A subunit-based influenza/SARS-CoV-2 Omicron combined vaccine induced potent protective immunity in BALB/c mice
Naru Zhang, Zihui Ye, Cun Li, and Jie Zhou contributed equally to this work.
Abstract
Infection with influenza A virus (IAV) and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) poses a significant risk to human life, health, and the global economy. Vaccination is one of the most effective strategies in the fight against infectious viruses. In this study, we, for the first time, have evaluated the immunogenicity and protective effect of an influenza/SARS-CoV-2 Omicron subunit combined vaccine adjuvanted with MF59 and administered to BALB/c mice. Results showed that the combined vaccine induced high levels of IgG, IgG1, and IgG2a antibodies, as well as influenza A H1N1/California/2009 virus-specific hemagglutination-inhibiting antibodies in BALB/c mice. Moreover, this subunit combined vaccine induced high titers of neutralization antibodies against SARS-CoV-2 Omicron sublineage BA.5 pseudovirus and effectively reduced the viral load of authentic SARS-CoV-2 Omicron sublineage BA.5.2 in the cell culture supernatants. These results suggested that this subunit combined vaccine achieved protective effect against both H1N1 A/California/07/2009 strain and SARS-CoV-2 Omicron BA.5.2 variant. It is therefore expected that this study will establish the scientific foundation for the next-step development of combined vaccines against other strains or variants of IAV and SARS-CoV-2.
1 INTRODUCTION
Coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has had a huge negative impact on global public health and economic development.1 Since its discovery, SARS-CoV-2 has evolved into several variants, and now Omicron is a major public health concern owing to its high infectivity and antibody evasion.2 Omicron variants BA.4 and BA.5 were reported to be more transmissible and resistant to immunity than previous variants, including Omicron BA.1 and most monoclonal antibodies.2 As of November 2, 2023, SARS-CoV-2 and its variants had caused 771 679 618 confirmed cases and 6 977 023 deaths globally.3 Influenza A virus (IAV), a common respiratory infectious virus, causes severe respiratory illnesses in 3−5 million people and 290 000−650 000 deaths yearly worldwide.4 IAV is one of the most commonly recognized respiratory viruses identified as coinfected with SARS-CoV-2.5 Studies have shown that coinfection with SARS-CoV-2 during the acute stage of IAV infection further increases the risk of multiple tissue or organ disease in patients, leading to the occurrence of higher rates of severe illness and mortality.6-8 Vaccination is one of the most effective measures to prevent respiratory infectious viruses.
Seasonal vaccination against IAV could significantly decrease the number of individuals getting sick or death from the infection.9 Also, COVID-19 vaccination has effectively reduced severe cases and mortality rate.10, 11 Many dual-vaccination strategies are currently in the developmental stage, such as the virion-based vaccine,12, 13 receptor-binding domain (RBD)-based vaccine,14 RBD-conjugated inactivated IAV,15 mRNA vaccine,8 chimpanzee adenovirus 68 (AdC68)-based vaccine,16 and recombinant VSV-based bivalent vaccine.17 Similarly, we imagined the advantages of a combined SARS-CoV-2/IAV subunit vaccine in the fight against COVID-19 and influenza, including the safety and ease of producing such subunit vaccine on a large scale.
Accordingly, in this study, the immunogenicity and protective effect of an influenza/SARS-CoV-2 Omicron subunit combined vaccine adjuvanted with MF59 were evaluated in BALB/c mice. The combined vaccine induced high levels of IgG, IgG1, and IgG2a antibodies, as well as influenza A H1N1/California/2009 virus-specific hemagglutination-inhibiting antibodies in BALB/c mice. Furthermore, the subunit combined vaccine induced high titers of neutralizing antibodies against SARS-CoV-2 Omicron sublineage BA.5 pseudovirus and effectively reduced the viral load of SARS-CoV-2 Omicron BA.5.2 variant in the cell culture supernatants. Our study further demonstrated that the subunit combined vaccine achieved a double protective effect against influenza H1N1 A/California/07/2009 virus and SARS-CoV-2 Omicron BA.5.2 variant infection.
2 MATERIALS AND METHODS
2.1 Proteins, reagents, cell lines, and viruses
HA, SARS-CoV-2 Omicron sublineage BA.5 S, and RBD proteins were purchased from SinoBiological. MF59 adjuvant was prepared according to our previously published paper.18 Horseradish peroxidase (HRP)-labeled goat anti-mouse IgG and 3,3′,5,5′-Tetramethylbenzidine (TMB) were purchased from Beyotime. HRP-labeled goat anti-mouse IgG1 and IgG2a were purchased from Abcam. VeroE6-TMPRSS2 cells were purchased from the American Type Culture Collection. Dulbecco's Modified Eagle's Medium (DMEM) was purchased from Meilun. Fetal bovine serum (FBS) was purchased from Gibco. Experiments involving the SARS-CoV-2 Omicron sublineage BA.5.2 and H1N1 A/California/07/2009 strain were conducted by following the approved standard operating procedures of the biosafety level 3 facility at the University of Hong Kong.
2.2 Animal vaccination and sample collection
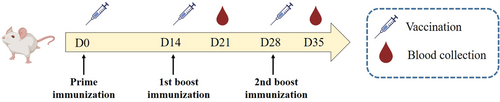
Twenty BALB/c mice were randomly divided into four groups (n = 5). Mice were intramuscularly immunized as we previously described.18 Briefly, each mouse in Group 1 was immunized with 100 µL PBS as a control, while that in Group 2 or 3 was immunized with 5 µg HA or SARS-CoV-2 Omicron BA.5 S diluted in 50 µL PBS formulated with an equal volume of MF59, respectively. Each mouse in Group 4 was immunized with 5 µg HA plus 5 µg SARS-CoV-2 Omicron BA.5 S diluted in 50 µL PBS formulated with an equal volume of MF59. As shown in Figure 1, all mice were immunized three times at 2-week intervals. Serum was isolated from the collected blood 1 week after each immunization, heat-inactivated at 56°C for 30 min, and then divided and frozen at −20°C in the refrigerator for further use.
2.3 Enzyme-linked immunosorbent assay (ELISA)
ELISA was used to detect HA or SARS-CoV-2 Omicron BA.5 S-RBD-specific IgG, IgG1, and IgG2a antibodies in mouse sera. ELISA plates were precoated with 2 µg/mL HA or SARS-CoV-2 Omicron BA.5 S-RBD protein overnight at 4°C. The plates were washed with PBS containing 0.05% Tween-20 (PBST) and then blocked with PBS containing 2% skimmed milk powder at 37°C for 1 h. After washing the plates with PBST, serially diluted mouse serum was added into the plates, followed by incubation at 37°C for 1 h. After again washing with PBST, HRP-conjugated goat anti-mouse IgG, IgG1, or IgG2a was added to the plates, followed again by incubation at 37°C for 1 h. After washing, TMB was added to the plates. Finally, 1 N H2SO4 was added to the plates to terminate the reaction, and the absorbance value was measured at 450 nm by microplate reader. Endpoint titers were expressed as the highest reciprocal serum dilution exhibiting an absorbance of 450 nm >2.1-fold over the background values.
2.4 Hemagglutination inhibition (HAI) assay
HAI of immunized mouse sera was performed as previously described.14 First, serum samples were treated with receptor-destroying enzymes (Denka Seiken) overnight at 37°C and heat-inactivated at 56°C for 30 min. Second, 25 µL of eight HAU of A/California/07/2009 (H1N1) virus were added to an equal volume of twofold serially diluted mouse sera in wells of a U-bottom 96-well microtiter plate and incubated for 45 min to 1 h. Finally, 0.55% turkey erythrocytes were added into the wells, followed by further incubation at room temperature for 30 min. HAI titers were defined as the reciprocal of the highest serum dilution required for complete HAI.
2.5 SARS-CoV-2 Omicron sublineage BA.5 pseudovirus production and neutralization assay
Generation of pseudotyped SARS-CoV-2 Omicron sublineage BA.5 was performed as previously described.19, 20 Briefly, the backbone plasmid of pNL4-3.Luc.R-E- was cotransfected with the plasmid of pcDNA3.1-SARS-CoV-2 Omicron BA.5-S into HEK-293T cells by using VigoFect transfection reagent (Vigorous Biotechnology). Cell culture supernatant containing the pseudoviruses after centrifuging at 3000 rpm for 10 min was harvested 48 h posttransfection. The pseudotyped viruses were stored at −80°C until use. Detection of neutralizing antibodies against pseudotyped SARS-CoV-2 Omicron BA.5 variant was conducted by neutralization assay. Briefly, mouse sera were heat-inactivated at 56°C for 30 min. Huh-7 cells were seeded in wells of a 96-well cell culture plate at a density of 1 × 104/well. Serially diluted mouse sera were incubated with the pseudotyped SARS-CoV-2 Omicron BA.5 for 30 min. Then, the mixture of mouse sera and pseudoviruses was transferred to a 96-well cell culture plate. After 12 h, the cell culture supernatant was discarded, and cell culturing continued with fresh DMEM containing 5% FBS for 48 h. Then, 1X lysis buffer was added to the wells for 30 min, the lysate was transferred to a 96-well half-area white plate, and luciferase activity was detected by using a Firefly luciferase assay kit (Promega). Luciferase values were measured using a SpectraMax i3x multimode microplate reader (Molecular Devices). Neutralizing antibody titers (NT50) were defined as the serum dilutions that could reduce 50% relative luminescence units compared to virus control wells.
2.6 SARS-CoV-2 Omicron sublineage BA.5.2 viral load reduction assay
Viral load reduction assay was performed on VeroE6-TMPRSS2 cells. Threefold serially diluted mouse serum samples were preincubated with 1000 PFU of authentic SARS-CoV-2 Omicron sublineage BA.5.2 at 37°C for 1 h. Following preincubation, a 100 μL serum-virus mixture was added gently to VeroE6-TMPRSS2 cells. The inoculated cells were incubated at 37°C for 1 h. Afterwards, the infectious inoculum was aspirated, washed and replaced with fresh medium containing the serially diluted mouse serum. Forty-eight hours later, supernatant from the infected cells was harvested for qRT-PCR analysis of viral load. Briefly, 50 µL of viral supernatant were lysed with 200 µL of AVL buffer and then extracted for total RNA using the QIAamp viral RNA mini kit (Qiagen). Real-time one-step qRT-PCR was used for quantitation of viral load using the QuantiNova Probe RT-PCR kit (Qiagen) with the LightCycler 480 real-time PCR system (Roche). Each 20 µL reaction mixture contained 10 µL of 2 x QuantiNova Probe RT-PCR master mix, 1.2 µL of RNase-free water, 0.2 µL of QuantiNova Probe RT-Mix, 1.6 µL each of 10 µM forward and reverse primer, 0.4 µL of 10 µM probe, and 5 µL of extracted RNA as the template. Reactions were incubated at 45°C for 10 min for reverse transcription and 95°C for 5 min for denaturation, followed by 45 cycles of 95°C for 5 s and 55°C for 30 s. Signal detection and measurement were performed in each cycle after the annealing step. The cycling profile ended with a cooling step at 40°C for 30 s. Primers and probe sequences were against the RNA-dependent RNA polymerase/Helicase (RdRP/Hel) gene region of SARS-CoV-2 Omicron BA.5.2: forward primer: 5′-CGCATACAGTCTTRCAGGCT-3′; reverse primer: 5′-GTGTGATGTTGAWATGACATGGTC-3′; specific probe: 5′-FAM TTAAGATGTGGTGCTTGCATACGTAGAC-IABkFQ-3′.
2.7 Statistical analysis
All statistical analyses were performed with GraphPad Prism 8 software. Statistical significance was calculated by two-way ANOVA. p Values less than 0.05 were considered statistically significant.
3 RESULTS
3.1 Influenza/SARS-CoV-2 Omicron subunit combined vaccine induced high levels of IgG antibody titers
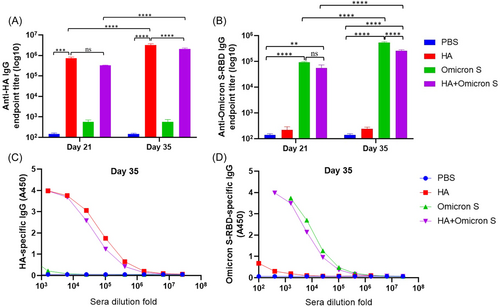
BALB/c mice were intramuscularly immunized with PBS, HA, SARS-CoV-2 Omicron BA.5 S, or HA + SARS-CoV-2 Omicron BA.5 S adjuvanted with MF59, respectively, three times at 2-week intervals. Serum samples were collected 1 week after each immunization for HA or SARS-CoV-2 Omicron BA.5 S-RBD-specific IgG detection. Based on mouse sera collected on Day 21, 1 week after the first boost immunization, as shown in Figure 2A, a high level of HA-specific IgG was observed in mice immunized with HA with no significant difference between the HA and HA + SARS-CoV-2 Omicron BA.5 S immunized groups. The level of IgG was significantly increased on Day 35, 1 week after the second boost immunization, in mice immunized with HA and HA + SARS-CoV-2 Omicron BA.5 S, respectively. Similarly, based on mouse sera collected on Day 21, as shown in Figure 2B, a high level of SARS-CoV-2 Omicron BA.5 S-RBD-specific IgG was observed in mice immunized with SARS-CoV-2 Omicron BA.5 S with no significant difference between the SARS-CoV-2 Omicron BA.5 S and HA + SARS-CoV-2 Omicron BA.5 S immunized groups. The level of IgG was significantly increased on Day 35 in mice immunized with SARS-CoV-2 Omicron BA.5 S and HA + SARS-CoV-2 Omicron BA.5 S, respectively. As shown in Figure 2C,D, mouse sera collected on Day 35 showed good binding ability with coated HA and Omicron S-RBD proteins.
3.2 Influenza/SARS-CoV-2 Omicron subunit combined vaccine induced high levels of IgG1 and IgG2a antibodies in mice
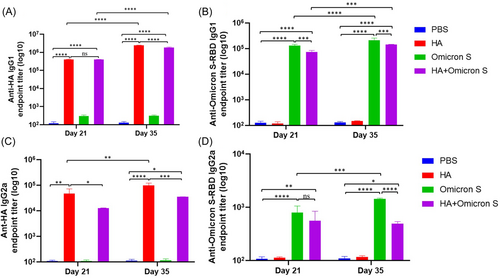
Endpoint titers of HA or SARS-CoV-2 Omicron BA.5 S-RBD-specific IgG1 and IgG2a in immunized mouse sera on Days 21 and 35 were also detected by ELISA. As shown in Figure 3A, a quite high level of HA-specific IgG1 was observed in mice immunized with HA with no significant difference between the HA and HA + SARS-CoV-2 Omicron BA.5 S immunized groups. The level of IgG1 was significantly increased on Day 35 in mice immunized with HA and HA + SARS-CoV-2 Omicron BA.5 S, respectively. As shown in Figure 3B, a high level of SARS-CoV-2 Omicron BA.5 S-RBD-specific IgG1 was observed in mice immunized with SARS-CoV-2 Omicron BA.5 S. The level of IgG1 was significantly increased on Day 35 in mice immunized with SARS-CoV-2 Omicron BA.5 S and HA + SARS-CoV-2 Omicron BA.5 S, respectively. As shown in Figure 2C,D, HA or SARS-CoV-2 Omicron BA.5 S-RBD-specific IgG2a in immunized mouse sera on Day 21 was detected. Moreover, the level of IgG2a was significantly increased on Day 35 in mice immunized with HA and SARS-CoV-2 Omicron BA.5 S, respectively. Importantly, the influenza/SARS-CoV-2 Omicron subunit combined vaccine induced balanced Th1/Th2 cellular immune response in BALB/c mice.
3.3 Influenza/SARS-CoV-2 Omicron subunit combined vaccine induced high levels of H1N1 A/California/07/2009 virus-specific hemagglutination-inhibiting antibodies in mice
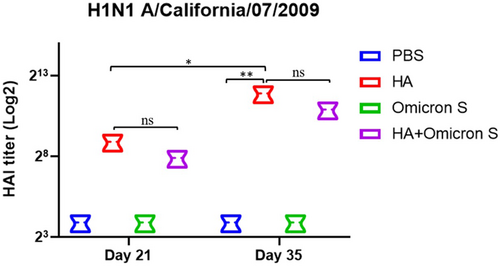
The H1N1 A/California/07/2009 virus-specific hemagglutination-inhibiting antibody titers in immunized mouse sera on Days 21 and 35 were detected by HAI assay. As shown in Figure 4, a quite high level of H1N1 A/California/07/2009 virus-specific HAI titer was detected in mice immunized with HA with no significant difference between the HA and HA + SARS-CoV-2 Omicron BA.5S immunized groups. HAI titer was significantly increased on Day 35 in mice immunized with HA, again with no significant difference between the HA and HA + SARS-CoV-2 Omicron BA.5 S immunized groups.
3.4 Influenza/SARS-CoV-2 Omicron subunit combined vaccine induced high titers of neutralizing antibodies against SARS-CoV-2 Omicron BA.5 pseudovirus and effectively reduced the viral load of authentic SARS-CoV-2 Omicron BA.5.2 in the cell culture supernatants
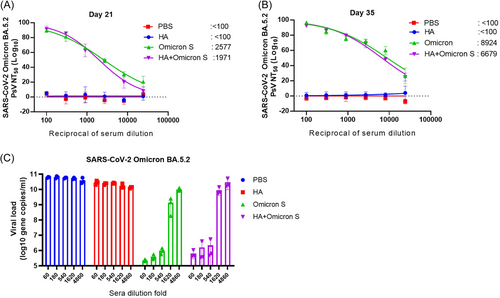
We next detected the neutralizing antibodies of immunized mouse sera collected on Days 21 and 35 against pseudotyped SARS-CoV-2 Omicron BA.5 variant. As shown in Figure 5A, Omicron S and HA+Omicron S immunized mouse sera collected on Day 21 elicited high titers of Omicron BA.5-specific neutralizing antibodies (2577 and 1977, respectively). The neutralizing antibody titers in the sera of mice immunized with Omicron S or HA+Omicron S collected on Day 35 increased significantly (8924 and 6679, respectively) (Figure 5B). We further detected the antiviral effect of mouse sera collected on Day 35 against SARS-CoV-2 Omicron BA.5.2 strain on VeroE6-TMPRSS2 cells. As shown in Figure 5C, both Omicron S and HA+Omicron S immunized mouse sera effectively reduced the viral load of SARS-CoV-2 Omicron BA.5.2 in cell culture supernatants on Day 35, and such reduction was dose-dependent.
4 DISCUSSION
The COVID-19 pandemic caused by SARS-CoV-2 and its variants continues to pose a threat to human health globally. Although vaccination brought the pandemic under control to some extent, the large number of breakthrough infection cases may require annual COVID-19 vaccination. IAV is a common respiratory infectious virus that causes severe respiratory illnesses worldwide. WHO updates vaccines to prevent seasonal influenza epidemics every year. It was reported that the coinfection rate of SARS-CoV-2 and IAV during the COVID-19 pandemic was as high as 49.8%.21 To date, no clinically effective prophylactics are available for the prevention of both SARS-CoV-2 and IAV infections, highlighting a need for the development of combined vaccines. The production of a combined influenza/SARS-CoV-2 Omicron vaccine capable of protecting from both IAV- and SARS-CoV-2-induced disease may reduce the cost, inconvenience, and administration burden of multiple independent vaccines. In our study, the immunogenicity and protective effect of the influenza/SARS-CoV-2 Omicron subunit combined vaccine adjuvanted with MF59 were evaluated in BALB/c mice. Data showed that the combined vaccine induced high levels of IgG, IgG1, and IgG2a antibodies, as well as influenza A H1N1/California/2009 virus-specific hemagglutination-inhibiting antibodies in BALB/c mice. Furthermore, the subunit combined vaccine induced high titers of neutralizing antibodies against SARS-CoV-2 Omicron BA.5 pseudovirus infection and effectively reduced the viral load of authentic SARS-CoV-2 Omicron BA.5.2 variant in the cell culture supernatants.
In general, a subunit vaccine consisting of B cell epitopes-containing viral surface proteins, like influenza virus HA and SARS-CoV S protein, is expected to induce mainly B cell immunity, but limited T cell immunity. Given that the subunit vaccines consisting of T cell epitopes-containing viral nucleocapsid protein, like influenza virus NP and SARS-CoV N protein, is more effective to induce T cell immunity that can provides broader spectrum and longer protection than B cell immunity to combat the influenza virus and SARS-CoV Omicron subvariants with many mutations in their surface proteins, we will add some T cell epitopes in influenza virus NP and SARS-CoV N protein to the new vaccine designed, to develop a next generation subunit-based Influenza/SARS-CoV-2 Omicron combined vaccine in the near future.
In conclusion, our study educed evidence showing the influenza/SARS-CoV-2 Omicron subunit combined vaccine to be a potential interventional candidate against COVID-19 and pandemic influenza coinfection, thus deserving of further translational development.
AUTHOR CONTRIBUTIONS
Naru Zhang, Haijun Han, Shibo Jiang, and Jie Zhou conceived the idea and designed the experiments. Zihui Ye, Cun Li, Jie Zhou, Wei Xue, Luying Xiang, Yuewen Chen, Rouhan Ye, and Jingyin Dong performed the experiments. Naru Zhang and Zihui Ye analyzed the data. Naru Zhang wrote the draft and Jie Zhou, Shibo Jiang, and Haijun Han revised the manuscript. All authors have read and agreed to the published version of the manuscript.
ACKNOWLEDGMENTS
This work was supported by Hangzhou Science and Technology Bureau as well as the Scientific Research Foundation (grant No. J-202106) and Innovation Training Program (grant No. X202301126) of Hangzhou City University to H. H. and N. Z.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
Six-week-old specific pathogen-free (SPF) female BALB/c mice were purchased from Hangzhou Paisiao Biotechnology Co., Ltd., and all mice were in good health. Animal studies were conducted in strict accordance with the recommendations of the Institutional Animal Care and Use Committee (IACUC), and the animal protocols were approved by the Animal Experimentation Ethics Committee of the School of Medicine, Hangzhou City University.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.




