM/biotin-labeled HCV-RT-MCDA amplicons were integrated with SA-AuNPs (Figure 2A-2). At the detection region (NC membrane), anti-FAM was fixed at the test line and applied to capture FAM-labeled HCV-RT-MCDA amplicons, biotin-BSA was anchored at the control line to stop SA-AuNPs (Figure 2A-3). Interpretation of the HCV-RT-MCDA results by AuNPs-LFB is shown in Figure 2A-4. For a positive outcome, both the control and test lines turned red, whereas only the control line turned red for a negative result.
The workflow of HCV-RT-MCDA-AuNPs-LFB assay was shown in Figure 2B, the HCV genomic RNA was isolated rapidly by nucleic acid releasing agent within 5 min (Figure 2B-1). The RT-MCDA technique was used for rapidly and specifically amplifying target gene 5′UTR within 25 min at a constant temperature (68°C). The two core primers D1 and C1 were labeled at the 5′ ends with FAM and biotin, respectively. The amplicons were simultaneously labeled with the two signal molecules (Figure 2B-2). Finally, the MCDA amplicons were visually analyzed through AuNPs-LFB within 2 min (Figure 2B-3).
3.2 Verification of HCV-RT-MCDA products
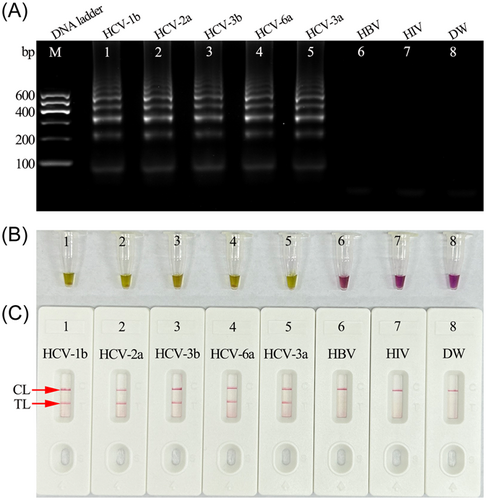
To confirm the feasibility of the HCV-RT-MCDA reaction system, the reaction mixtures were incubated at a constant temperature of 65°C for 1 h. The products were analyzed using 2% agarose gel electrophoresis, colorimetric indicator (L-HNB), and AuNPs-LFB (Figure 3A–C). The templates from five synthetic HCV-5′UTR plasmids (subtypes 1b, 2a, 3b, 6a, and 3a) were amplified and showed positive results through each analysis method. No amplification was observed for the negative controls (HBV and HIV) and blank control (DW). These data indicated that the MCDA primer set devised for the 5′UTR gene was an appropriate candidate for development of the HCV-RT-MCDA-AuNPs-LFB assay.
3.3 Optimal amplification temperature for HCV-RT-MCDA-AuNPs-LFB assay
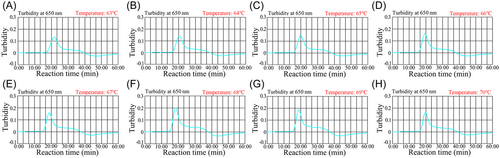
An appropriate reaction temperature is critical for high efficiency MCDA amplification. In this study, the HCV-RT-MCDA reaction temperature was optimized by performing the amplification at a temperature ranging from 63°C to 70°C (Figure 4A−H). The HCV-5′UTR-plasmid template concentration used for the detection was 2.0 × 103 copies/test. A real-time turbidimeter was used to analyze the HCV-RT-MCDA amplicons. Robust amplification for the HCV-RT-MCDA assays occurred at 68°C (Figure 4F). Hence, 68°C was considered to be the appropriate reaction temperature and used for the following HCV-RT-MCDA-AuNPs-LFB assay.
3.4 Sensitivity of the HCV-RT-MCDA-AuNPs-LFB assay
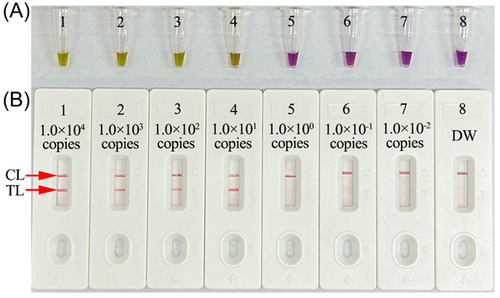
Serial dilutions of the HCV-5′UTR plasmids were used as templates to test the LoD of the assay. HCV-RT-MCDA amplifications were performed at 68°C for 1 h, and the MCDA amplicons were analyzed with colorimetric indicator (L-HNB) and AuNPs-LFB. The LoD of HCV-RT-MCDA-AuNPs-LFB was 10 copies per test (Figure 5). The results obtained using AuNPs-LFB (Figure 5B) were in accordance with those obtained with colorimetric indicator (L-HNB) (Figure 5A).
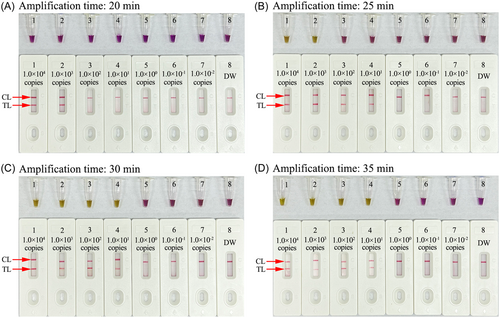
3.5 Optimal reaction time for HCV-RT-MCDA-AuNPs-LFB assay
The optimal reaction time for the assay at the RT-MCDA amplification stage was tested in the range of 20–35 min with 5 min intervals (Figure 6A−D). The amplicons were monitored with AuNPs-LFB and colorimetric indicator (L-HNB). The LoD of 10 copies of the HCV-5′UTR plasmid was tested when the amplification time was 25 min using AuNPs-LFB (Figure 6B). However, the LoD required 30 min when using L-HNB (Figure 6C). The whole detection procedure for HCV-RT-MCDA-AuNPs-LFB diagnosis, including rapid template isolation (5 min), RT-MCDA amplification (25 min), and result readout with AuNPs-LFB (<2 min), can be completed within 35 min.
3.6 Specificity of HCV-RT-MCDA-AuNPs-LFB assay
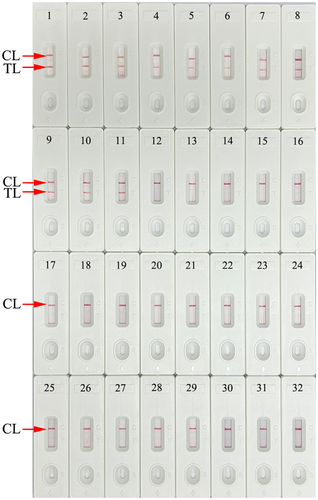
The specificity of the assay was evaluated using HCV 5′UTR plasmids (subtypes 1b, 2a, 3b, 6a, and 3a), positive HCV clinical samples (confirmed by RT-qPCR), and 20 other pathogens (Table 2). All tests were performed under the optimal reaction conditions, and the results were analyzed using AuNPs-LFB. Only the HCV 5′UTR plasmids and strains showed a positive outcome, while the other 20 non-HCV pathogens and blank control (DW) were negative (Figure 7 and Table 2). These data demonstrated that the HCV-RT-MCDA-AuNPs-LFB assay had good selectivity and did not produce cross-reactivity with non-HCV pathogens.
3.7 Feasibility of HCV-RT-MCDA-AuNPs-LFB assay for clinical specimens
To evaluate the suitability of HCV-RT-MCDA-AuNPs-LFB assay as a useful tool for HCV diagnosis, 83 serum samples from patients with suspected HCV infection were analyzed simultaneously by the novel assay and traditional HCV-RT-qPCR method. Thirty-seven samples tested as HCV-positive using the HCV-RT-MCDA-AuNPs-LFB assay. All the positive samples were confirmed by RT-qPCR. In addition, all positive samples were measured using Sanger sequencing at Dian Medical Laboratory Center to confirm their subtypes. Fifteen samples were tested as subtype 1b, 11 as subtype 2a, four as subtype 3b, four as subtype 6a, three as subtype 3a, and one as subtype 1a (Tables 3 and 4). Compared with traditional HCV-RT-qPCR technique, our assay's sensitivity and specificity were 100% (95% CI: 90.51%−100%) and 100% (95% CI: 92.29%−100.00%), respectively (Table 3).
| HCV-MCDA-AuNPs-LFB | HCV RT-qRCR (reference method) | Sensitivity | Specificity | ||||
|---|---|---|---|---|---|---|---|
| Positive | Negative | Total | Value | 95% CI | Value | 95% CI | |
| Positive | 37 (subtype 1b, 15; subtype 2a, 11; subtype 3b, 4; subtype 6a, 4; subtype 3a, 3) |
0 | 37 | 100.00% | 90.51%−100.00% | 100% | 92.29%− 100.00% |
| Negative | 0 | 46 | 46 | ||||
| Total | 37 | 46 | 83 | ||||
- Abbreviations: HCV, hepatitis C virus; MCDA, multiple cross displacement amplification; RT-qPCR, reverse transcription-polymerase chain reaction.
| Sample no | RT-qPCR result(copies) | HCV-RT-MCDA-AuNPs-LFB | HCV Genotype |
|---|---|---|---|
| Test 1 | 1.45 × 106 | + | 2a |
| Test 2 | 1.08 × 103 | + | 1b |
| Test 3 | 7.21 × 105 | + | 1b |
| Test 4 | 4.30 × 106 | + | 1b |
| Test 5 | 3.22 × 103 | + | 1b |
| Test 6 | 3.15 × 104 | + | 3a |
| Test 7 | 1.65 × 104 | + | 2a |
| Test 8 | 6.16 × 105 | + | 2a |
| Test 9 | 3.21 × 106 | + | 6a |
| Test 10 | 4.24 × 107 | + | 1b |
| Test 11 | 3.59 × 104 | + | 6a |
| Test 12 | 2.15 × 106 | + | 2a |
| Test 13 | 2.84 × 104 | + | 3b |
| Test 14 | 2.49 × 105 | + | 1b |
| Test 15 | 1.51 × 106 | + | 1b |
| Test 16 | 3.56 × 104 | + | 2a |
| Test 17 | 1.76 × 107 | + | 2a |
| Test 18 | 1.73 × 105 | + | 1b |
| Test 19 | 1.08 × 107 | + | 1b |
| Test 20 | 3.18 × 104 | + | 1b |
| Test 21 | 3.31 × 106 | + | 6a |
| Test 22 | 6.14 × 105 | + | 3b |
| Test 23 | 3.20 × 106 | + | 3a |
| Test 24 | 1.86 × 104 | + | 2a |
| Test 25 | 4.26 × 107 | + | 3a |
| Test 26 | 7.91 × 104 | + | 3b |
| Test 27 | 6.32 × 106 | + | 1b |
| Test 28 | 4.13 × 106 | + | 6a |
| Test 29 | 2.81 × 105 | + | 3b |
| Test 30 | 5.23 × 103 | + | 2a |
| Test 31 | 2.18 × 105 | + | 2a |
| Test 32 | 7.51 × 105 | + | 1b |
| Test 33 | 5.18 × 105 | + | 1b |
| Test 34 | 1.12 × 107 | + | 2a |
| Test 35 | 1.17 × 105 | + | 1b |
| Test 36 | 4.09 × 104 | + | 1b |
| Test 37 | 9.32 × 105 | + | 2a |
| Test 38−83 | − | − |
- Note: The RT-qPCR diagnosis was carried out using commercial real-time TaqMan PCR Kit (Xi'an Tianlong Technology Co., Ltd.). The concentrations of HCV more than 45 copies will be considered as a positive result according to the manufacturer's instructions. The HCV-RT-qPCR positive samples were analyzed with Sanger sequencing (Dian Medical Laboratory Center Co., Ltd.).
- Abbreviations: HCV, hepatitis C virus; MCDA, multiple cross displacement amplification; RT-PCR, reverse transcription-polymerase chain reaction; +, positive; −, negative.
4 DISCUSSION
In this study, a novel HCV nucleic acid diagnosis platform, HCV-RT-MCDA-AuNPs-LFB assay, which integrated RT-MCDA amplification with AuNPs-LFB detection, was successfully developed for rapid, specific, visual, and user-friendly diagnosis of HCV. The feasibility of the novel assay was verified using clinical samples from individuals with suspected HCV infection.
Currently, HCV RNA detection with PCR-related techniques is the gold standard for the diagnosis of active HCV infection.10, 24 However, these techniques require advanced infrastructure and trained operators, and are not feasible for resource-limited regions. Here, the HCV-RT-MCDA-AuNPs-LFB assay used the novel isothermal technique RT-MCDA for amplifying the target sequence within a short time (25 min) at a constant temperature (68°C). RT-MCDA can be performed isothermally with simple instruments, such as a water bath, metal bath, heating block, or even a thermos cup. The HCV-RT-MCDA amplification products were visually and rapidly interpreted using AuNPs-LFB. The crude nucleic acid was sufficient for the MCDA reaction owing to the Bst 4.2 DNA/RNA polymerase, which has fewer inhibitors than the Taq DNA polymerase used in traditional PCR systems.25, 26 Hence, the novel assay is simple and time-saving, and the whole process, including genomic RNA extraction (~5 min), RT-MCDA reaction (25 min), and AuNPs-LFB visual results identification (less than 2 min), can be finished within 35 min.
The HCV-specific amplicons were generated in the RT-MCDA system using only Bst 4.2 DNA/RNA polymerase with strand displacement activity and a set of 10 specific primers that span 10 unique fragments of the target gene. The MCDA primers included two displacement primers (F1 and F2), two cross primers (CP1 and CP2), and six amplification primers (C1, C2, D1, D2, R1, and R2). The principle of the MCDA reaction has been reported previously.11-13 In the current study, the MCDA primer set was successfully designed based on the 5′UTR gene from the five prevalent HCV subtypes (1b, 2a, 3b, 6a, and 3a) in China.21, 22 The RT-MCDA reaction condition was optimized at 68°C for 25 min. The specificity of the HCV-RT-MCDA-AuNPs-LFB diagnostic platform was tested on HCV strains and other pathogens. As expected, the assay specifically identified HCV, and had no cross-reaction with other pathogens (Table 2 and Figure 7). Sensitivity analysis indicated that the HCV-RT-MCDA-AuNPs-LFB assay was able to detect as few as 10 copies per test (Figure 5). To verify the feasibility of the HCV-RT-MCDA-AuNPs-LFB assay, 83 clinical samples from patients with suspected HCV infection were tested simultaneously with the novel assay and traditional RT-qPCR method. The results confirmed that the novel assay effectively identified HCV in clinical samples. Isothermal amplification techniques, such as RT-loop-mediated isothermal amplification and RT-recombinase polymerase amplification, have also been reported to diagnose HCV. Hongjaisee et al. used a method based on RT-loop-mediated isothermal amplification and identified 10–100 ng per reaction.27 Chia et al. applied an assay based on RT-recombinase polymerase amplification and identified an LoD of 25 copies per test.28 It was obvious that the novel assay in this study was more sensitive than the approaches previously reported.
For visual and rapid analysis, AuNPs-LFB diagnostic platform was applied in the present study. AuNPs-LFB is a paper-based biosensor that is a convenient POC testing technique on account of its high sensitivity and specificity, rapid detection, ease of use, low cost, and no need for trained personnel.29, 30 AuNPs-LFB strips have been extensively used to diagnose various analytes, such as infectious pathogens, cancer biomarkers, and foodborne microbes.18 Here, the AuNPs-LFB diagnostic platform comprised four sections: a sample pad, conjugation pad, detection zone (NC membrane), and absorption pad (Figure 2A). The sample pad was formed by cellulose fibers, and loaded at the beginning of the platform, which was ideal for transporting HCV-RT-MCDA products to the next biosensor components. The conjugate pad was the second component to be encountered by the samples, and SA-AuNPs were fabricated as the biosensor of this component. The NC membrane (also termed detection zone) was the third part of the biosensor where the signal was generated. BSA-biotin and anti-FAM were immobilized to the control line and test line of the NC membrane, respectively. For a negative result, only crimson dye SA-AuNPs were captured at the control line and presented with a red band. For a positive outcome, the FAM/biotin-labeled HCV-RT-MCDA amplicons were stopped by anti-FAM at the test line, and the SA-AuNPs were also stopped at the control line. The final part of the biosensor was the absorbent pad, which served as bibulous paper to prompt flow of the HCV-RT-MCDA amplicons from the sample pad to the detection zone. In the current study, agarose gel electrophoresis, real-time turbidity, and colorimetric indicator L-HNB were also used to detect HCV-RT-MCDA amplicons. However, the two former approaches need expensive facilities and are not suitable for a POC diagnostic platform. The L-HNB method was visual and equipment-free, but need more amplification time for detection of the LoD (Figure 6B,C), and the visual result was ambiguous when the amplicons were low.23, 25 Our AuNPs-LFB strip was convenient operation and inexpensive (approximately US$2.0 per test). Hence, the approximate total cost of each HCV-RT-MCDA-AuNPs-LFB detection, including genomic RNA extraction (US $0.5), RT-MCDA reaction (US $1.5), and AuNPs-LFB detection (US $2.0) was US $4.0.
There were some limitations to the present study. First, the MCDA primers only specifically identified HCV dominant subtypes in China (1b, 2a, 3b, 6a, and 3a). Further refinement of the HCV-RT-MCDA primers is needed because HCV has high genetic heterogeneity and various subtypes. Second, identifying the HCV-RT-MCDA results by AuNPs-LFB required opening of the reaction tube, which increased the risk of carry-over contamination. To improve the test for clinical implementation and reduce aerosol contamination, it will be necessary to refine the system to avoid opening of the MCDA reaction tube.
5 CONCLUSIONS
Here, for the first time, we integrated RT-MCDA isothermal amplification with AuNPs-LFB detection platform to develop a novel HCV-RT-MCDA-AuNPs-LFB assay for rapid and visual identification of HCV in clinical application. The analytical data indicated that our assay had a 10-copy LoD and showed no cross-reactivity with other microbes. The entire diagnostic procedure can be performed within 35 min without the need for expensive facilities and trained personnel. Hence, the HCV-RT-MCDA-AuNPs-LFB assay is a valuable tool for sensitive, specific, visual, cost-saving, and rapid detection of HCV, and has potential as a POC diagnostic platform for screening and early clinical detection of HCV infection.
AUTHOR CONTRIBUTIONS
Xu Chen, Zhenghua Xiao, and Qingxue Zhou were involved in study conceptualization, supervision, and project administration. Shilei Dong, Yuanfang Shi, Zengguang Wu, Xue Wu, Xiaoyan Zeng, Xinggui Yang, and Qi Zhao performed experiments and data curation. Xu Chen, Zhenghua Xiao, and Qingxue Zhou involved in study funding acquisition and methodology. Yuanfang Shi, Zengguang Wu, Xue Wu, Qi Zhao, and Zhenghua Xiao were involved in validation studies and visualization. Xu Chen and Zhenghua Xiao was involved in writing—original draft. Xu Chen and Qingxue Zhou were involved in writing—review and editing.
ACKNOWLEDGMENTS
This work was supported by the Medical Scientific Research Foundation of Zhejiang Province (Grant No. 2023KY995), Zhejiang Provincial Natural Science Foundation of China (Grant No. LTGY23H190004), the Program of Science and Technology of Guizhou Provincial Health Commission (Grant No. gzwjkj2022-1-497), Guizhou Provincial Key Technology R&D Program (Grant No. Qian Ke He Support [2023] General 242), the Program of Key Laboratory of Higher Education in Guizhou Province (Grant No. Qian Jiao Ji [2023]017), the NATCM's Project of High-level Construction of Key TCM Disciplines (Grant No. zyyzdxk-2023187), and the Program of Scientific and Technological of Guiyang City (Grant No. Zhu Ke He (2024) 2-34).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
This study was approved by the Human Ethics Committee of the Second Affiliated Hospital, Guizhou University of Traditional Chinese Medicine (Approval No. KYW2022034), and complied with the Declaration of Helsinki. Before our team obtained clinical samples/isolates and conducted this research, any personal patient identifiers were removed. Patient informed consent was waived by the ethics committee.
Open Research
DATA AVAILABILITY STATEMENT
The data sets used and/or analyzed during the current study are available from the corresponding author on reasonable request.




