Non-liver-related mortality in the DAA era: Insights from post-SVR patients with and without previous HCC history
Abstract
Background and Aims
Mortality after sustained virological response (SVR) with interferon-free direct-acting antiviral (IFN-free DAA) therapy is crucial for optimizing post-SVR patient care, but it remains unclear, especially regarding non-liver-related mortality.
Methods
Consecutive post-SVR patients from 14 institutions were stratified into three cohorts: A (without advanced fibrosis and without prior HCC), B (with advanced fibrosis and without prior HCC), and C (curative HCC treatment). We assessed mortality (per 1000 person-years [/1000PY]) post-SVR. Mortality rates were compared between cohorts A and B and the general population using age- and sex-adjusted standardized mortality ratio (SMR). Comparison of survival between each cohort was performed using propensity-score (PS) matching with sex, age, and comorbidity.
Results
In cohort A (n = 762; median age, 65 years), 22 patients died (median follow-up, 36 months); all-cause mortality was 10.0/1000PY, with 86.4% non-liver-related deaths. In cohort B (n = 519; median age, 73 years), 27 patients died (median follow-up, 39 months); all-cause mortality was 16.7/1000PY, with 88.9% non-liver-related deaths. In both cohorts, malignant neoplasm was the most common cause of death; all-cause mortality was comparable to that of the general population (SMR: 0.96 and 0.92). In cohort C (n = 108; median age, 75 years), 15 patients died (median follow-up, 51 months); all-cause mortality was 36.0/1000PY, with 53.3% liver-related deaths. PS matching showed no significant survival differences between cohorts A and B, both of which had better survival than cohort C.
Conclusions
Mortality varies based on HCC history in the DAA era; nevertheless, attention should be paid to non-liver-related deaths in all post-SVR patients.
Abbreviations
-
- CH
-
- chronic hepatitis
-
- EHC
-
- extrahepatic cancer
-
- EHM
-
- extrahepatic manifestation
-
- HCC
-
- hepatocellular carcinoma
-
- IFN-free DAA
-
- interferon-free direct-acting antiviral
-
- LC
-
- liver cirrhosis
-
- SMR
-
- standardized mortality ratio
-
- SVR
-
- sustained virological response
1 INTRODUCTION
According to the World Health Organization, the number of patients infected with hepatitis C virus (HCV) has risen to 58 million worldwide, with 1.5 million newly infected patients every year.1 HCV infection triggers a persistent state of liver inflammation, which can result in the development of chronic hepatitis (CH), liver cirrhosis (LC), or even hepatocellular carcinoma (HCC) over 20–30 years.2, 3 Recently, anti-HCV therapy has changed considerably with the introduction of direct-acting antivirals (DAAs). The development of interferon-free DAA (IFN-free DAA) therapy has enabled highly effective HCV elimination, with infected individuals achieving a sustained virological response (SVR) in >95% of patients. In addition, the patient population eligible for IFN-free DAA therapy has significantly shifted from patients who were eligible for conventional IFN-based therapy and has expanded to include cases such as elderly patients, those with LC, and patients with renal dysfunction where IFN-based treatment was challenging.4-6 It is highly plausible that the mortality rate and causes of death of patients who achieved SVR (post-SVR patients) in the IFN-free DAA era differ from those reported in the IFN era.
SVR cases have shown improved all-cause and liver-related mortality compared with non-SVR (or nontreatment) cases.7, 8 However, there are limited studies analyzing mortality and cause of death, including non-liver-related death after SVR with IFN-free DAA therapy. HCV induces an unfavorable environment not only in the liver tissue but also systemically, resulting in the development of extrahepatic manifestations (EHMs), including cardiovascular diseases, type 2 diabetes, chronic nephritis,9, 10 and extrahepatic cancers (EHCs), such as colorectal cancer and lymphoma.11-14 The frequency of these EHMs is >70% in patients with chronic HCV infection at the initial visit15 and can affect non-liver-related mortality.16, 17 Considering that the indications for IFN-free DAA therapy have shifted to older patients, the importance of non-liver-related mortality cannot be ignored in mortality analyses following SVR, and further research is needed for clarity.
The final verdict derived from the accumulation of many meta-analyses indicated that IFN-free DAAs improve the survival prognosis of patients with curative HCC treatment by achieving SVR.18, 19 Thus, the induction of DAA therapy for patients post-curative HCC treatment was accelerated in the real world, since IFN-free DAA therapy can be completed in a short duration (8–12 weeks) and generates a high response rate even in LC patients.20 However, only few reports exist on the mortality of post-SVR patients treated with IFN-free DAA stratified by HCC treatment history.
In this study, we aimed to elucidate the mortality of post-SVR patients in the DAA era, stratifying patients by the presence or absence of fibrosis progression or HCC treatment history and investigating whether the mortality rate of post-SVR patients without prior HCC treatment history was equivalent to that of the general population.
2 PATIENTS AND METHODS
2.1 Data collection and patient follow-up course
This study was a multi-institutional joint historical cohort study, which was approved by the Institutional Review Board of Nagasaki University Hospital (approval no. 22121902) and conducted in accordance with the 1975 Declaration of Helsinki. The study protocol was published and guaranteed opt-out opportunities were advertised on the website of our hospital, in accordance with IRB instructions.
This study was conducted using a database of patients treated with IFN-free DAA therapy, from 14 Nagasaki-based main liver disease institutions with resident hepatologists. In the database, the clinical background, blood test data (at the initiation of IFN-free DAA therapy, at the end of therapy, and SVR), the incidence of death, cause of death, and whether HCC occurred after IFN-free DAA therapy initiation was registered. SVR was determined based on HCV RNA negativity at 12 weeks after the end of therapy. The selection of IFN-free DAA regimens was at the discretion of the physician based on the drug label and the Japanese Society of Hepatology guidelines at treatment initiation.21 All patients were evaluated for HCC using imaging tests such as abdominal ultrasonography, contrast-enhanced computed tomography (enhanced CT), and contrast-enhanced gadolinium ethoxybenzyl-diethylenetriaminepentaacetic acid-enhanced magnetic resonance imaging (EOB-MRI) before the initiation of IFN-free DAA therapy. After achieving SVR, follow-ups were performed every 3–6 months, and imaging tests were performed at least every 6 months to evaluate the occurrence of HCC. The presence of advanced fibrosis/LC was diagnosed by pathological evaluation (METAVIR F3 or F4) in patients who underwent liver biopsy and by a fibrosis-4 (Fib-4) score of >3.25,22 liver stiffness measurement >12.5,23 assessment for the symptoms of liver failure (ascites, varices, and encephalopathy), and morphological evaluation (blunted nodular edge and splenomegaly) using imaging tests in patients who did not undergo liver biopsy. The Child–Pugh grade was determined based on data at the initiation of IFN-free DAA therapy.
2.2 Study participants and cohort classification
The inclusion criteria were as follows: (1) patients aged 18 years or older who received IFN-free DAA therapy were enrolled between September 2014 and December 2020; (2) patients who achieved SVR with IFN-free DAA therapy. Patients who met the following criteria were excluded from the study: (1) HCV reinfection after liver transplantation; (2) loss to follow-up (LTFU) before SVR determination; (3) non-SVR cases, (4) cases of co-infection with hepatitis B virus or human immunodeficiency virus, (5) LTFU cases with no hospital visits after SVR determination; and (6) cases of non-curative HCC at the initiation of IFN-free DAA therapy.
Curative HCC was defined as a case in which all the following criteria were met: (1) last session of HCC treatment before IFN-free DAA therapy was surgery, radiofrequency ablation, or curative stereotactic body radiation therapy (SBRT), (2) enhanced CT or EOB-MRI at the initiation of IFN-free DAA therapy showed no HCC suspectable lesions, such as indicated by arterial enhancement or delayed washout, and (3) no HCC occurred until 180 days after initiation of IFN-free DAA therapy.
Patients were stratified into three groups according to the presence or absence of fibrosis progression or prior HCC treatment history, as follows: cohort A, patients without advanced fibrosis/LC and without HCC before IFN-free DAA therapy; cohort B, patients with advanced fibrosis/LC and without HCC before IFN-free DAA therapy; and cohort C, patients who underwent curative HCC treatment before IFN-free DAA therapy.
2.3 Data end and study end point
For the patients whose follow-up had continued after SVR, the “data end” date was defined as December 31, 2021. For patients LTFU by December 31, 2021, after SVR, the “data end” date was defined as the date of the last visit to the hospital. However, for patients who were transferred to other hospitals during the follow-up period after SVR, we investigated the occurrence and cause of death and defined “data end” date as the last confirmed date by December 31, 2021.
The primary endpoint of this study was all-cause, liver-related, and non-liver-related mortality post-SVR. The factors contributing to all-cause mortality in cohorts A, B, and C at the initiation of IFN-free DAA therapy were analyzed. Furthermore, the mortality rate of cohorts A and B adjusted for sex and age was compared with that of the general population.
2.4 Statistical analysis
Categorical data are presented as numbers (percentages), and continuous data are presented as medians (1st–3rd interquartile range [IQR]). The chi-square test with Yates' continuity correction was used to analyze categorical data. To compare the differences between the two groups, the Mann–Whitney U test was used for continuous data. To compare differences among the three groups, the Kruskal–Wallis test (KW test) was used for continuous data, and the Dunn–Bonferroni test was used to test for multiple comparisons. Survival time and time to HCC occurrence after SVR were calculated as the period from the date of SVR to that of the event or data end. Multivariate Cox proportional hazards models were used to analyze factors contributing to all-cause mortality in each cohort. For comparisons between each cohort, propensity scores (PS) were calculated using multiple logistic regression to match clinical backgrounds; 1:1 matching was performed with 0.1-caliper width. Variables for PS matching included sex, age, and presence or absence of comorbidity (hypertension, dyslipidemia, and diabetes mellitus) for matching between each cohort. Mortality rates for cohorts A and B, adjusted for sex and age, were compared with those of the general Japanese population using the standardized mortality ratio (SMR). The SMR was calculated as the ratio of the total number of deaths in cohorts A and B to the total expected number of deaths for all sexes and ages. Based on population sex- and age-specific mortality rates, vital statistics were obtained from the 2020 Population Survey Report published by the Ministry of Health, Labour, and Welfare.24
3 RESULTS
SVR was determined in 1540 of 1685 cases, excluding 31 post-liver transplant cases and 114 LTFU cases (Figure 1). Among them, SVR was achieved in 1504 (97.7%) cases. The 36 cases of non-SVR included 5 non-responders, 13 breakthroughs, and 18 relapse cases. The highest percentage of non-SVR cases was observed in patients treated with daclatasvir plus asunaprevir (Supporting Information: Figure 1). After excluding 9 patients coinfected with hepatitis B or human immunodeficiency virus, 41 patients who did not return to the hospital after SVR, and 65 patients with non-curative HCC at the initiation of IFN-free DAA therapy, 1389 cases were included in the analysis. Overall, 762 patients were stratified into cohort A (post-SVR patients without advanced fibrosis/LC and without prior HCC), 519 patients into cohort B (post-SVR patients with advanced fibrosis/LC and without prior HCC), and 108 patients into cohort C (post-SVR patients with prior curative HCC treatment).
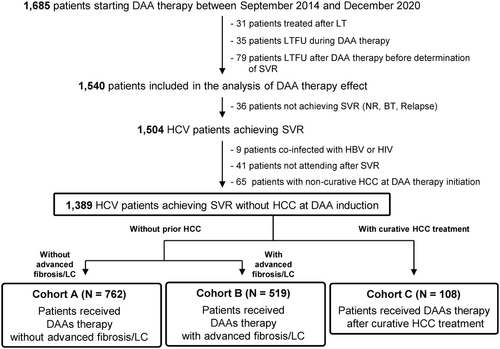
Patient characteristics at initiation of IFN-free DAA therapy are presented in Table 1. Cohort C had a higher proportion of male patients than cohorts A and B (proportion of male patients: A, 43.7%; B, 41.0%; and C, 52.8%; p = 0.079). Patients in cohort A were younger in age than those in cohorts B and C (median age: A, 65 years; B, 73 years; and C, 75 years; p < 0.001). Regarding comorbidities (hypertension, diabetes mellitus, and dyslipidemia), patients in cohort A had a significantly lower prevalence of hypertension than those in cohorts B and C (p = 0.005). In cohort C, advanced fibrosis or LC was observed in 79.6% of patients; the final HCC treatment before IFN-free DAA therapy initiation was radiofrequency ablation in 66 patients, surgical resection in 30 patients, and curative SBRT in 12 patients. There were significant differences in the de novo HCC rates after SVR between cohorts A and B (cohort A: 3 years, 1.5% [95% confidence interval, CI: 0.5–2.6] and 5 years, 4.7% [2.2–7.2]; cohort B: 3 years, 9.1% [95% CI: 6.2–12.0] and 5 years, 14.3% [10.0–18.5]; p < 0.001) (Supporting Information: Figure 2A). HCC recurrence rates in cohort C were as follows: 1 year, 19.0% (95% CI: 11.5–26.5); 3 years, 40.6% (30.9–50.3); and 5 years, 53.2% (42.5–63.9) (Supporting Information: Figure 2B).
| Numbers (%) or Median (1st–3rd IQR) | ||||
|---|---|---|---|---|
| Variable | Cohort A (n = 762) | Cohort B (n = 519) | Cohort C (n = 108) | p Value |
| Sex (male/female) | 333 (43.7)/429 (56.3) | 213 (41.0)/306 (59.0) | 57 (52.8)/51 (47.2) | 0.079 |
| Age, years | 65 (56–73) | 73 (66–79) | 75 (68–80) | <0.001 |
| BMI, kg/m2 | 22.5 (20.2–25.0) | 22.2 (20.0–24.2) | 22.0 (20.5–24.4) | 0.195 |
| Hypertension | 350 (45.9) | 279 (53.8) | 63 (58.3) | 0.005 |
| Diabetes mellitus | 96 (12.6) | 89 (17.2) | 19 (17.6) | 0.053 |
| Dyslipidemia | 99 (13.0) | 47 (9.1) | 11 (10.2) | 0.093 |
| Metabolome factora | 0.156 | |||
| 0 | 343 (45.0) | 198 (38.2) | 37 (34.3) | |
| 1 | 310 (40.7) | 239 (46.1) | 53 (49.1) | |
| 2 | 92 (12.1) | 70 (13.5) | 14 (13.0) | |
| 3 | 17 (2.2) | 12 (2.3) | 4 (3.7) | |
| Advanced fibrosis or LC | 0 (0) | 519 (100) | 86 (79.6) | <0.001 |
| CP grade, A/B or C | 762 (100)/0 (0) | 497 (95.8)/22 (4.2) | 98 (90.7)/10 (9.26) | <0.001 |
| Fib-4 index | 2.00 (1.44–2.56) | 4.97 (3.83–6.62) | 5.65 (3.21–8.78) | <0.001 |
| ALBI score | −2.86 (−3.04 to 2.65) | −2.59 (−2.82 to 2.28) | −2.40 (−3.04 to 2.65) | <0.001 |
| HCV RNA, log IU/mL | 6.2 (5.6–6.7) | 6.1 (5.5–6.5) | 5.9 (5.3–6.3) | <0.001 |
| Serotype GT1 | 545 (73.6) | 390 (77.8) | 85 (86.7) | 0.154 |
| Previous IFN (+) | 183 (24.0) | 124 (23.9) | 29 (26.9) | 0.797 |
| Plt, ×104/mm3 | 18.2 (15.6–22.4) | 11.2 (8.6–13.9) | 10.9 (7.9–15.1) | <0.001 |
| INR | 1.00 (0.97–1.05) | 1.07 (1.02–1.12) | 1.08 (1.04–1.15) | <0.001 |
| T.bil, mg/dL | 0.7 (0.5–0.9) | 0.8 (0.6–1.0) | 0.8 (0.6–1.0) | <0.001 |
| ALT, IU/mL | 30 (21–49) | 44 (28–74) | 43 (28–65) | <0.001 |
| γGTP, IU/mL | 26 (18–46) | 34 (22–58) | 35 (22–50) | <0.001 |
| Alb, g/dL | 4.2 (4.0–4.4) | 3.9 (3.6–4.2) | 3.7 (3.4–4.0) | <0.001 |
| eGFR, mL/min/1.73 m2 | 72.8 (61.1–83.5) | 66.7 (55.0–80.1) | 65.9 (57.5–78.0) | <0.001 |
| LDL-C, mg/dL | 106 (86–126) | 84 (70–102) | 87 (71–101) | <0.001 |
| HDL-C, mg/dL | 57 (45–68) | 51 (42–63) | 50 (40–60) | 0.016 |
| TG, mg/dL | 95 (72–126) | 93 (70–121) | 94 (71–134) | 0.540 |
| HbA1c, % | 5.7 (5.4–6.0) | 5.6 (5.3–6.0) | 5.7 (5.2–6.0) | 0.242 |
| AFP, ng/mL | 3.7 (2.5–5.7) | 6.2 (3.5–12.4) | 7.1 (3.8–14.6) | <0.001 |
| DCP, mAU/mL | 20 (17–26) | 19 (15–25) | 21 (16–26) | 0.062 |
- Note: Categorical data are presented as numbers (ratio) of patients and numerical data as median (1st–3rd IQR).
- Abbreviations: AFP, α-fetoprotein; ALT, alanine aminotransferase; DCP, des-γ-carboxyprothrombin; IFN, interferon; IQR, interquartile range; HCV, hepatitis C virus; LC, liver cirrhosis.
- a “Metabolome factor” represents the sum of comorbidities due to hypertension, diabetes mellitus, and dyslipidemia.
3.1 Mortality and cause of death in each cohort
3.1.1 Cohort A
During a median follow-up of 36 months (IQR: 13–55 months), death occurred in 22 (1.7%) of 762 patients in cohort A. Liver-related and non-liver-related deaths accounted for 13.6% (n = 3) and 86.4% (n = 19) of all deaths, respectively (Table 2). Among the non-liver-related causes of death, malignant neoplasms were predominant (8 of 19 cases, 42.1%).
| Cohort A | Cohort B | Cohort C | |||
|---|---|---|---|---|---|
| Cause of death | N (%) | Cause of death | N (%) | Cause of death | N (%) |
| Liver related | |||||
| HCC | 2 (9.1) | HCC | 1 (3.7) | HCC | 7 (46.7) |
| Liver abscess | 1 (4.5) | Liver failure | 1 (6.7) | ||
| Non-liver related | |||||
| Malignant neoplasma | 8 (36.3) | Malignant neoplasmb | 12 (44.4) | Heart disease | 2 (13.3) |
| Heart disease | 4 (18.2) | Pneumonia | 3 (11.1) | Pneumonia | 2 (13.3) |
| Pneumonia | 1 (4.5) | Heart disease | 1 (3.7) | Sepsis (Cholangitis) | 1 (6.7) |
| CVD | 1 (4.5) | CVD | 1 (3.7) | ||
| Unknown | 5 (22.7) | Die of old age | 1 (3.7) | ||
| Renal failure | 1 (3.7) | ||||
| Others | 2 (7.4) | ||||
| Unknown | 3 (11.1) | ||||
| Unknown | |||||
| 0 (0) | 2 (7.4) | 2 (13.3) | |||
- Abbreviations: CVD, cerebrovascular disease; HCC, hepatocellular carcinoma.
- a Types of malignant neoplasms in the eight cases were lung cancer in four patients, colon cancer in two, endometrial cancer in one, and cervical cancer in one.
- b Types of malignant neoplasms in the 12 cases were lung cancer in two patients, gallbladder cancer in two, and others (colon cancer, cholangiocarcinoma, breast cancer, pancreatic cancer, diffuse large B-cell lymphoma, acute myeloid leukemia, neuroendocrine cancer, and endometrial cancer) in one patient each.
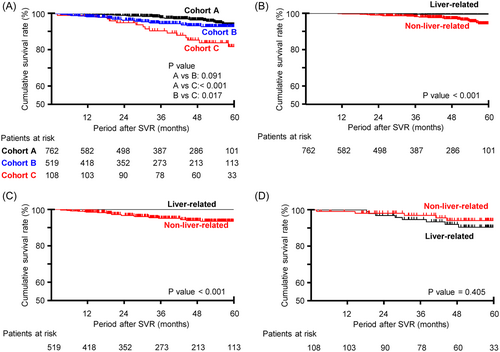
The 5-year cumulative survival rate for all-cause death was 93.7% (95% CI: 90.6–96.6), with an all-cause mortality rate of 10.0/1000 person-years (/1000PY) (Figure 2A, Table 3). Non-liver-related mortality was significantly higher than liver-related mortality (p < 0.001) (Figure 2B).
| Mortality rate (per 1000 person-years) | |||
|---|---|---|---|
| Cohort A (N = 762) | Cohort B (N = 519) | Cohort C (N = 108) | |
| All-cause | 10.00 | 16.71 | 35.98 |
| Liver related | 1.36 | 0.62 | 19.19 |
| Non-liver related | 8.64 | 14.9 | 11.99 |
| Unknown | 0 | 1.24 | 4.80 |
3.1.2 Cohort B
During a median follow-up of 39 months (IQR: 17–58 months), death occurred in 27 (5.2%) of 519 patients in cohort B. Liver-related and non-liver-related deaths accounted for 3.7% (n = 1) and 88.9% (n = 24) of all deaths, respectively (Table 2). Among the non-liver-related causes of death, malignant neoplasms were predominant (12 of 24 cases, 50.0%).
The 5-year cumulative survival rate for all-cause death was 92.9% (95% CI: 90.2–95.7), with an all-cause mortality rate of 16.7/1000 person-years (/1000PY) (Figure 2A, Table 3). Non-liver-related mortality was significantly higher than liver-related mortality (p < 0.001) (Figure 2C).
3.1.3 Cohort C
During a median follow-up of 51 months (IQR: 32–63, maximum 75 months), death occurred in 15 (13.9%) of 108 patients in cohort C. Liver-related and non-liver-related deaths accounted for 53.3% (n = 8), 33.3% (n = 5), and 13.3% (n = 2) of all deaths, respectively (Table 2). Among the liver-related causes of death, HCC was the most common (seven out of eight cases, 87.5%).
The 5-year cumulative survival rate for all-cause death was 81.9% (95% CI: 73.3–90.5), with an all-cause mortality rate of 36.0/1000PY (Figure 2A, Table 3). The 5-year cumulative survival rate for liver-related death was 90.4% (95% CI: 84.0–96.8), with a mortality rate of 19.2/1000PY, whereas the 5-year cumulative survival rate for non-liver-related death was 94.1% (95% CI: 89.0–99.2), with a mortality rate of 12.0/1000PY. There was no significant difference in liver-related and non-liver-related mortality (p = 0.405) (Figure 2D).
3.2 Contributing factors at DAA induction to poor long-term survival in each cohort
In cohort A, the univariate analysis showed that the factors at the initiation of IFN-free DAA therapy contributing to poor long-term survival after SVR were older age, higher Fib-4 index, higher ALBI scores, lower albumin levels, and lower estimated glomerular filtration rate (eGFR). In the multivariate analysis, older age (hazard ratio [HR]: 1.098; 95% CI: 1.026–1.176; p = 0.007) and lower eGFR (HR: 0.955; 95% CI: 0.927–0.984, p = 0.003) were significant factors contributing to poor long-term survival after SVR (Table 4 and Supporting Information: Table I).
| Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|
| Variable | Category | HR (95% CI) | p Value | HR (95% CI) | p Value |
| Cohort A | |||||
| Age, years | Continuous | 1.111 (1.056–1.168) | <0.001 | 1.098 (1.026–1.176) | 0.007 |
| Fib-4 index | Continuous | 2.930 (1.239–6.934) | 0.014 | 1.929 (0.693–5.366) | 0.208 |
| ALBI score | Continuous | 1.533 (1.033–2.275) | 0.034 | 8.324 (0.075–922.6) | 0.378 |
| Albumin, g/dL | Continuous | 0.179 (0.062–0.517) | 0.001 | 1.164 (0.023–58.12) | 0.940 |
| eGFR, mL/min/1.73 m2 | Continuous | 0.955 (0.935–0.975) | <0.001 | 0.955 (0.927–0.984) | 0.003 |
| Cohort B | |||||
| Age, years | Continuous | 1.079 (1.023–1.139) | 0.005 | 1.079 (1.022–1.138) | 0.006 |
| Dyslipidemia | (+) | 2.675 (1.010–7.086) | 0.048 | 2.551 (0.961–6.769) | 0.060 |
| Cohort C | |||||
| Number of prior HCC treatment sessions | 2 ≤ (ref 1) | 3.738 (1.274–10.97) | 0.016 | 7.106 (1.846–27.36) | 0.004 |
| Albumin, g/dL | Continuous | 0.408 (0.174–0.957) | 0.039 | 0.700 (0.157–3.111) | 0.639 |
| LDL-C, mg/dL | Continuous | 0.971 (0.945–0.997) | 0.028 | 0.981 (0.950–1.012) | 0.226 |
- Abbreviations: CI, confidence interval; eGFR, estimated glomerular filtration rate; HCC, hepatocellular carcinoma; HR, hazard ratio; LDL-C, low-density lipoprotein cholesterol.
In cohort B, the univariate analysis showed that the factors at the initiation of IFN-free DAA therapy contributing to poor long-term survival after SVR were older age and the presence of dyslipidemia. In the multivariate analysis, older age (HR: 1.079; 95% CI: 1.022–1.138, p = 0.006) was the only significant factor contributing to poor long-term survival after SVR (Table 4 and Supporting Information: Table II).
In cohort C, the univariate analysis revealed that the factors present at initiation of IFN-free DAA therapy that contributed to poor long-term survival after SVR were multiple (more than twice) HCC treatment sessions, lower albumin levels, and lower low-density lipoprotein cholesterol (LDL-C) levels. In the multivariate analysis, only multiple HCC treatment sessions were a significant factor contributing to poor long-term survival after SVR (HR: 7.106; 95% CI: 1.846–27.36, p = 0.004) (Table 4 and Supporting Information: Table III).
3.3 Comparison of all-cause mortality between each cohort after PS matching
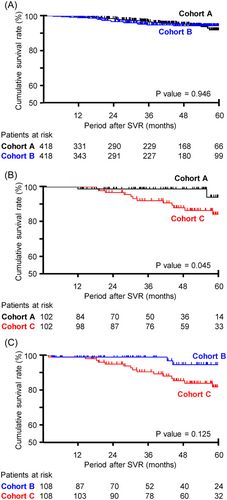
The survival rate of each cohort was compared after PS matching using variables including sex, age, and presence or absence of comorbidity. Characteristics of study participants of each cohort after PS matching are shown in Table 5. As shown in Figure 3, there were no significant differences in the survival rate between cohorts A and B (p = 0.946). Cohort A had significantly lower mortality than cohort C (p = 0.045), while cohort B tended to have low mortality compared to cohort C (p = 0.125).
| Variable | Numbers (%) or median (1st–3rd IQR) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Cohort A versus B | Cohort A versus C | Cohort B versus C | |||||||
| Cohort A (n = 418) | Cohort B (n = 418) | p Value | Cohort A (n = 102) | Cohort C (n = 102) | p Value | Cohort B (n = 108) | Cohort C (n = 108) | p Value | |
| Sex (female) | 248 (59.3) | 247 (59.1) | 1.000 | 51 (50.0) | 50 (49.0) | 1.000 | 47 (43.5) | 51 (47.2) | 0.682 |
| Age, years | 71 (64–76) | 71 (64–76) | 0.928 | 74 (68–80) | 75 (68–80) | 0.951 | 75 (67–81) | 75 (68–80) | 0.980 |
| BMI, kg/m2 | 21.9 (19.9–24.3) | 22.4 (20.2–24.2) | 0.295 | 22.2 (20.3–24.5) | 21.9 (20.5–24.4) | 0.820 | 22.4 (20.3–24.6) | 22.0 (20.5–24.4) | 0.731 |
| Hypertension | 211 (50.5) | 214 (51.2) | 0.890 | 60 (58.8) | 60 (58.8) | 0.887 | 56 (51.9) | 63 (58.3) | 0.411 |
| Diabetes mellitus | 61 (14.6) | 65 (15.6) | 0.783 | 16 (15.7) | 18 (17.7) | 0.851 | 11 (10.2) | 19 (17.6) | 0.168 |
| Dyslipidemia | 39 (9.3) | 42 (10.1) | 0.815 | 9 (8.8) | 10 (9.8) | 1.000 | 11 (10.2) | 11 (10.2) | 0.822 |
| Metabolome factora | 0.952 | 0.064 | 0.144 | ||||||
| 0 | 176 (42.1) | 171 (40.9) | 40 (39.2) | 35 (34.3) | 42 (38.9) | 37 (34.3) | |||
| 1 | 180 (43.1) | 182 (43.5) | 44 (43.1) | 50 (49.0) | 54 (50.0) | 53 (49.1) | |||
| 2 | 55 (13.2) | 56 (13.4) | 13 (12.8) | 13 (12.8) | 12 (11.1) | 14 (13.0) | |||
| 3 | 7 (1.7) | 9 (2.2) | 5 (4.9) | 4 (3.9) | 0 (0) | 4 (3.7) | |||
| Advanced fibrosis or LC | 0 (0) | 418 (100) | <0.001 | 0 (0) | 81 (79.4) | <0.001 | 108 (100) | 86 (79.6) | <0.001 |
| CP grade (B or C) | 0 (0) | 16 (3.83) | <0.001 | 0 (0) | 9 (8.8) | 0.006 | 4 (3.7) | 10 (9.3) | 0.167 |
| Fib–4 index | 2.16 (1.72–2.68) | 4.98 (3.80–6.69) | <0.001 | 2.29 (1.72–2.74) | 5.80 (3.20–8.79) | <0.001 | 4.84 (3.77–6.44) | 5.65 (3.21–8.78) | 0.480 |
| ALBI score | −2.82 (−2.99 to −2.59) | −2.59 (−2.83 to −2.27) | <0.001 | −2.78 (−2.99– to −2.59) | −2.39 (−2.68 to −2.02) | <0.001 | −2.61 (−2.83 to −2.27) | −2.40 (−2.70 to −2.03) | 0.004 |
- Note: Categorical data are presented as numbers (ratio) of patients and numerical data as median (1st–3rd IQR).
- Abbreviations: IQR, interquartile range; LC, liver cirrhosis.
- a “Metabolome factor” represents the sum of comorbidities due to hypertension, diabetes mellitus, and dyslipidemia.
3.4 Comparison of all-cause mortality in cohorts A and B and the general population
The all-cause mortality in cohorts A and B was compared with that of the general population after adjusting for sex and age. The SMR (number of deaths/expect number of deaths) was 0.96 for cohort A and 0.92 for cohort B (Supporting Information: Table IV), indicating that all-cause mortality was not higher in post-SVR patients without prior HCC treatment history than in the general population.
4 DISCUSSION
Herein, we present the results of a historical cohort study evaluating mortality after SVR with IFN-free DAA therapy in the real world, stratified by the progression of fibrosis and prior HCC treatment history. Post-SVR patients with non-curative HCC treatment were excluded to the greatest extent by verifying the details of the last HCC treatment before IFN-free DAA therapy and by retrospectively excluding HCC cases occurring up to 180 days after the initiation of IFN-free DAA therapy. In addition, the comorbidities, which affect all-cause mortality,25 were examined and included in the study. We found that there were differences in mortality and causes of death between post-SVR patients with and without prior HCC treatment history in the DAA era. However, the progression of fibrosis did not have an impact on mortality in post-SVR patients without HCC treatment history. Most importantly, non-liver-related mortality was revealed to be significant and could not be ignored in post-SVR patients, irrespective of the progression of fibrosis or prior HCC treatment history.
In post-SVR patients without prior HCC, non-liver-related death was the main cause of death; its incidence was 6.4-fold higher in cohort A and 24-fold higher in cohort B than that of liver-related death. Among the non-liver-related causes of death, malignant neoplasm was predominant in both cohorts A and B. Contrastingly, in post-SVR patients with prior curative HCC treatment (cohort C), liver-related death was the main cause of death and HCC was the most common cause of death. However, non-liver-related mortality was unexpectedly high, accounting for approximately 40% of post-SVR patients. On comparing each cohort using PS matching based on age, gender, and comorbidities, cohort C showed significantly poorer prognosis than cohorts A and B, while no significant difference in prognosis was observed between cohorts A and B. Finally, comparison of all-cause mortality of post-SVR patients without prior HCC treatment (cohorts A and B) with that of the general population showed that all-cause mortality was slightly lower in post-SVR patients.
Age was the common factor affecting all-cause mortality in cohorts A and B. The cause of death was primarily non-liver-related, with the most common cause being malignant neoplasms, as in the general population. Additionally, the SMR was not inferior to that of the general population. These results suggest that post-SVR patients treated with IFN-free DAAs without prior HCC in the real world have the same mortality rates and causes of death as those of the general population. HCC accounted for 3 (6.1%) of 49 deaths, suggesting that careful follow-ups are necessary to prevent death caused by HCC after SVR, as recommended in the guidelines.26, 27 However, it should be noted that death from EHC, cardiovascular diseases, or pneumonia is more common than death from HCC.
Malignant neoplasm excluding HCC was the predominant cause of death, accounting for 36.3% of deaths in cohort A and 44.4% of deaths in cohort B. IFN-free DAA therapy, unlike IFN-based therapy, was introduced in older patients, which might increase the mortality associated with malignant neoplasm. Obi et al. analyzed the frequency of all organ malignancies in 651 post-SVR patients (26.1% LC cases and 6% cases treated for curative HCC)28 and showed that the incidence of EHC in post-SVR patients was almost the same as that in patients with HCC, indicating that careful screening for EHC is necessary to extend life expectancy in post-SVR patients. Additionally, approximately half of the cases of lung and colorectal cancer were diagnosed at stages 3 and 4 and had high mortality rates, which was consistent with our findings, wherein they were the predominant causes of death in malignant neoplasms. Similar to that recommended for the general population, health checkups and screening tests, particularly focusing on the occurrence of lung and colorectal cancers, are necessary to extend the life expectancy of post-SVR patients.
Cohort B had higher all-cause mortality than cohort A, which is considered to reflect a difference in non-liver-related mortality rather than in liver-related mortality. However, after PS matching for age, gender, and comorbidities, there were no differences in survival in the two cohorts. These findings suggest that the patient background, such as older age and a high prevalence of comorbidities in cohort B, result in higher all-cause mortality, rather than the progression of fibrosis. This is supported by the results of the multivariate analysis using a combined cohort comprising both cohorts A and B, which revealed that the only factor contributing to poor survival rate was older age and not the progression of fibrosis (data not shown). However, it might be superficial to conclude the impact of fibrosis progression on non-liver-related mortality, because the results vary depending on the study participants.
In cohort C, liver-related mortality was higher than the non-liver-related mortality, and HCC was the predominant cause of death. Even after curative treatment, the rate of liver-related deaths due to HCC recurrence remains high in the DAA era, consistent with the predominant cause of liver-related death in post-SVR patients in the IFN era.29 Nevertheless, non-liver-related mortality was unexpectedly high, accounting for approximately 40% of post-SVR patients. The impact of HCC treatment history on non-liver-related mortality after SVR by IFN-free DAA therapy has not been fully discussed, because many large population-based studies investigating the mortality of post-SVR patients excluded patients with HCC treatment history from the analysis or included unstratified.8, 30-32 To our knowledge, there has been only one report by D'Ambrosio et al. that investigated liver- and non-liver-related mortality separately in patients with curative HCC who achieved SVR with IFN-free DAA therapy,33 which was valuable when applied to our results. They similarly analyzed the liver-related and non-liver-related mortality in 636 cirrhosis patients who achieved SVR with IFN-free DAA therapy, stratifying them according to the previous HCC treatment history. In their report, the cumulative 5-year mortality rates for liver-related and non-liver-related cases in the post-SVR patients by IFN-free DAA therapy with curative HCC treatment (67 cases) were 12.1% and 7.7%, respectively. In our study, the 5-year cumulative mortality rates of the liver-related and non-liver-related patients in cohort C were 9.6% and 5.9%, respectively. Although the mortality rate was lower than that reported in the previous study, perhaps because of the inclusion of CH cases in our study, the proportions of liver- and non-liver-related deaths showed similar trends. One possible reason for the relatively high incidence of non-liver-related deaths even in post-SVR patients receiving curative HCC treatment might be that they were older than post-SVR patients without a history of HCC. In the present study, the median age of cohort C (75 years) was higher than that of Cohort A and B (65 and 73 years). Similarly, the median age of post-SVR patients with curative treatment in the D'Ambrosio report was higher (75 years) than that of patients without a history of HCC. Conversely, in most reports analyzing patients who achieved SVR with IFN-based therapy after curative HCC treatment, the median age was 60–65 years,34, 35 which was younger than that of patients in whom IFN-free DAA therapy was introduced. In the DAA era, the incidence of non-liver-related deaths is high, even in post-SVR patients with curative HCC treatment, and should not be ignored in the follow-up.
This study has some limitations. First, the number of follow-up patients and median follow-up period after SVR in this study is still considered insufficient for assessing mortality in post-SVR patients. A study with a greater number of patients and a longer follow-up period after SVR is desirable for mortality analysis. However, the relatively small number of follow-up patients allows for detailed accumulation of data, such as causes of death, enabling a clearer understanding of the real-world mortality of post-SVR patients in the DAA era. Second, mortality and causes of death after SVR were analyzed only in the Japanese population. The age distribution and medical examination rate of health checkups and screening tests are different across countries; thus, mortality and causes of death should be analyzed in a nationwide study.
In conclusion, the mortality analysis of post-SVR patients receiving IFN-free DAA therapy showed that non-liver-related death is an important factor, which should be the focus of clinical attention in the DAA era, possibly owing to the increased number of older patients being introduced to IFN-free DAA therapy. These findings indicate that health checkups and screening tests, in addition to follow-ups for HCC occurrence, are becoming more important in the DAA era than in the IFN era.
AUTHOR CONTRIBUTIONS
All authors contributed to the study conceptualization and design. Data collection was performed by all authors, and analysis was performed by Satoshi Miuma and Kazuhiko Arima. The first draft of the manuscript was written by Satoshi Miuma. All authors commented on previous versions of the manuscript and approved the final manuscript.
ACKNOWLEDGMENTS
The authors would like to thank their colleagues at the hospital who participated in the Nagasaki Association for the Study of Liver Disease (NASLDs). This study was partially supported by a Grant-in-Aid for Research from the Japan Agency for Medical Research and Development (JP23fk0210113).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.




