Consistency in human papillomavirus type detection between self-collected vaginal specimens and physician-sampled cervical specimens
Abstract
With the rising need for accessible cervical cancer screening, self-sampling methods offer a promising alternative to traditional physician-led sampling. This study aims to evaluate the efficacy of the HygeiaTouch Self Sampling Kit for Women in detecting human papillomavirus (HPV) types and predicting cervical lesions. We studied the concordance in identifying high-risk HPV (hrHPV) types between samples collected by physicians and those self-collected by women using a self-sampling kit for validation. Women aged 21–65, fitting into specific categories based on their cervical health history were eligible. Cohen's kappa coefficient to gauge concordance between the two specimen types and relative accuracy metrics in identifying cervical intraepithelial neoplasia (CIN) were also calculated, with physician-sampled specimens serving as a reference. A total of 1210 participants from three institutes were involved. The self-sampling kit closely matched the physician-led method in terms of collecting valid specimens (100% vs. 100%), identifying hrHPV types (kappa: 0.75, 95% confidence interval [95% CI]: 0.72–0.79; agreement: 87.7%, 95% CI: 85.8–89.6) and predicting CIN grade 2 or worse (CIN2+) (relative sensitivity: 0.949, relative accuracy: 0.959). Kappa values varied between 0.71 and 0.83 for different hrHPV types and combinations, with an overall value 0.75 (95% CI: 0.72–0.79) signifying robust compatibility between the two methods. Our study underscores the potential of the HygeiaTouch Self Sampling Kit as a reliable, efficient, and user-friendly alternative to traditional sampling methods. This suggests that self-sampling could be pivotal in expanding cervical cancer screening accessibility and enhancing detection rates.
1 INTRODUCTION
Cervical cancer, which is predominantly associated with persistent high-risk human papillomavirus (hrHPV) infection, remains a significant medical challenge despite the implementation of HPV vaccination. It stands as the fourth most prevalent cancer in women worldwide. An estimated 600 000 new cases and approximately 340 000 deaths were projected for 2020.1 In 2019, the “Global Strategy towards the Elimination of Cervical Cancer as a Public Health Problem” was initiated by the World Health Organization and partnering with various United Nations agencies and other entities.2, 3 Among the three strategic goals set for 2020–2030, one emphasizes the necessity for expansive screening: aiming for 70% of women to be screened using a high-quality test by the ages of 35 and 45.
Screening for cervical cancer via hrHPV tests demonstrates enhanced sensitivity and negative predictive accuracy compared to cytological methods.4-8 As of 2021, 48 countries endorsed primary HPV-focused screening, either independently or alongside other tests such as cytology.9 Of these, 17 have integrated self-sampling into national policies or guidelines, targeting underserved groups or as a chief screening methodology. Self-collection devices include FLOQSwabs® (Copan Italia S.p.A.), Evalyn®Brush (Rovers Medical), and HerSwab™ (Eve Medical)10-16 are available currently. While ample evidence supports the comparable efficacy of vaginal self-sampling with physician-sampling in hrHPV detection, there remains an emerging requirement to appraise self-collection tools regarding safety, user comfort, and simplicity. In this context, we undertook a clinical trial to assess the performance of a novel self-collection kit for HPV typing against the outcomes from physician-sampled specimens.
2 MATERIALS AND METHODS
2.1 Study objectives
The primary objective was to assess the concordance in identifying hrHPV types between samples acquired by physicians and those self-collected. This “concordance” was determined as either (1) the detection of any hrHPV type in both physician-sampled cervical specimens and self-collected vaginal specimens or (2) the non-detection of any hrHPV type in both sets of specimens. Furthermore, the alignment for specific hrHPV types, evident as identifying a particular hrHPV type in an individual's self-collected and physician-sampled specimens, was computed.
2.2 Study population
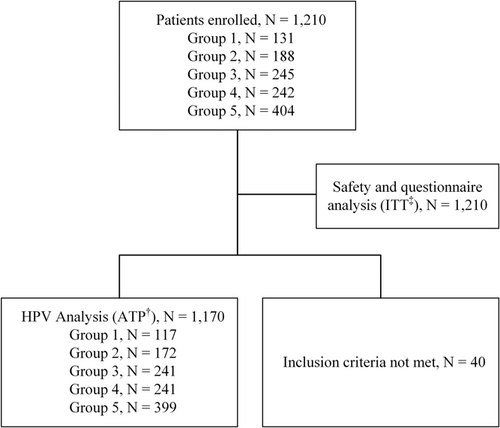
Eligible participants were women aged 21–65 with a uterine cervix and no previous or current radiotherapy for cervical tumors. Those who underwent subtotal hysterectomies were allowed. Participants could be in any of the following five groups: (1) no history or current cervical intraepithelial lesions or cervical malignancy; (2) prior history of low-grade cervical lesion or cytology within the last 3–12 months, such as atypical squamous cells of undetermined significance (ASCUS), cervical intraepithelial neoplasia grade 1 (CIN1), or atypical glandular cells (AGC); (3) had prior treatment for high-grade cervical lesions or a history of high-grade cytology, such as atypical squamous cells cannot exclude high-grade squamous intraepithelial lesion (ASC-H), high-grade squamous intraepithelial lesion (HSIL), cervical intraepithelial neoplasia grade 2 (CIN2), cervical intraepithelial neoplasia grade 3 (CIN3), cervical carcinoma in situ (CIN3), squamous cell carcinoma (SCC), atypical glandular cells favor neoplasm (AGCFN), adenocarcinoma in situ (AIS), or cervical adenocarcinoma; (4) current low-grade cervical histology or cytology, such as ASCUS, CIN1 or AGC; (5) a presence of high-grade cervical histology or cytology, including ASC-H, dysplasia cannot exclude HSIL, CIN2, CIN3, CIS, SCC, AGCFN, AIS, or cervical adenocarcinoma. In the context of defining time, the term “current” pertains to the time of enrollment in the trial, while “prior” denotes a period exceeding 90 days before enrollment.
Women who had undergone total hysterectomy, those with congenital cervical anomalies, those pregnant during the clinic visit, those diagnosed with cervicitis and treatment required, or had recent cervical surgery other than a biopsy within the past 90 days were not eligible. Women who had radiotherapy or were receiving radiation to the uterus, cervix, or vagina were excluded. Participants with unprotected sexual activity within 48 h, excessive vaginal discharge due to ovulation or inflammation, or those with vaginal medicine or menstruating could join once these conditions were no longer present.
Planned enrollment numbers for the respective categories were 120 for group 1 (expected 10% HPV positivity), 180 for group 2 (70% expected positivity), 240 for group 3, 240 for group 4, and 420 for group 5, with estimated HPV positivity rates of 50%, 70%, and 90%, respectively. The total projected enrollment was set at 1200 participants, anticipating an overall HPV positivity rate of 67%.
2.3 Sample collection and kits
The HygeiaTouch Self Sampling Kit for Women (Hygeia Touch Inc.) features a sterile vaginal applicator with a soft, highly absorbent foam affixed to a biocompatible handle. Designed for comfort, the applicator collects cells from the vaginal fornix via the foam on its distal end. Participants were directed to self-collect specimens before undergoing sampling by a physician to reduce the potential bias that could arise from having two separate samplings on the same day. After signing consent, each participant received the kit and an illustrated instruction, complemented by a QR-linked instruction video. Once collected, the specimen was placed into a sample-collection tube at room temperature and sent to the lab within 2 days.
For comparison, the LIBO Cytology Brush Kit (Iron Will Biomedical Technology) allowed investigators to collect samples from the cervix. After sample collection, the brush tip was break and put into a 2 mL phosphate buffered saline (PBS) sealed tube before transfer. Colposcopy, cervical biopsy, or other diagnostic procedure(s) were undertaken subsequently.
To ensure anonymity, tubes were marked only with pre-coded stickers. While the code on PBS tube linked to the enrolled group, the code on the tube for self-collected specimens showed randomized codes generated from SAS version 9.4 (SAS Institute Inc.). Laboratory technicians remained unaware of sample pairings.
2.4 HPV genotyping procedure
The self-collected specimen was washed from the applicator after adding 10 mL PBS buffer into the sample collection tube and vortex for 30 s. The physician sampled specimen was washed from the brush tip after vortex for 30 s. All the samples were stored at −20°C until further analysis. Whole genomic DNA was extracted using the QIAGEN QIAamp DNA Mini Kit (Qiagen). Twenty nanograms purified DNA was stored at −20°C until HPV genotyping using the DR. HPV Genotyping IVD Kit (DR. Chip Biotech, Inc.) at the Laboratory of DR. Chip Biotech, Inc. Briefly, The kit is an in vitro diagnostic device for the detection of 27 HPV types including 27 HPV types (6/11/16/18/31/33/35/39/45/51/52/53/54/56/58/59/61/62/66/68/69/70/72/73/81/82/84). The initial denaturation cycle was carried out at 95°C for 10 min, and the elongation cycle was at 72°C for 7 min. PCR was performed of 35 cycles of amplification (95°C for 30 s, 50°C for 30 s, 72°C for 50 s) After amplification, the HPV DNA amplicons were hybridized with an oligonucleotide array that was pre-spotted on DR. HPV Chip, then the colorimetric development and signal were captured and analyzed by DR. Aim Reader and DR. Aim Soft (DR. Chip Biotechnology Incorporation, Taiwan).17
2.5 Data analysis
The primary end-point was degree of concordance in detecting hrHPV (HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68) between the physician-sampled specimens and the self-collected specimens. Concordance between the two specimen types was gauged by agreement and Cohen's kappa coefficient (κ), with various ranges indicating the strength of agreement: a slight agreement was suggested when the coefficient at 0–0.20, fair agreement at 0.21–0.40, moderate at 0.41–0.60, substantial at 0.61–0.80 as, and almost perfect at 0.81–1.18 The secondary endpoints were (1) percentage of the valid sample between self-collected and physician-sampled specimens; (2) agreement of all (N = 27) HPV types between the paired samples; (3) adverse events associated with sample collection; (4) questionnaire of the appreciation and satisfaction of using self-collecting vaginal samples using “HygeiaTouch Self Sampling Kit for Women” through a short movie and a brief illustration; (5) correlation between histological diagnosis and HPV types. Secondary endpoints (3) and (4) will be analyzed in a separate report. The relative accuracy, specificity, sensitivity, positive predictive value (PPV), and negative predictive value (NPV) of HPV in identifying CIN histology in self-collected specimens were calculated using physician-sampled specimens as the reference. Histological results were determined according to the most severe diagnosis. Data analytics were performed using SAS, version 9.4 (SAS Institute Inc.).
3 RESULTS
From May 29, 2020, to November 19, 2021, we enrolled 1210 patients across three medical centers. All participants were considered for safety evaluation. However, 40 participants were not included in the HPV agreement assessment: 13 didn't meet the study's inclusion criteria, while 27 were recruited before the official approval of the trial agreement document from one of the study centers (Figure 1). The participants' median age was 45.0 years, with an age range from 21.0 to 65.9 years, and 29.8% were post-menopausal (Table 1). Among those who underwent a cervical biopsy, 76.8% (507 out of 650) were diagnosed with CIN1 or a more severe histology.
| Characteristics | Group 1a | Group 2a | Group 3a | Group 4a | Group 5a | Total |
|---|---|---|---|---|---|---|
| Age (year) | ||||||
| Mean ± SD | 46.0 ± 8.4 | 45.6 ± 9.7 | 46.5 ± 10.1 | 45.2 ± 10.8 | 44.3 ± 10.5 | 45.3 ± 10.2 |
| Median, range | 46.0 (24.0–63.0) | 45.0 (25.0–65.0) | 45.0 (26.0–65.4) | 46.0 (21.0–65.0) | 43.0 (23.0–65.9) | 45.0 (21.0–65.9) |
| Menopause, n (%) | ||||||
| No | 85 (72.6) | 126 (73.3) | 160 (66.4) | 162 (67.2) | 288 (72.2) | 821 (70.2) |
| Yes | 32 (27.4) | 46 (26.7) | 81 (33.6) | 79 (32.8) | 111 (27.8) | 349 (29.8) |
| Past histology,b n (%) | ||||||
| CIN1 | 0 | 116 (67.4) | 6 (2.5) | 38 (15.8) | 19 (4.8) | 179 (15.3) |
| CIN2 | 0 | 9 (5.2) | 73 (30.3) | 4 (1.7) | 14 (3.5) | 100 (8.5) |
| CIN3 | 0 | 9 (5.2) | 150 (62.2) | 3 (1.2) | 20 (5.0) | 182 (15.6) |
| LSIL | 0 | 2 (1.2) | 0 | 4 (1.7) | 0 | 6 (0.5) |
| HSIL | 0 | 2 (1.2) | 6 (2.5) | 2 (0.8) | 5 (1.3) | 15 (1.3) |
| Squamous cell carcinoma | 0 | 0 | 5 (2.1) | 0 | 1 (0.3) | 7 (0.6) |
| Adenocarcinoma | 0 | 0 | 1 (0.4) | 0 | 0 | 1 (0.1) |
| None | 117 (100) | 34 (19.8) | 0 | 190 (78.8) | 340 (85.2) | 680 (58.1) |
| Current histology,c n (%) | ||||||
| Condyloma | 0 | 0 | 1 (0.4) | 9 (3.7) | 0 | 10 (0.9) |
| CIN1 | 0 | 19 (11.0) | 4 (1.7) | 102 (42.3) | 35 (8.8) | 160 (13.7) |
| CIN2 | 0 | 2 (1.2) | 5 (2.1) | 17 (7.1) | 97 (24.3) | 121 (10.3) |
| CIN3 | 0 | 0 | 9 (3.7) | 7 (2.9) | 133 (33.3) | 149 (12.7) |
| Adenocarcinoma in situ | 0 | 0 | 0 | 0 | 8 (2.0) | 8 (0.7) |
| Squamous cell carcinoma | 0 | 0 | 0 | 0 | 46 (11.3) | 46 (3.9) |
| Adenosquamous cell carcinoma | 0 | 0 | 0 | 0 | 2 (0.5) | 2 (0.2) |
| Adenocarcinoma | 0 | 0 | 0 | 0 | 18 (4.5) | 18 (1.5) |
| Clear cell carcinoma | 0 | 0 | 0 | 0 | 2 (0.5) | 2 (0.2) |
| Carcinoma NOS | 0 | 0 | 0 | 0 | 1 (0.3) | 1 (0.1) |
| CIN/malignancy (−) | 0 | 10 (5.8) | 11 (4.6) | 69 (28.6) | 53 (13.3) | 143 (12.2) |
| No biopsy | 117 (100) | 141 (82.0) | 211 (87.6) | 37 (15.4) | 4 (1.0) | 510 (43.6) |
- Abbreviations: Carcinoma NOS, Carcinoma not otherwise specified; CIN, cervical intraepithelial neoplasia; HSIL, high-grade squamous intraepithelial lesion; LSIL, low-grade squamous intraepithelial lesion.
- a Group 1: no history or current cervical intraepithelial lesions or cervical malignancy. Group 2: prior history of low-grade cervical lesion or cytology within the last 3–12 months, such as ASCUS, CIN1, or AGC. Group 3: had prior treatment for CIN2/3 or a history of high-grade cytology, such as ASC-H, HSIL, CIN2, CIN3, SCC, AGC favor neoplasm, AIS, or cervical adenocarcinoma. Group 4: current low-grade cervical histology or cytology, such as ASCUS, CIN1 or AGC. Group 5: a presence of high-grade cervical histology or cytology, including ASC-H, dysplasia cannot exclude HSIL, CIN2, CIN3, CIS, SCC,
- b Histology ≥90 days before enrollment.
- c Histology at enrollment.
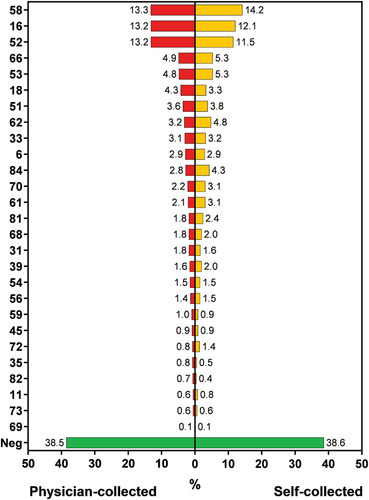
The self-sampling kit closely matched the physician-led method in terms of collecting valid specimens (100% vs. 100%). HPV types 58, 16, and 52 were the leading genotypes, followed by types 66, 53, and 18 (Figure 2 and Table S1). Out of the 1170 patients who met the eligibility criteria, 611 were found to have hrHPV in the physician-sampled specimens, while 581 were found to have hrHPV in the self-collected specimens. Multiple hrHPV types were presented in 18.7% (114/611) physician-sampled specimens and in 18.2% (106/581) self-collected specimens. The agreement of hrHPV between the physician-sampled specimens and self-collected specimens was evenly distributed in groups with an overall agreement of 87.7% (95% confidence interval [CI]: 85.8%–89.6%) (Table 2). The presence of any of the 27 HPV types was 61.5% (720/1170) in the physician-sampled specimens and 61.4% (718/1170) of the self-collected specimens, with an overall agreement 88% (95% CI: 86.2–89.9) (Table S2). Out of the 71 participants showing inconsistency, the types detected in physician-sampled but not in the self-collected specimens were HPV52 (n = 16), HPV16 (n = 12) and HPV58 (n = 12), HPV18 (n = 11), etc. In contrast, of the 69 participants with HPV types detected in the self-collected but not in the physician-sampled specimens were HPV58 (n = 18), HPV52 (n = 8), HPV84 (n = 7), HPV62 (n = 6), HPV70 (n = 6), HPV66 (n = 4), etc.
| Physician-sampled | Overall agreementa | p valueb | ||
|---|---|---|---|---|
| Self-collected | Positive | Negative | (%) (95% CI) | |
| Group 1c | ||||
| Positive | 8 | 10 | 87.2 (81.1–93.2) | <0.0001 |
| Negative | 5 | 94 | ||
| Group 2c | ||||
| Positive | 73 | 8 | 89.0 (84.3–93.6) | <0.0001 |
| Negative | 11 | 80 | ||
| Group 3c | ||||
| Positive | 66 | 23 | 81.3 (76.4–86.3) | <0.0001 |
| Negative | 22 | 130 | ||
| Group 4c | ||||
| Positive | 106 | 10 | 89.6 (85.8–93.5) | <0.0001 |
| Negative | 15 | 110 | ||
| Group 5c | ||||
| Positive | 271 | 6 | 90.0 (87.0–92.9) | <0.0001 |
| Negative | 34 | 88 | ||
| Total | ||||
| Positive | 524 | 57 | 87.7 (85.8–89.6) | <0.0001 |
| Negative | 87 | 502 | ||
- Note: hrHPV: high risk HPV including HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, 68.
- Abbreviations: CI, confidence interval; HPV, human papillomavirus; hrHPV, high-risk HPV.
- a The overall agreement was defined as either (1) presence of any hrHPV type both in the paired physician-sampled cervical specimen and self-collected vaginal specimen or (2) the absence of hrHPV both in the paired physician-sampled cervical specimen and self-collected vaginal specimen.
- b Pearson's Chi-square test was used to test for differences between two methods.
- c Group 1: no history or current cervical intraepithelial lesions or cervical malignancy. Group 2: prior history of low-grade cervical lesion or cytology within the last 3–12 months, such as ASCUS, CIN1, or AGC. Group 3: had prior treatment for CIN2/3 or a history of high-grade cytology, such as ASC-H, HSIL, CIN2, CIN3, SCC, AGC favor neoplasm, AIS, or cervical adenocarcinoma. Group 4: current low-grade cervical histology or cytology, such as ASCUS, CIN1 or AGC. Group 5: a presence of high-grade cervical histology or cytology, including ASC-H, dysplasia cannot exclude HSIL, CIN2, CIN3, CIS, SCC, AGCFN, AIS, or cervical adenocarcinoma.
The agreements and Cohen's kappa coefficients of types 16, 18, 16, or 18, hrHPV types other than 16 or 18, and all hrHPV types are 0.83, 0.71, 0.80, 0.74, and 0.75, respectively (Table 3). The kappa values suggested substantial to almost perfect agreements.
| Physician-sampled specimens | ||||
|---|---|---|---|---|
| Self-collected specimens | Positive | Negative | Agreementa (%) (95% CI) | Kappa statistic (95% CI) |
| HPV 16 | ||||
| Positive | 125 | 16 | 96.2 (95.1–97.3) | 0.83 (0.78–0.88) |
| Negative | 29 | 1000 | ||
| HPV 18 | ||||
| Positive | 32 | 7 | 97.9 (97.0–98.7) | 0.71 (0.60–0.82) |
| Negative | 18 | 1113 | ||
| HPV 16/18b | ||||
| Positive | 156 | 20 | 94.6 (93.3–95.9) | 0.80 (0.75–0.85) |
| Negative | 43 | 951 | ||
| hrHPV non-16/18 | ||||
| Positive | 380 | 69 | 87.7 (86.0–89.7) | 0.74 (0.70–0.78) |
| Negative | 73 | 648 | ||
| All hrHPV | ||||
| Positive | 524 | 57 | 87.7 (85.8–89.6) | 0.75 (0.72–0.79) |
| Negative | 87 | 502 | ||
- Abbreviations: CI, confidence interval; HPV, human papillomavirus; hrHPV, high-risk HPV.
- a Defined as either (1) presence of the specified HPV type(s) in the paired physician-sampled specimen and self-collected specimen or (2) absence of the specified HPV type(s) in both physician-sampled specimen and self-collected specimen.
- b HPV type 16 or 18.
The efficacy of HPV in identifying CIN1+ (CIN1 or more severe) histology through self-collected specimens was 78.5%, closely aligning with the 81.3% achieved with physician-sampled specimens and corresponds to a relative accuracy of 0.97 (95% CI: 0.91–1.03) (Table 4). In the realm of CIN3+ (CIN3 or more severe) detection, self-collected specimens exhibited a sensitivity of 84.7%, which was almost equivalent to the 88.9% marked by the physician-sampled specimens (relative sensitivity: 0.953 [95% CI: 0.885–1.026]; relative accuracy: 0.972 [95% CI: 0.900–1.051]). Moreover, for CIN3+, the specificity, PPV, NPV and accuracy obtained from self-collected specimens were consistent with those from physician-sampled specimens, with relative accuracies being 0.98, 0.96, 0.98, and 0.97, respectively. Such findings highlight that the HygeiaTouch Self Sampling Kit for Women holds comparable efficacy in predicting various degree of cervical lesions to the conventional physician-led sampling using a cytology brush (Table 4).
| Histology | Self-collected (% [95% CI]) | Physician-sampled (% [95% CI]) | Relative accuracy (95% CI) |
|---|---|---|---|
| CIN1 or worse | |||
| Sensitivity | 391/498 (78.5 [74.9–82.1]) | 405/498 (81.3 [77.9–84.7]) | 0.965 (0.907–1.028) |
| Specificity | 340/655 (51.9 [48.1– 55.7]) | 353/655 (53.9 [50.1–57.7]) | 0.963 (0.870–1.067) |
| PPV | 391/706 (55.4 [51.7–59.0]) | 405/707 (57.3 [53.6–60.9]) | 0.967 (0.882–1.060) |
| NPV | 340/447 (76.1 [72.1– 80.0]) | 353/4469 (79.1 [75.4–82.9]) | 0.961 (0.896–1.031) |
| Accuracy | 731/1153 (63.4 [60.6–66.2]) | 758/1153 (65.7 [63.0–68.5]) | 0.964 (0.908–1.025) |
| CIN2 or worse | |||
| Sensitivity | 279/330 (84.5 [80.6–88.4]) | 294/330 (89.1 [85.7–92.5]) | 0.949 (0.894–1.007) |
| Specificity | 396/823 (48.1 [44.7–51.5]) | 410/823 (49.8 [46.4–53.2]) | 0.966 (0.875–1.066) |
| PPV | 279/706 (39.5 [35.9–43.1]) | 294/707 (41.6 [38.0–45.2]) | 0.950 (0.838–1.078) |
| NPV | 396/447 (88.6 [85.6–91.5]) | 410/446 (91.9 [89.4–94.5]) | 0.964 (0.923–1.006) |
| Accuracy | 675/1153 (58.5 [55.7–61.4]) | 704/1153 (61.1 [58.2–63.9]) | 0.959 (0.897–1.025) |
| CIN3 or worse | |||
| Sensitivity | 183/216 (84.7 [79.9–89.5]) | 192/216 (88.9 [84.7–93.1]) | 0.953 (0.885–1.026) |
| Specificity | 414/937 (44.2 [41.0–47.4]) | 422/937 (45.0 [41.9–48.2]) | 0.981 (0.887–1.085) |
| PPV | 183/706 (25.9 [22.7–29.2]) | 192/707 (27.2 [23.9–30.4]) | 0.955 (0.802–1.135) |
| NPV | 414/447 (92.6 [90.2–95.0]) | 422/446 (94.6 [92.5–96.7]) | 0.979 (0.946–1.013) |
| Accuracy | 597/1153 (51.8 [48.9–54.7]) | 614/1153 (53.3 [50.4–56.1]) | 0.972 (0.900–1.051) |
- Abbreviations: CI, confidence interval; NPV, negative predictive value; PPV, positive predictive value.
4 DISCUSSION
Taiwanese women aged 30–69 have approximately a 30% yearly and 69% triennial cervical cancer screening rate, mostly through the Pap tests.19 These tests have been provided free of charge by the government to women aged 30 and above since 1995. Despite the implementation of a national HPV vaccination program for schoolgirls aged 13–15 in 2018, the majority of women aged 40 and older have not been vaccinated against HPV. Consequently, it is anticipated that cervical cancer screening will remain essential for this demographic in the foreseeable future.20 Introducing self-collection of vaginal specimens with easy-to-use procedures for HPV DNA detection could enhance cervical screening uptake. Earlier meta-analyses by Arbyn et al.21 showed that the sensitivity of HPV DNA testing on self-collected samples was lower than physician-sampled specimens (ratio 0.88 for CIN2+ [95% CI: 0.85–0.91]; ratio 0.89 for CIN3+ [95% CI: 0.83–0.96]). Another systemic review found a pooled Cohen's kappa of 0.66 (95% CI: 0.61–0.71) using PCR-based evaluations.22 Our research demonstrates that the identification of hrHPV types showed substantial agreement (kappa: 0.75, agreement: 87.7%), and the prediction of CIN demonstrated high relative accuracy (0.949 for CIN2+ [95% CI: 0.894–1.007]; 0.953 for CIN3+ [95% CI: 0.885–1.026]). Kappa values varied between 0.71 and 0.83 for different hrHPV types and combinations, with an overall kappa value 0.75 (95% CI: 0.72–0.79) signifying robust compatibility between the two methods. The relative accuracies in recognizing CIN stood 0.95 or above. Arbyn et al.'s23 recent meta-analysis of HPV test agreement indicated that pooled overall agreement was 88.7% (95% CI: 86.3%–90.0%), positive agreement was 84.6% (95% CI: 79.7%–88.7%). In a separate meta-analysis, the reported sensitivities were 77% (95% CI: 69%–82%) for CIN2+ detection from self-samples and 93% (95% CI: 89%–96%) from physician samples.24
Our trial flagged 140 discordant HPV positive results; 71 were negative in self-collected yet positive in physician-sampled specimens, while 69 positives in the self-collected were negative in physician-sampled specimens. This disparity was notably higher among participants previously treated for cervical lesions. Of the discordant cases, physician-sampled specimens harbored more HPV52/16/18, while self-collected specimens captured more noncarcinogenic HPV strains (e.g., HPV62, 84), possibly due to the prevalence of α3/α15 HPV strains in vaginal samples.25 Besides, the self-sampling kit tested in our study showed excellent efficiency of collecting valid specimens (self-collected 100% vs. physician-sampled 100%), while in an Australian study comparing a flocked swab self-sampling device and 6 HPV assays, proportion of invalid results were significantly higher (5 of 6 kits) in self-collected than physician-sampled specimens.13
In this investigation, we adopted a paired study design to assess sampling method agreement. To avoid bias from dual samplings on the same day and to counter potential cervical tissue damage during sampling, participants self-collected first before undergoing physician sampling. A standout strength of our trial is the ability to assess collection method efficacy in populations with a high prevalence of high-grade lesions, facilitating a robust sensitivity evaluation.
5 CONCLUSIONS
In this study, the HygeiaTouch Self Sampling Kit for Women, when employed for self-collection of vaginal cytology specimens, closely matched the physician-led cervical cytology sampling in terms of identifying HPV types and predicting cervical lesions. This compatibility was underscored by kappa values ranging from substantial to nearly perfect. These findings, in conjunction with other studies, emphasize the viability of self-sampling as an efficient, dependable, and user-friendly alternative to traditional physician-led sampling. This suggests a promising avenue to expand screening accessibility and improve cervical cancer detection rates.
AUTHOR CONTRIBUTIONS
Hung-Hsueh Chou: conceptualization, methodology, formal analysis, investigation, writing-review & editing. Chung-Yao Yang: formal analysis, writing-review & editing. Angel Chao: investigation, writing-review & editing. Hao Lin: investigation, writing-review & editing. Yu-Che Ou: investigation, writing-review & editing. Chien-Hsing Lu: investigation, writing-review & editing. Shih-Tien Hsu: investigation, writing-review & editing. Yu-Hsiang Shih: investigation, writing-review & editing. Huei-Jean Huang: investigation, writing-review & editing. Cheng-Tao Lin: investigation, writing-review & editing. Min-Yu Chen: investigation, writing-review & editing. Lou Sun: investigation, writing-review & editing. Ching-Chou Tsai: investigation, writing-review & editing. Hung-Chun Fu: investigation, writing-review & editing. Kuan-Gen Huang: investigation, writing-review & editing. Kai-Yun Wu: investigation, writing-review & editing. Chen-Hsuan Wu: investigation, writing-review & editing. Wu-Chiao Hsieh: investigation, writing-review & editing. Yi-Ting Huang: investigation, writing-review & editing. Liang-Hsuan Chen: investigation, writing-review & editing. Lan-Yan Yang: formal analysis, writing-review & editing. Wei-Yang Chang: formal analysis, writing-review & editing. Ting-Chang Chang: conceptualization, methodology, formal analysis, writing-review & editing. Chyong-Huey Lai: conceptualization, methodology, formal analysis, writing-review & editing.
ACKNOWLEDGMENTS
This work was funded by HygeiaTouch Inc. (Taipei, Taiwan).
CONFLICT OF INTEREST STATEMENT
CY Yang and TC Chang are employees of Hygeia Touch Inc. (Taipei, Taiwan).
ETHICS STATEMENT
This study was approved by the Institutional Review Boards of Chang Gung Medical Foundation (IRB No. 20200618A0) and Taichung Veterans General Hospital (IRB No. SF20101B). The ClinicalTrials.gov identifier of this trial is NCT04472377.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding authors upon reasonable request.




